Phage SPO1 Protein Gp49 Is a Novel RNA Binding Protein That Is Involved in Host Iron Metabolism
Abstract
1. Introduction
2. Results
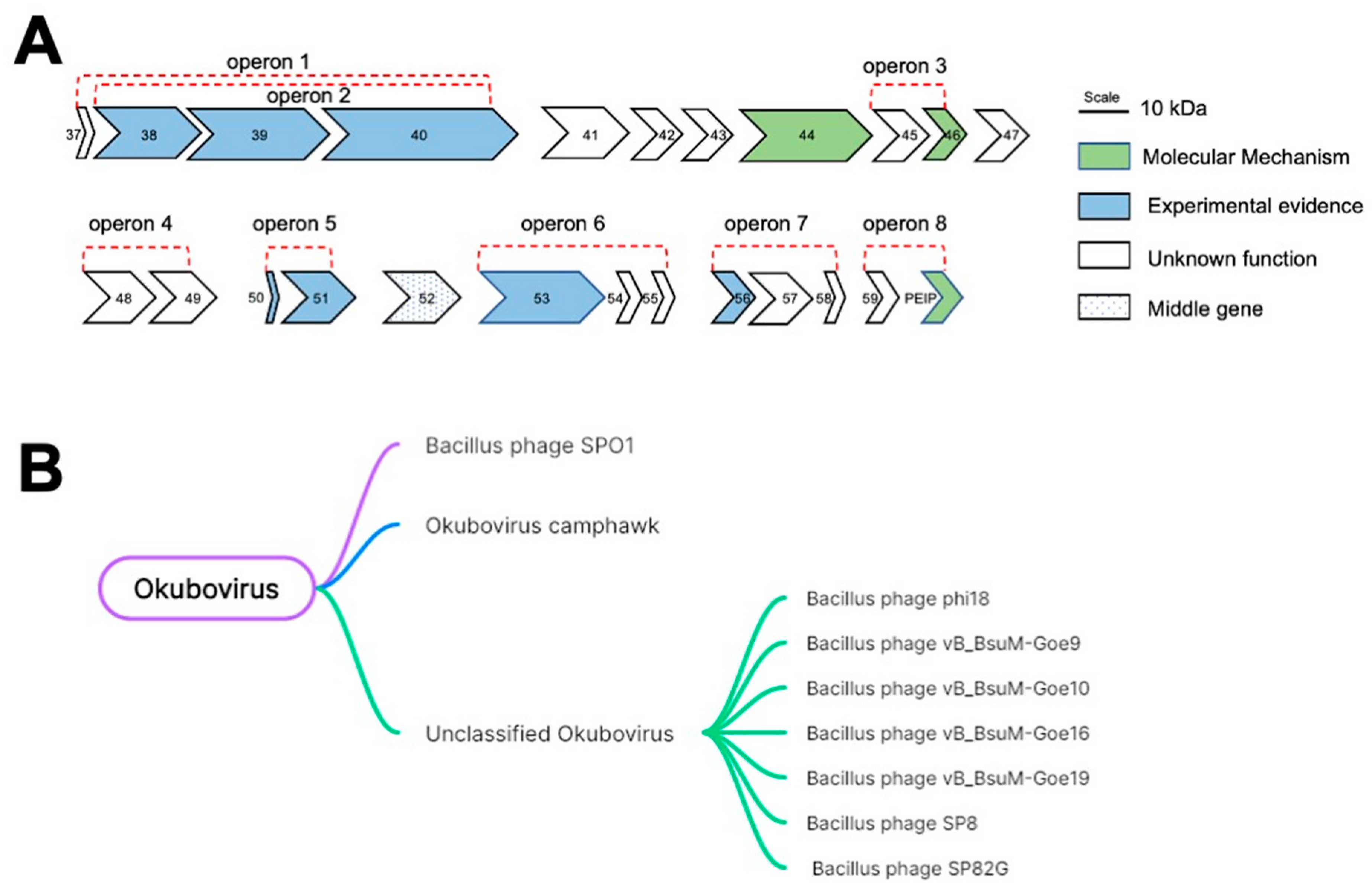
2.1. Gp49 Is Only Conserved in Bacillus Phages and Might Be Involved in Host Nucleic Acid Metabolism
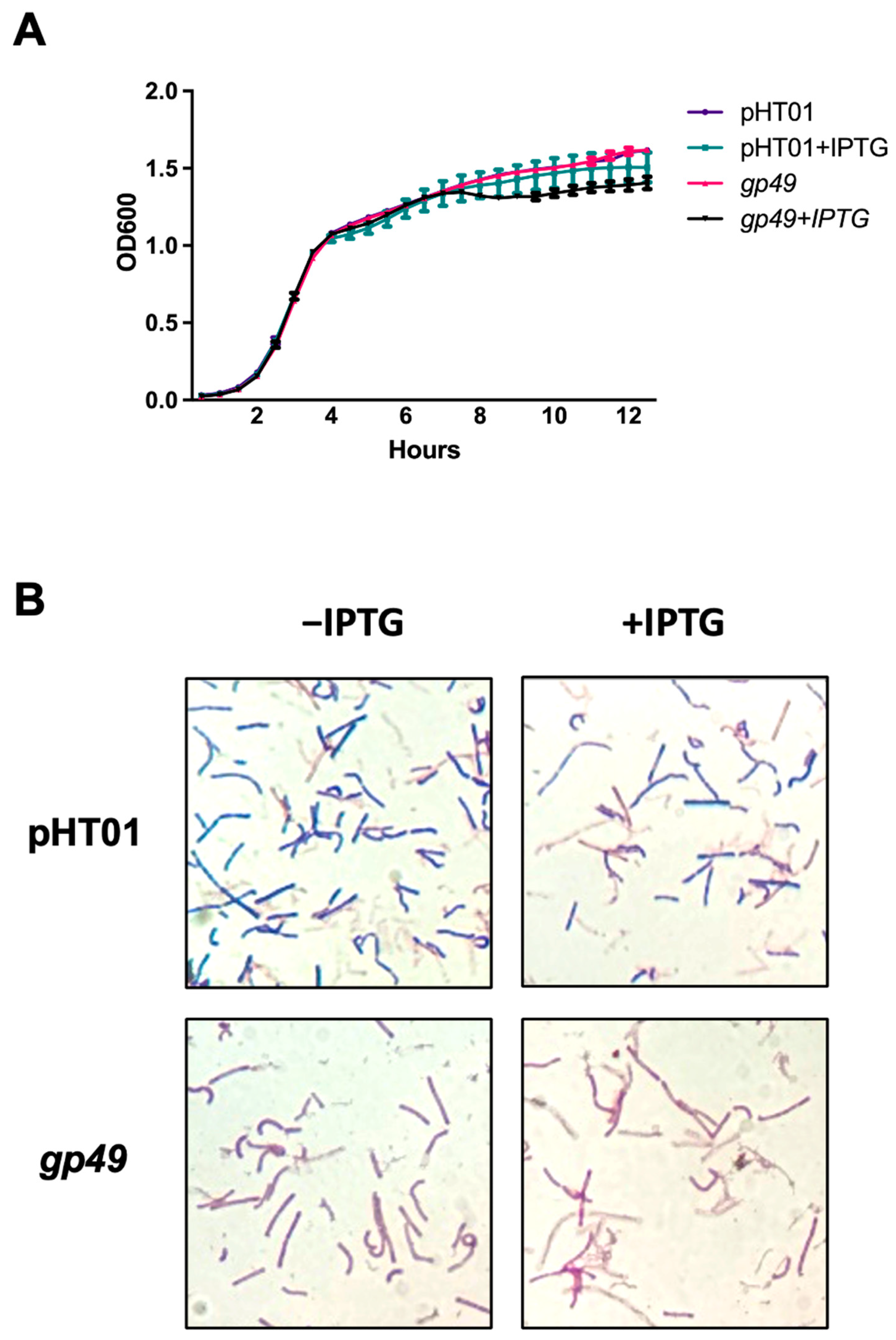
2.2. Gp49 Does Not Affect the Growth of B. subtilis in Stationary Phase
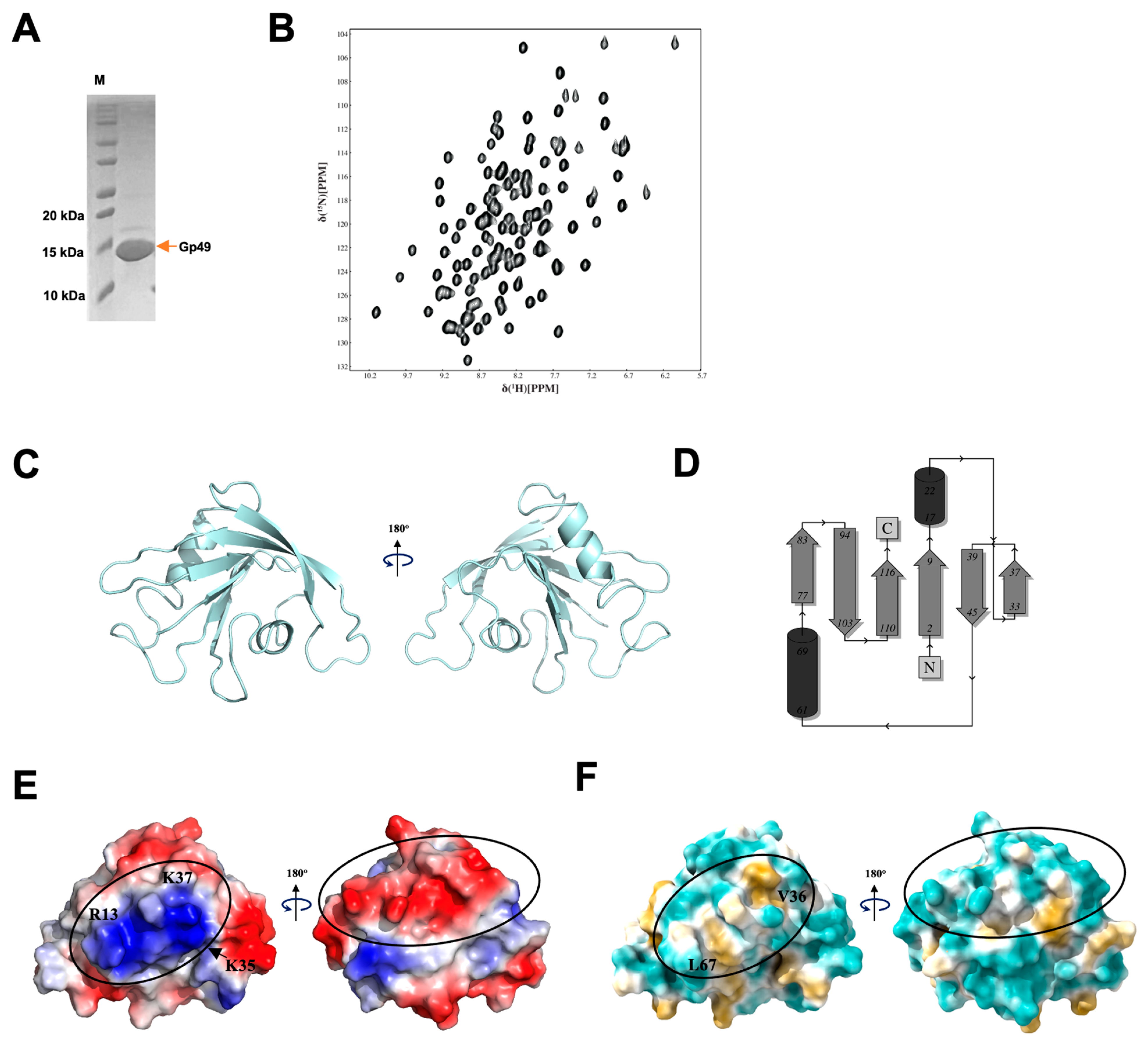
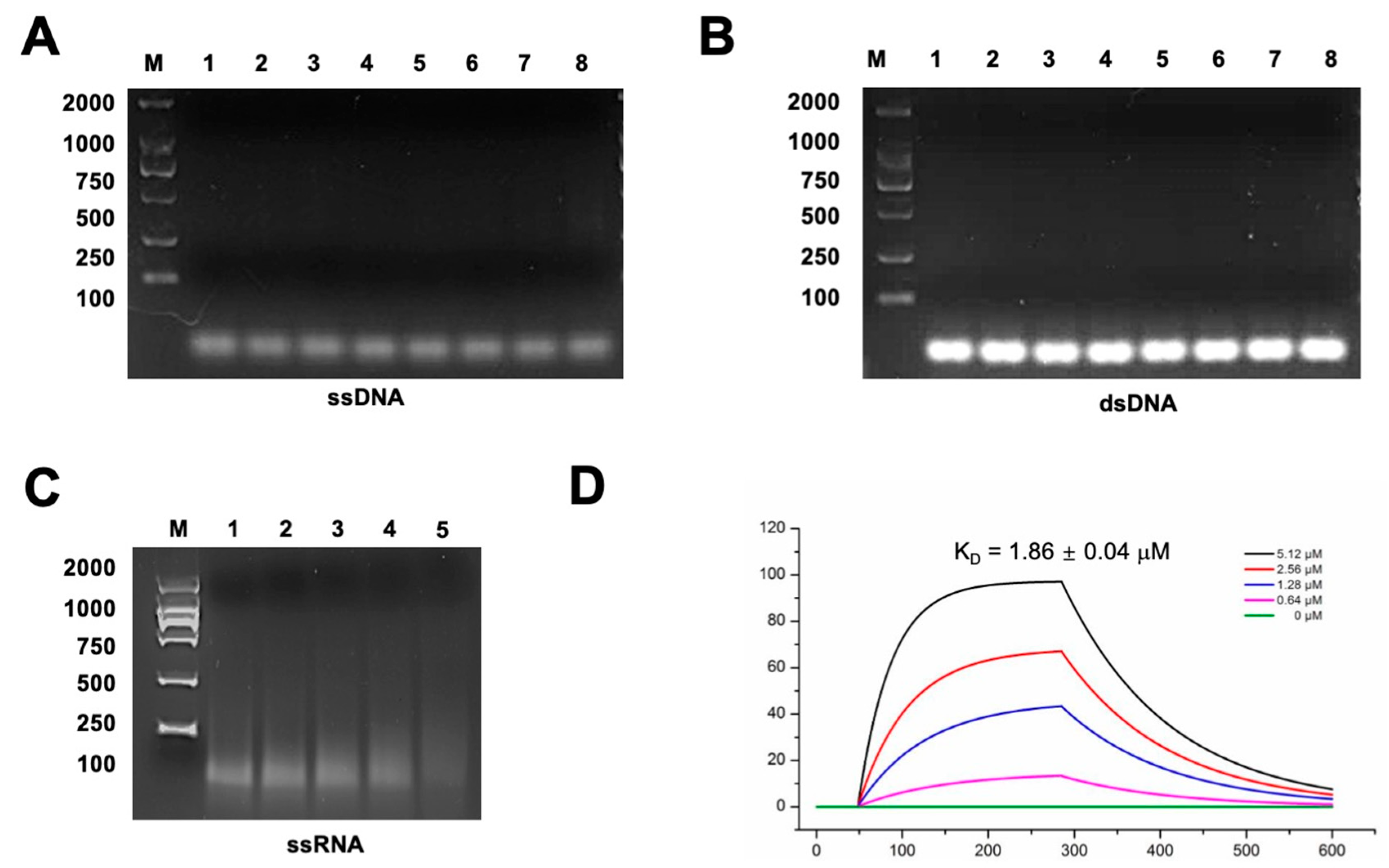
2.3. Gp49 Has a Putative Nucleic Acid Binding Motif and Binds to Single-Stranded RNA
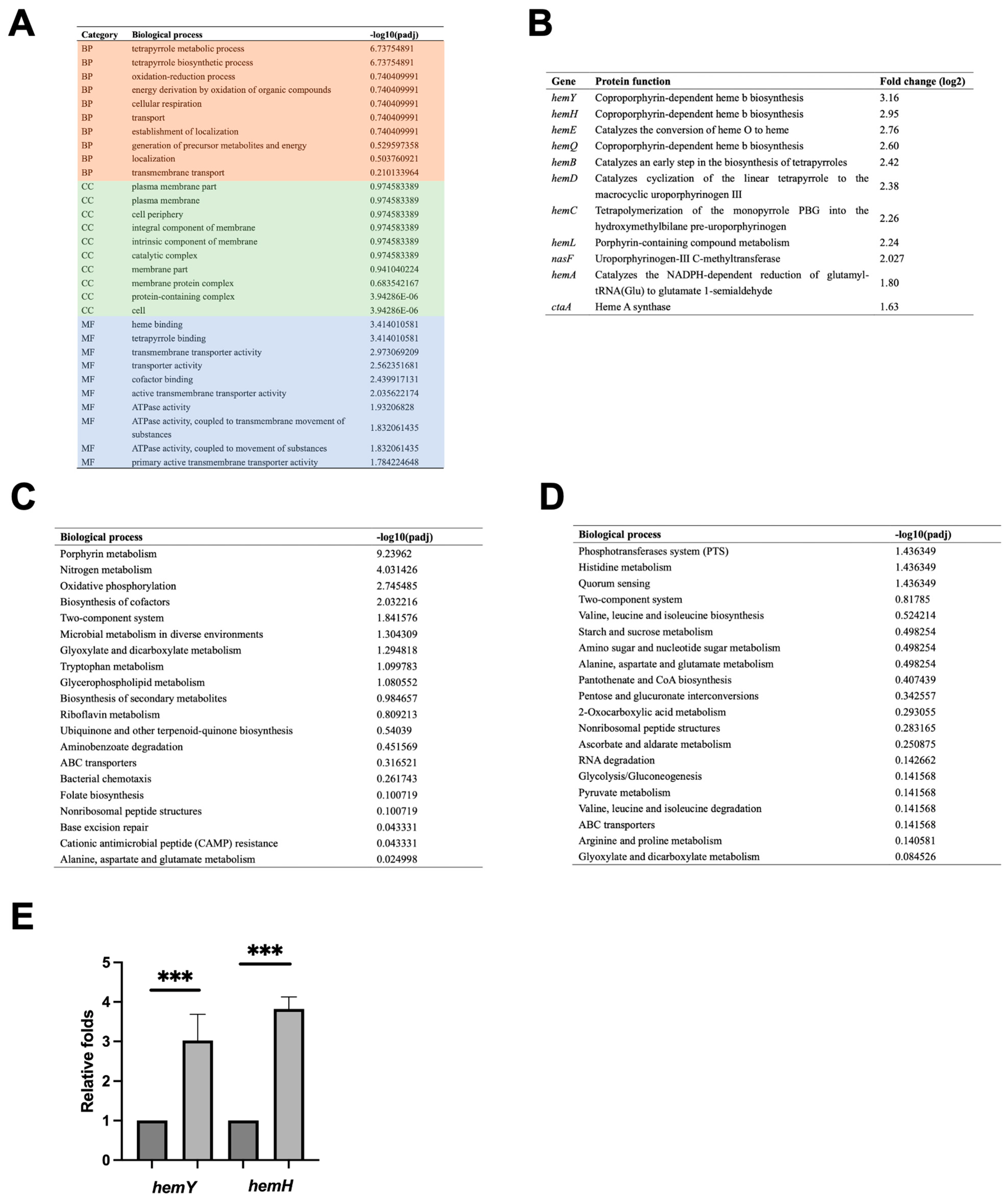
2.4. Gp49 Upregulates Host Iron Metabolism
3. Discussion
4. Material and Methods
4.1. Bacterial Growth Curve and Gram Staining
4.2. Protein Expression and Purification
4.3. Structure Determination
4.4. RNA Synthesis
4.5. Electrophoretic Mobility Shift Assay (EMSA)
4.6. Surface Plasmon Resonance
4.7. RNA Extraction and RNA-Seq Analysis
4.8. Real-Time Quantitative PCR (RT-qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okubo, S.; Yanagida, T.; Fujita, D.J.; Olsson-Wilhelm, B.M. The genetics of bacteriophage SPO1. Biken J. 1972, 15, 81–97. [Google Scholar] [PubMed]
- Stewart, C.R.; Casjens, S.R.; Cresawn, S.G.; Houtz, J.M.; Smith, A.L.; Ford, M.E.; Peebles, C.L.; Hatfull, G.F.; Hendrix, R.W.; Huang, W.M.; et al. The genome of Bacillus subtilis bacteriophage SPO1. J. Mol. Biol. 2009, 388, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, J.; Lavigne, R.; Loessner, M.J.; Ackermann, H.W. The SPO1-related bacteriophages. Arch. Virol. 2010, 155, 1547–1561. [Google Scholar] [CrossRef]
- Hoet, P.P.; Coene, M.M.; Cocito, C.G. Replication cycle of Bacillus subtilis hydroxymethyluracil-containing phages. Annu. Rev. Microbiol. 1992, 46, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Sayre, M.H.; Geiduschek, E.P. TF1, the bacteriophage SPO1-encoded type II DNA-binding protein, is essential for viral multiplication. J. Virol. 1988, 62, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Mulvenna, N.; Sanz-Hernandez, M.; Zhang, P.; Li, Y.; Ma, J.; Wang, Y.; Matthews, S.; Wigneshweraraj, S.; et al. A Bacteriophage DNA Mimic Protein Employs a Non-specific Strategy to Inhibit the Bacterial RNA Polymerase. Front. Microbiol. 2021, 12, 692512. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Wang, Y.; Du, K.; Wang, Z.; Yu, J.; Chang, G.; Matthews, S.; Wang, H.; Liu, B. Bacteriophage protein Gp46 is a cross-species inhibitor of nucleoid-associated HU proteins. Proc. Natl. Acad. Sci. USA 2022, 119, e2116278119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, S.; Wang, Y.; Wang, Z.; Mulvenna, N.; Yang, H.; Zhang, P.; Chen, H.; Li, Y.; Wang, H.; et al. Bacteriophage protein PEIP is a potent Bacillus subtilis enolase inhibitor. Cell Rep. 2022, 40, 111026. [Google Scholar] [CrossRef]
- Mulvenna, N.; Hantke, I.; Burchell, L.; Nicod, S.; Bell, D.; Turgay, K.; Wigneshweraraj, S. Xenogeneic modulation of the ClpCP protease of Bacillus subtilis by a phage-encoded adaptor-like protein. J. Biol. Chem. 2019, 294, 17501–17511. [Google Scholar] [CrossRef]
- Bhambhani, A.; Iadicicco, I.; Lee, J.; Ahmed, S.; Belfatto, M.; Held, D.; Marconi, A.; Parks, A.; Stewart, C.R.; Margolin, W.; et al. Bacteriophage SP01 Gene Product 56 Inhibits Bacillus subtilis Cell Division by Interacting with FtsL and Disrupting Pbp2B and FtsW Recruitment. J. Bacteriol. 2020, 203, e00463-20. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodriguez-Quinones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Dautant, A.; Meyer, J.B.; Yariv, J.; Precigoux, G.; Sweet, R.M.; Kalb, A.J.; Frolow, F. Structure of a monoclinic crystal from of cyctochrome b1 (Bacterioferritin) from E. coli. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Svistunenko, D.A.; Wilson, M.T.; Hemmings, A.M.; Moore, G.R.; Le Brun, N.E. Bacterial iron detoxification at the molecular level. J. Biol. Chem. 2020, 295, 17602–17623. [Google Scholar] [CrossRef] [PubMed]
- Heffron, J.; McDermid, B.; Mayer, B.K. Bacteriophage inactivation as a function of ferrous iron oxidation. Environ. Sci. Water Res. Technol. 2019, 5, 1309–1317. [Google Scholar] [CrossRef]
- Sampath, A.; Stewart, C.R. Roles of genes 44, 50, and 51 in regulating gene expression and host takeover during infection of Bacillus subtilis by bacteriophage SPO1. J. Bacteriol. 2004, 186, 1785–1792. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Smith, M.C.M.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary relationships among diverse bacteriophages and prophages: All the world’s a phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197. [Google Scholar] [CrossRef]
- Miller, E.S.; Kutter, E.; Mosig, G.; Arisaka, F.; Kunisawa, T.; Ruger, W. Bacteriophage T4 genome. Microbiol. Mol. Biol. R 2003, 67, 86–156. [Google Scholar] [CrossRef]
- Holm, L. Benchmarking fold detection by DaliLite v.5. Bioinformatics 2019, 35, 5326–5327. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D 2004, 60, 2256–2268. [Google Scholar] [CrossRef]
- Govindarajan, D.K.; Meghanathan, Y.; Sivaramakrishnan, M.; Kothandan, R.; Muthusamy, A.; Seviour, T.W.; Kandaswamy, K. Enterococcus faecalis thrives in dual-species biofilm models under iron-rich conditions. Arch. Microbiol. 2022, 204, 710. [Google Scholar] [CrossRef]
- Hong, H.A.; Khaneja, R.; Tam, N.M.; Cazzato, A.; Tan, S.; Urdaci, M.; Brisson, A.; Gasbarrini, A.; Barnes, I.; Cutting, S.M. Bacillus subtilis isolated from the human gastrointestinal tract. Res. Microbiol. 2009, 160, 134–143. [Google Scholar] [CrossRef]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of Bacillus subtilis Strains on Intestinal Barrier Function and Inflammatory Response. Front. Immunol. 2019, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhu, F.; Li, Y.; Pan, S.L.; Wang, H.L.; Yang, Z.; Wang, Z.H.; Hu, Z.Y.; Yu, J.F.; Barritt, J.D.; et al. Bacteriophages allow selective depletion of gut bacteria to produce a targeted-bacterium-depleted mouse model. Cell Rep. Methods 2022, 2, 100324. [Google Scholar] [CrossRef]
- Borodovich, T.; Shkoporov, A.N.; Ross, R.P.; Hill, C. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol. Rep. 2022, 10, goac012. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Yip, T.K.; Myles, B.; Laughlin, L. Roles of genes 38, 39, and 40 in shutoff of host biosyntheses during infection of Bacillus subtilis by bacteriophage SPO1. Virology 2009, 392, 271–274. [Google Scholar] [CrossRef][Green Version]
- Caza, M.; Kronstad, J.W. Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front. Cell. Infect. Microbiol. 2013, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Chareyre, S.; Mandin, P. Bacterial Iron Homeostasis Regulation by sRNAs. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M. Bacterioferritin: Structure, Dynamics, and Protein-Protein Interactions at Play in Iron Storage and Mobilization. Acc. Chem. Res. 2017, 50, 331–340. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef]
- Cherayil, B.J. The role of iron in the immune response to bacterial infection. Immunol. Res. 2011, 50, 1–9. [Google Scholar] [CrossRef]
- Binnenkade, L.; Teichmann, L.; Thormann, K.M. Iron triggers lambdaSo prophage induction and release of extracellular DNA in Shewanella oneidensis MR-1 biofilms. Appl. Environ. Microbiol. 2014, 80, 5304–5316. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Hu, Z.; Kang, Y.; Gao, J.; Chen, H.; Liu, H.; Wang, Y.; Liu, B. Phage SPO1 Protein Gp49 Is a Novel RNA Binding Protein That Is Involved in Host Iron Metabolism. Int. J. Mol. Sci. 2023, 24, 14318. https://doi.org/10.3390/ijms241814318
Yang Y, Hu Z, Kang Y, Gao J, Chen H, Liu H, Wang Y, Liu B. Phage SPO1 Protein Gp49 Is a Novel RNA Binding Protein That Is Involved in Host Iron Metabolism. International Journal of Molecular Sciences. 2023; 24(18):14318. https://doi.org/10.3390/ijms241814318
Chicago/Turabian StyleYang, Yanan, Zhenyue Hu, Yue Kang, Juanjuan Gao, Huan Chen, Hui Liu, Yawen Wang, and Bing Liu. 2023. "Phage SPO1 Protein Gp49 Is a Novel RNA Binding Protein That Is Involved in Host Iron Metabolism" International Journal of Molecular Sciences 24, no. 18: 14318. https://doi.org/10.3390/ijms241814318
APA StyleYang, Y., Hu, Z., Kang, Y., Gao, J., Chen, H., Liu, H., Wang, Y., & Liu, B. (2023). Phage SPO1 Protein Gp49 Is a Novel RNA Binding Protein That Is Involved in Host Iron Metabolism. International Journal of Molecular Sciences, 24(18), 14318. https://doi.org/10.3390/ijms241814318





