Recent Advances in Bacterial Persistence Mechanisms
Abstract
:1. Introduction
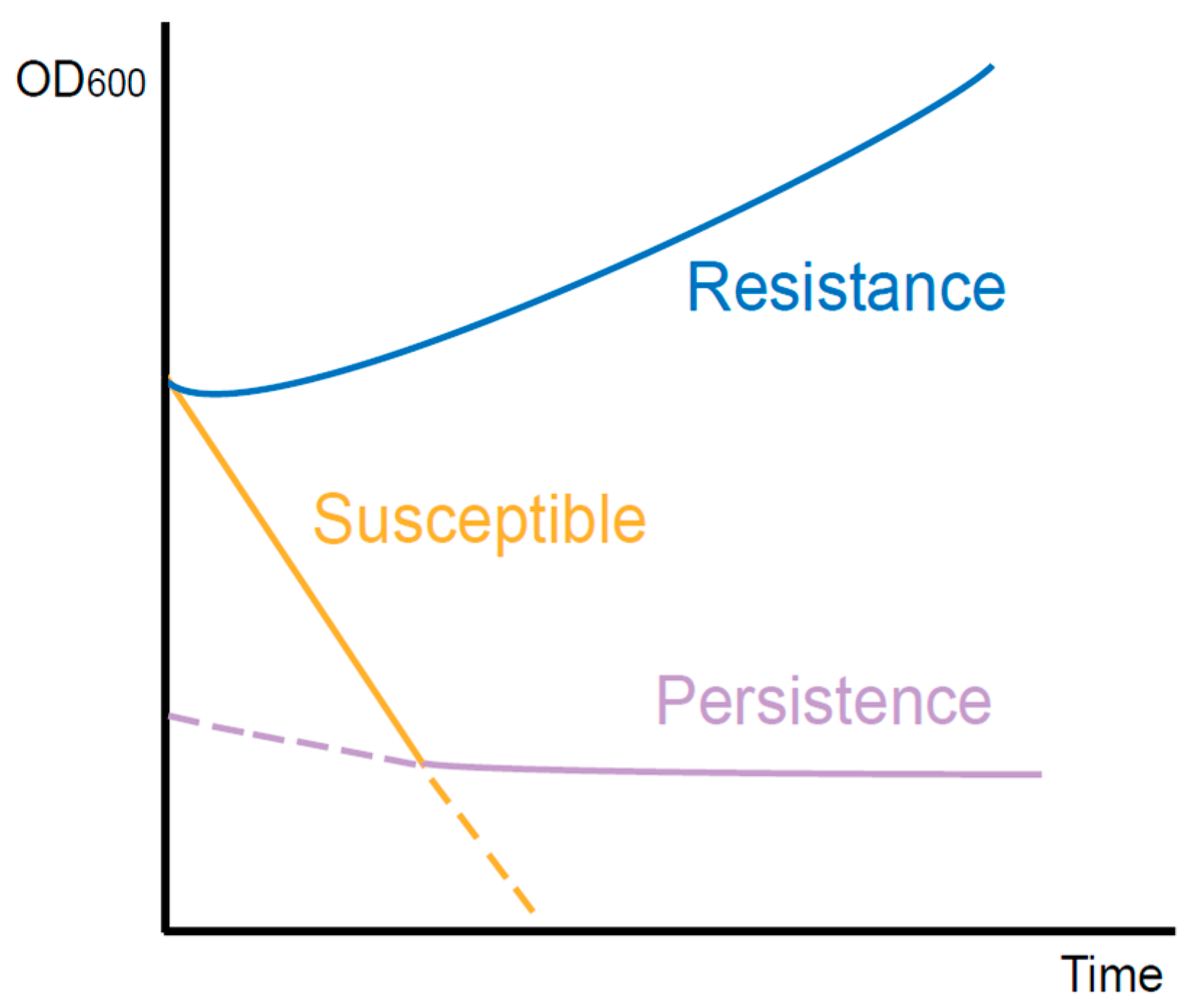
2. The Difference between Persistence and Resistance
3. Research on the Mechanism of Bacterial Persistence
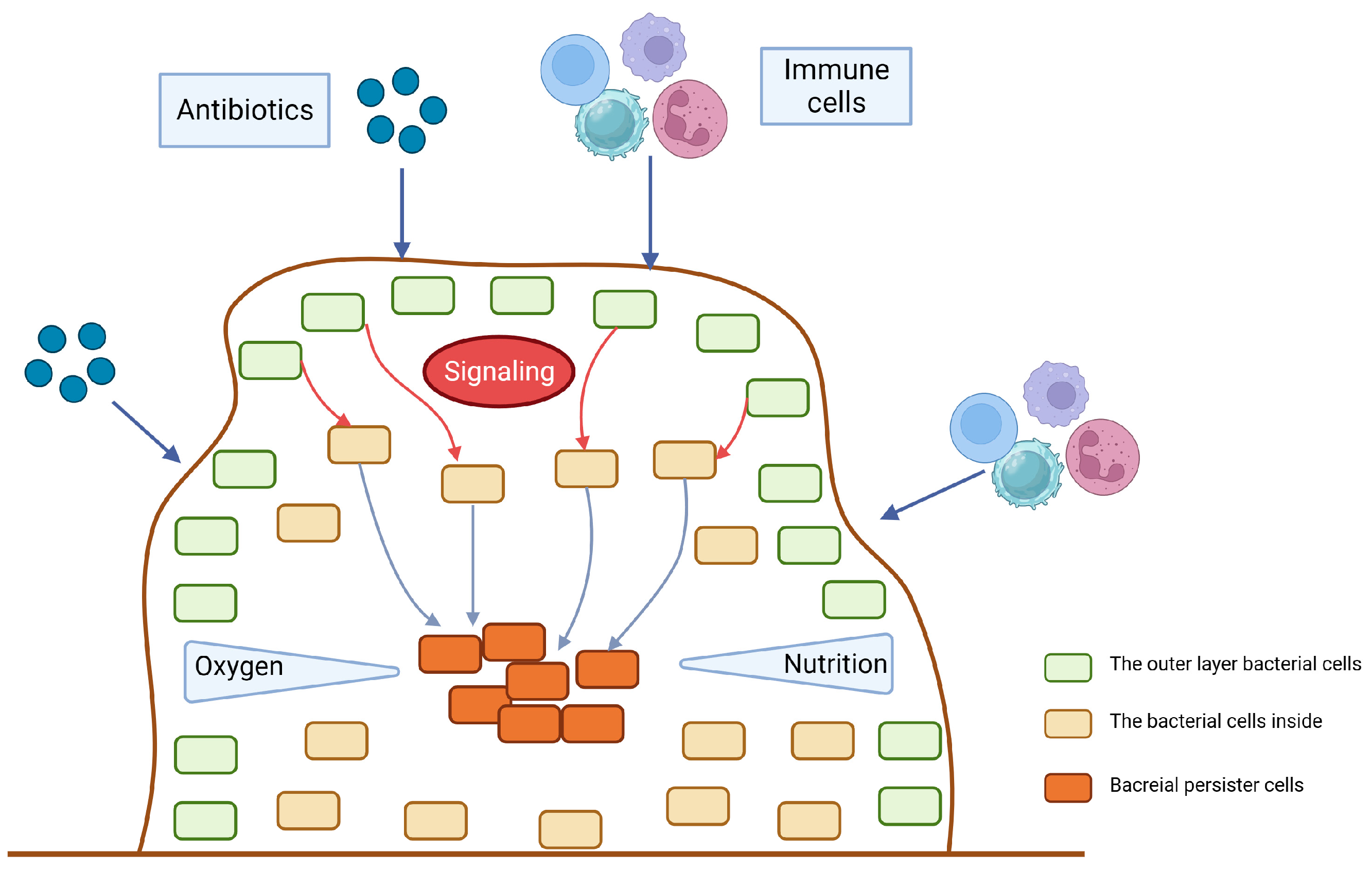
3.1. Biofilm
3.2. Toxin–Antitoxin Modules
3.3. ppGpp and Stringent Response
3.4. SOS Response
4. Treatment of Persisters
| Strategies | Targets/Options | Examples |
|---|---|---|
| Directly target the existing persisters | Bacterial membranes and cell walls | AM-0016 [72], boromycin [73] |
| Drug combination | Daptomycin combined with ceftaroline [74] | |
| Anti-cancer drugs | Mitomycin C [75] | |
| Block persister formation | SOS response | Engineered bacteriophage [76] |
| Biofilm formation | Quercetin [78], Dispersin B [79], peptide 1018 [80] | |
| Before the infection | Vaccination [4] | |
| Resuscitate persisters | Signaling pathways | Cis-2-decenoic acid [81] |
| Stimulate antibiotic influx | Silver [83] |
5. Recent Progress of Technologies on Persister Studies
5.1. Enrichment Methods
5.2. From the Single-Cell Level
6. Remaining Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bigger, J.W. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 1944, 244, 497–500. [Google Scholar] [CrossRef]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Zhao, Z.; Pu, Y.; Bai, F. Bacterial persistence. Sci. China Chem. 2014, 57, 1625–1633. [Google Scholar] [CrossRef]
- Gollan, B.; Grabe, G.; Michaux, C.; Helaine, S. Bacterial Persisters and Infection: Past, Present, and Progressing. Annu. Rev. Microbiol. 2019, 73, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Peyrusson, F.; Varet, H.; Nguyen, T.K.; Legendre, R.; Sismeiro, O.; Coppee, J.Y.; Wolz, C.; Tenson, T.; Van Bambeke, F. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat. Commun. 2020, 11, 2200. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Kim, J.S.; Yamasaki, R.; Song, S.; Zhang, W.; Wood, T.K. Single cell observations show persister cells wake based on ribosome content. Environ. Microbiol. 2018, 20, 2085–2098. [Google Scholar] [CrossRef]
- Rollin, G.; Tan, X.; Tros, F.; Dupuis, M.; Nassif, X.; Charbit, A.; Coureuil, M. Intracellular Survival of Staphylococcus aureus in Endothelial Cells: A Matter of Growth or Persistence. Front. Microbiol. 2017, 8, 1354. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Xu, T.; Zhang, W.H.; Zhang, Y. Molecular mechanisms of bacterial persistence and phenotypic antibiotic resistance. Yi Chuan 2016, 38, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Beroz, F.; Yan, J.; Sabass, B.; Stone, H.A.; Bassler, B.L.; Wingreen, N.S.; Meir, Y. Verticalization of bacterial biofilms. Nat. Phys. 2018, 14, 954–960. [Google Scholar] [CrossRef]
- Helaine, S.; Cheverton, A.M.; Watson, K.G.; Faure, L.M.; Matthews, S.A.; Holden, D.W. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 2014, 343, 204–208. [Google Scholar] [CrossRef]
- Burmolle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homoe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef]
- Cendra, M.D.M.; Blanco-Cabra, N.; Pedraz, L.; Torrents, E. Optimal environmental and culture conditions allow the in vitro coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in stable biofilms. Sci. Rep. 2019, 9, 16284. [Google Scholar] [CrossRef]
- Conlon, B.P. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: An investigation of persister cells, their formation and their role in S. aureus disease. Bioessays 2014, 36, 991–996. [Google Scholar] [CrossRef]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Ziemyte, M.; Carda-Dieguez, M.; Rodriguez-Diaz, J.C.; Ventero, M.P.; Mira, A.; Ferrer, M.D. Real-time monitoring of Pseudomonas aeruginosa biofilm growth dynamics and persister cells’ eradication. Emerg. Microbes Infect. 2021, 10, 2062–2075. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Uzoechi, S.C.; Abu-Lail, N.I. Variations in the Morphology, Mechanics and Adhesion of Persister and Resister E. coli Cells in Response to Ampicillin: AFM Study. Antibiotics 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Joshi-Datar, A.; Lepine, F.; Bauerle, E.; Olakanmi, O.; Beer, K.; McKay, G.; Siehnel, R.; Schafhauser, J.; Wang, Y.; et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 2011, 334, 982–986. [Google Scholar] [CrossRef]
- Ernst, C.M.; Braxton, J.R.; Rodriguez-Osorio, C.A.; Zagieboylo, A.P.; Li, L.; Pironti, A.; Manson, A.L.; Nair, A.V.; Benson, M.; Cummins, K.; et al. Adaptive evolution of virulence and persistence in carbapenem-resistant Klebsiella pneumoniae. Nat. Med. 2020, 26, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Harrison, E.M.; Bi, D.; Tai, C.; He, X.; Ou, H.Y.; Rajakumar, K.; Deng, Z. TADB: A web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res. 2011, 39, D606–D611. [Google Scholar] [CrossRef] [PubMed]
- Hayes, F.; Kedzierska, B. Regulating toxin-antitoxin expression: Controlled detonation of intracellular molecular timebombs. Toxins 2014, 6, 337–358. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Shakespeare, L.J.; Jorgensen, M.G.; Gerdes, K. Bacterial persistence by RNA endonucleases. Proc. Natl. Acad. Sci. USA 2011, 108, 13206–13211. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, Targets, and Triggers: An Overview of Toxin-Antitoxin Biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Jurenas, D.; Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Biology and evolution of bacterial toxin-antitoxin systems. Nat. Rev. Microbiol. 2022, 20, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, N.; Knapen, W.J.; Kint, C.I.; Liebens, V.; Van den Bergh, B.; Dewachter, L.; Michiels, J.E.; Fu, Q.; David, C.C.; Fierro, A.C.; et al. Obg and Membrane Depolarization Are Part of a Microbial Bet-Hedging Strategy that Leads to Antibiotic Tolerance. Mol. Cell 2015, 59, 9–21. [Google Scholar] [CrossRef]
- Keren, I.; Shah, D.; Spoering, A.; Kaldalu, N.; Lewis, K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 2004, 186, 8172–8180. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Piro, K.M.; Xu, W.; Hansen, S.; Lewis, K.; Brennan, R.G. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 2009, 323, 396–401. [Google Scholar] [CrossRef]
- Korch, S.B.; Henderson, T.A.; Hill, T.M. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol. Microbiol. 2003, 50, 1199–1213. [Google Scholar] [CrossRef]
- Shah, D.; Zhang, Z.; Khodursky, A.; Kaldalu, N.; Kurg, K.; Lewis, K. Persisters: A distinct physiological state of E. coli. BMC Microbiol. 2006, 6, 53. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef] [PubMed]
- Donegan, N.P.; Cheung, A.L. Regulation of the mazEF toxin-antitoxin module in Staphylococcus aureus and its impact on sigB expression. J. Bacteriol. 2009, 191, 2795–2805. [Google Scholar] [CrossRef]
- Hooven, T.A.; Catomeris, A.J.; Bonakdar, M.; Tallon, L.J.; Santana-Cruz, I.; Ott, S.; Daugherty, S.C.; Tettelin, H.; Ratner, A.J. The Streptococcus agalactiae Stringent Response Enhances Virulence and Persistence in Human Blood. Infect. Immun. 2018, 86, e00612-17. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Buchad, H.; Gajjar, D. Pseudomonas aeruginosa persister cell formation upon antibiotic exposure in planktonic and biofilm state. Sci. Rep. 2022, 12, 16151. [Google Scholar] [CrossRef]
- Boutte, C.C.; Crosson, S. Bacterial lifestyle shapes stringent response activation. Trends Microbiol. 2013, 21, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tozawa, Y.; Nomura, Y. Signalling by the global regulatory molecule ppGpp in bacteria and chloroplasts of land plants. Plant Biol. (Stuttg) 2011, 13, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Viducic, D.; Ono, T.; Murakami, K.; Susilowati, H.; Kayama, S.; Hirota, K.; Miyake, Y. Functional analysis of spoT, relA and dksA genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol. Immunol. 2006, 50, 349–357. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Castro-Camargo, M.; Gerdes, K. (p)ppGpp Controls Bacterial Persistence by Stochastic Induction of Toxin-Antitoxin Activity. Cell 2018, 172, 1135. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.K.; Chan, E.W.; Chin, M.L.; Chan, R.C. Delineation of a bacterial starvation stress response network which can mediate antibiotic tolerance development. Antimicrob. Agents Chemother. 2010, 54, 1082–1093. [Google Scholar] [CrossRef]
- Chakraborty, A.; Halder, S.; Kishore, P.; Saha, D.; Saha, S.; Sikder, K.; Basu, A. The structure-function analysis of Obg-like GTPase proteins along the evolutionary tree from bacteria to humans. Genes. Cells 2022, 27, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, M.S.; Veress, A.; Harms, A.; Mitarai, N.; Semsey, S. Birth and Resuscitation of (p)ppGpp Induced Antibiotic Tolerant Persister Cells. Sci. Rep. 2019, 9, 6056. [Google Scholar] [CrossRef]
- Si, L.; Gu, J.; Wen, M.; Wang, R.; Fleming, J.; Li, J.; Xu, J.; Bi, L.; Deng, J. relA Inactivation Converts Sulfonamides Into Bactericidal Compounds. Front. Microbiol. 2021, 12, 698468. [Google Scholar] [CrossRef]
- Atkinson, G.C.; Tenson, T.; Hauryliuk, V. The RelA/SpoT homolog (RSH) superfamily: Distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE 2011, 6, e23479. [Google Scholar] [CrossRef]
- Baek, M.S.; Chung, E.S.; Jung, D.S.; Ko, K.S. Effect of colistin-based antibiotic combinations on the eradication of persister cells in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020, 75, 917–924. [Google Scholar] [CrossRef]
- Chowdhury, N.; Kwan, B.W.; Wood, T.K. Persistence Increases in the Absence of the Alarmone Guanosine Tetraphosphate by Reducing Cell Growth. Sci. Rep. 2016, 6, 20519. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, X.; Chen, Z.; Lyu, Z.; Lin, Z.; Zheng, J.; Wu, Y.; Deng, Q.; Yu, Z.; Zhang, Y.; et al. Staphylococcus aureus PhoU Homologs Regulate Persister Formation and Virulence. Front. Microbiol. 2020, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.G.; Ortiz, J.H.; Schneider, R.P.; Spira, B. phoU inactivation in Pseudomonas aeruginosa enhances accumulation of ppGpp and polyphosphate. Appl. Environ. Microbiol. 2015, 81, 3006–3015. [Google Scholar] [CrossRef] [PubMed]
- Ferullo, D.J.; Lovett, S.T. The stringent response and cell cycle arrest in Escherichia coli. PLoS Genet. 2008, 4, e1000300. [Google Scholar] [CrossRef]
- Prossliner, T.; Skovbo Winther, K.; Sorensen, M.A.; Gerdes, K. Ribosome Hibernation. Annu. Rev. Genet. 2018, 52, 321–348. [Google Scholar] [CrossRef]
- Krasny, L.; Gourse, R.L. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 2004, 23, 4473–4483. [Google Scholar] [CrossRef]
- Geiger, T.; Goerke, C.; Fritz, M.; Schafer, T.; Ohlsen, K.; Liebeke, M.; Lalk, M.; Wolz, C. Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect. Immun. 2010, 78, 1873–1883. [Google Scholar] [CrossRef]
- Shahi, I.; Llaneras, C.N.; Perelman, S.S.; Torres, V.J.; Ratner, A.J. Genome-Wide CRISPR-Cas9 Screen Does Not Identify Host Factors Modulating Streptococcus agalactiae beta-Hemolysin/Cytolysin-Induced Cell Death. Microbiol. Spectr. 2022, 10, e0218621. [Google Scholar] [CrossRef]
- Andresen, L.; Varik, V.; Tozawa, Y.; Jimmy, S.; Lindberg, S.; Tenson, T.; Hauryliuk, V. Auxotrophy-based High Throughput Screening assay for the identification of Bacillus subtilis stringent response inhibitors. Sci. Rep. 2016, 6, 35824. [Google Scholar] [CrossRef]
- Wexselblatt, E.; Oppenheimer-Shaanan, Y.; Kaspy, I.; London, N.; Schueler-Furman, O.; Yavin, E.; Glaser, G.; Katzhendler, J.; Ben-Yehuda, S. Relacin, a novel antibacterial agent targeting the Stringent Response. PLoS Pathog. 2012, 8, e1002925. [Google Scholar] [CrossRef]
- Podlesek, Z.; Zgur Bertok, D. The DNA Damage Inducible SOS Response Is a Key Player in the Generation of Bacterial Persister Cells and Population Wide Tolerance. Front. Microbiol. 2020, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Theodore, A.; Lewis, K.; Vulic, M. Tolerance of Escherichia coli to fluoroquinolone antibiotics depends on specific components of the SOS response pathway. Genetics 2013, 195, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Guerin, E.; Cambray, G.; Sanchez-Alberola, N.; Campoy, S.; Erill, I.; Da Re, S.; Gonzalez-Zorn, B.; Barbe, J.; Ploy, M.C.; Mazel, D. The SOS response controls integron recombination. Science 2009, 324, 1034. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.W.S.; Moldoveanu, A.L.; Sargen, M.; Ronneau, S.; Glegola-Madejska, I.; Beetham, C.; Fisher, R.A.; Helaine, S. The vulnerable versatility of Salmonella antibiotic persisters during infection. Cell Host Microbe 2021, 29, 1757–1773. [Google Scholar] [CrossRef]
- Little, J.W. Mechanism of specific LexA cleavage: Autodigestion and the role of RecA coprotease. Biochimie 1991, 73, 411–421. [Google Scholar] [CrossRef]
- McCourt, J.; O’Halloran, D.P.; McCarthy, H.; O’Gara, J.P.; Geoghegan, J.A. Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol. Lett. 2014, 353, 157–164. [Google Scholar] [CrossRef]
- Goormaghtigh, F.; Van Melderen, L. Single-cell imaging and characterization of Escherichia coli persister cells to ofloxacin in exponential cultures. Sci. Adv. 2019, 5, eaav9462. [Google Scholar] [CrossRef]
- Chakraborty, P.; Bajeli, S.; Kaushal, D.; Radotra, B.D.; Kumar, A. Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis. Nat. Commun. 2021, 12, 1606. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef]
- Mukherjee, D.; Zou, H.; Liu, S.; Beuerman, R.; Dick, T. Membrane-targeting AM-0016 kills mycobacterial persisters and shows low propensity for resistance development. Future Microbiol. 2016, 11, 643–650. [Google Scholar] [CrossRef]
- Moreira, W.; Aziz, D.B.; Dick, T. Boromycin Kills Mycobacterial Persisters without Detectable Resistance. Front. Microbiol. 2016, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.O.; Heil, E.L.; Covert, K.L.; Cluck, D.B. Treatment strategies for persistent methicillin-resistant Staphylococcus aureus bacteraemia. J. Clin. Pharm. Ther. 2018, 43, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.W.; Chowdhury, N.; Wood, T.K. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015, 17, 4406–4414. [Google Scholar] [CrossRef]
- Lu, T.K.; Collins, J.J. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 4629–4634. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Kaplan, J.B. Therapeutic potential of biofilm-dispersing enzymes. Int. J. Artif. Organs 2009, 32, 545–554. [Google Scholar] [CrossRef]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob. Agents Chemother. 2004, 48, 4360–4365. [Google Scholar] [CrossRef]
- Marques, C.N.; Morozov, A.; Planzos, P.; Zelaya, H.M. The fatty acid signaling molecule cis-2-decenoic acid increases metabolic activity and reverts persister cells to an antimicrobial-susceptible state. Appl. Environ. Microbiol. 2014, 80, 6976–6991. [Google Scholar] [CrossRef] [PubMed]
- Defraine, V.; Fauvart, M.; Michiels, J. Fighting bacterial persistence: Current and emerging anti-persister strategies and therapeutics. Drug Resist. Updat. 2018, 38, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra181. [Google Scholar] [CrossRef] [PubMed]
- Vega, N.M.; Allison, K.R.; Khalil, A.S.; Collins, J.J. Signaling-mediated bacterial persister formation. Nat. Chem. Biol. 2012, 8, 431–433. [Google Scholar] [CrossRef]
- Henry, T.C.; Brynildsen, M.P. Development of Persister-FACSeq: A method to massively parallelize quantification of persister physiology and its heterogeneity. Sci. Rep. 2016, 6, 25100. [Google Scholar] [CrossRef]
- Adikari, S.; Hong-Geller, E.; Micheva-Viteva, S. Methods for Enrichment of Bacterial Persister Populations for Phenotypic Screens and Genomic Studies. Methods Mol. Biol. 2021, 2357, 71–82. [Google Scholar] [CrossRef]
- Keren, I.; Minami, S.; Rubin, E.; Lewis, K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio 2011, 2, e00100–e00111. [Google Scholar] [CrossRef]
- Wang, C.; Chen, R.; Xu, J.; Jin, L. Single-cell Raman spectroscopy identifies Escherichia coli persisters and reveals their enhanced metabolic activities. Front. Microbiol. 2022, 13, 936726. [Google Scholar] [CrossRef]
- Windels, E.; Van den Bergh, B.; Michiels, J. Enrichment of Persister Cells Through Beta-Lactam-Induced Filamentation and Size Separation. Methods Mol. Biol. 2021, 2357, 63–69. [Google Scholar] [CrossRef]
- Micheva-Viteva, S.N.; Shakya, M.; Adikari, S.H.; Gleasner, C.D.; Velappan, N.; Mourant, J.R.; Chain, P.S.G.; Hong-Geller, E. A Gene Cluster That Encodes Histone Deacetylase Inhibitors Contributes to Bacterial Persistence and Antibiotic Tolerance in Burkholderia thailandensis. mSystems 2020, 5, e00609-19. [Google Scholar] [CrossRef]
- Johnson, P.J.; Levin, B.R. Pharmacodynamics, population dynamics, and the evolution of persistence in Staphylococcus aureus. PLoS Genet. 2013, 9, e1003123. [Google Scholar] [CrossRef] [PubMed]
- Dorr, T.; Vulic, M.; Lewis, K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 2010, 8, e1000317. [Google Scholar] [CrossRef] [PubMed]
- Windels, E.M.; Ben Meriem, Z.; Zahir, T.; Verstrepen, K.J.; Hersen, P.; Van den Bergh, B.; Michiels, J. Enrichment of persisters enabled by a ss-lactam-induced filamentation method reveals their stochastic single-cell awakening. Commun. Biol. 2019, 2, 426. [Google Scholar] [CrossRef] [PubMed]
- Iino, R.; Sakakihara, S.; Matsumoto, Y.; Nishino, K. Single-Cell Detection and Collection of Persister Bacteria in a Directly Accessible Femtoliter Droplet Array. Methods Mol. Biol. 2016, 1333, 101–109. [Google Scholar] [CrossRef]
- Hare, P.J.; LaGree, T.J.; Byrd, B.A.; DeMarco, A.M.; Mok, W.W.K. Single-Cell Technologies to Study Phenotypic Heterogeneity and Bacterial Persisters. Microorganisms 2021, 9, 2277. [Google Scholar] [CrossRef]
- Ma, P.; Amemiya, H.M.; He, L.L.; Gandhi, S.J.; Nicol, R.; Bhattacharyya, R.P.; Smillie, C.S.; Hung, D.T. Bacterial droplet-based single-cell RNA-seq reveals antibiotic-associated heterogeneous cellular states. Cell 2023, 186, 877–891. [Google Scholar] [CrossRef]
- Ueno, H.; Kato, Y.; Tabata, K.V.; Noji, H. Revealing the Metabolic Activity of Persisters in Mycobacteria by Single-Cell D(2)O Raman Imaging Spectroscopy. Anal. Chem. 2019, 91, 15171–15178. [Google Scholar] [CrossRef]
- Spanka, D.T.; Konzer, A.; Edelmann, D.; Berghoff, B.A. High-Throughput Proteomics Identifies Proteins With Importance to Postantibiotic Recovery in Depolarized Persister Cells. Front. Microbiol. 2019, 10, 378. [Google Scholar] [CrossRef]
- Iino, R.; Matsumoto, Y.; Nishino, K.; Yamaguchi, A.; Noji, H. Design of a large-scale femtoliter droplet array for single-cell analysis of drug-tolerant and drug-resistant bacteria. Front. Microbiol. 2013, 4, 300. [Google Scholar] [CrossRef]
- Dawson, E.; Simsek, E.; Kim, M. Observing Bacterial Persistence at Single-Cell Resolution. Methods Mol. Biol. 2021, 2357, 85–93. [Google Scholar] [CrossRef]
- Marro, F.C.; Laurent, F.; Josse, J.; Blocker, A.J. Methods to monitor bacterial growth and replicative rates at the single-cell level. FEMS Microbiol. Rev. 2022, 46, fuac030. [Google Scholar] [CrossRef]
- Potvin-Trottier, L.; Luro, S.; Paulsson, J. Microfluidics and single-cell microscopy to study stochastic processes in bacteria. Curr. Opin. Microbiol. 2018, 43, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Balomenos, A.D.; Stefanou, V.; Manolakos, E.S. Analytics and visualization tools to characterize single-cell stochasticity using bacterial single-cell movie cytometry data. BMC Bioinform. 2021, 22, 531. [Google Scholar] [CrossRef] [PubMed]
- Wakamoto, Y.; Dhar, N.; Chait, R.; Schneider, K.; Signorino-Gelo, F.; Leibler, S.; McKinney, J.D. Dynamic persistence of antibiotic-stressed mycobacteria. Science 2013, 339, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schulze-Makuch, D.; de Vera, J.P.; Cockell, C.; Leya, T.; Baque, M.; Walther-Antonio, M. The Development of an Effective Bacterial Single-Cell Lysis Method Suitable for Whole Genome Amplification in Microfluidic Platforms. Micromachines 2018, 9, 367. [Google Scholar] [CrossRef]
- Mohiuddin, S.G.; Kavousi, P.; Orman, M.A. Flow-cytometry analysis reveals persister resuscitation characteristics. BMC Microbiol. 2020, 20, 202. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wood, T.K. ppGpp ribosome dimerization model for bacterial persister formation and resuscitation. Biochem. Biophys. Res. Commun. 2020, 523, 281–286. [Google Scholar] [CrossRef]
- Wilmaerts, D.; Focant, C.; Matthay, P.; Michiels, J. Transcription-coupled DNA repair underlies variation in persister awakening and the emergence of resistance. Cell Rep. 2022, 38, 110427. [Google Scholar] [CrossRef]
- Mok, W.W.K.; Brynildsen, M.P. Timing of DNA damage responses impacts persistence to fluoroquinolones. Proc. Natl. Acad. Sci. USA 2018, 115, E6301–E6309. [Google Scholar] [CrossRef]
- Orman, M.A.; Brynildsen, M.P. Dormancy is not necessary or sufficient for bacterial persistence. Antimicrob. Agents Chemother. 2013, 57, 3230–3239. [Google Scholar] [CrossRef]
- Stapels, D.A.C.; Hill, P.W.S.; Westermann, A.J.; Fisher, R.A.; Thurston, T.L.; Saliba, A.E.; Blommestein, I.; Vogel, J.; Helaine, S. Salmonella persisters undermine host immune defenses during antibiotic treatment. Science 2018, 362, 1156–1160. [Google Scholar] [CrossRef] [PubMed]

| Technique | Principle | Advantages | Disadvantages | Application |
|---|---|---|---|---|
| BacDrop | Droplet-based technique for bacterial single-cell RNA-seq | Enables the massively parallel transcriptional profiling of millions of single bacterial cells | Prior knowledge of the genomes of interest species is required | Evaluate the heterogeneity in K. pneumoniae [96] |
| Single-cell Raman spectroscopy | Based on the D2O absorption rate and carbon-deuterium Raman band which reflects the metabolic activity of a single cell | A powerful tool to identify persisters and normal bacteria at the single-cell level to allow further downstream analyses | It is challenging to directly detect Raman spectra with the presence of non-persister cells | Evaluate the biochemical properties of E. coli and persisters [88] |
| A directly accessible femtoliter droplet array | A micron-sized femtoliter droplet array is fixed on a hydrophilic-in-hydrophobic micropatterned surface | (i) Individual droplets can be collected with a micropipette. (ii) This array can be used for gene and protein analyses | It is challenging to form a thin and uniform CYTOP layer as the surface | Collect the single cells of P. aeruginosa and microscopically observe them [94] |
| Fluorescence dilution | Uses a susceptible strain harboring a plasmid that encodes a dose-dependent tetracycline-inducible gfp gene | Directly visualize and measure bacterial replication | The bacterial strain is inserted with a reporter | Monitor the lysis of intracellular S. aureus [7] |
| Single-cell imaging using glass-bottom dishes and a nutrient agarose pad | A liquid culture is sandwiched in a glass bottom dish beneath the nutrient agarose pad which can maintain a consistent environment around the cells | Provides a long-term single-cell microscopy observation to capture high-quality quantitative information | The thickness of glass-bottom dishes needs to be adjusted to different microscopes | Characterize the lag phase and persistence of individual E. coli cells [100] |
| Microfluidics coupled to fluorescence microscopy | Microfabricated chamber holds cells against the glass imaging surface to maintain a single focal plane during perfusion-based imaging experiments, and psulA::gfp plasmid is used as a fluorescent reporter | Acquire quantitative data and visualize the nucleoids in individual cells | It is limited to morphological observations | Analyze the persistence of wild-type E. coli to ofloxacin during stationary growth and follow the dynamics of the SOS response [68] |
| ViSCAR | Useful information from complex time-lapse bacterial single-cell movies is transformed into a digital representation | It is an open-source software tool and provides multiple ways to visualize cell attribute trends | Data source should be based on the time-lapse microscopy live-cell imaging | Investigate the contribution of single-cell heterogeneity to emerging cellular phenotypes at different scales [103] |
| Atomic force microscopy | Silicon nitride cantilevers are selected to perform force measurements on the bacterial surface | Studying persister phenotypic means from a morphological level | AMF is limited to characterizing changes that occur on the bacterial surface | Quantify the contributions of the membrane surface properties of MDR-E. coli strains [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Liu, W.; Du, Q.; Zhang, H.; Han, D. Recent Advances in Bacterial Persistence Mechanisms. Int. J. Mol. Sci. 2023, 24, 14311. https://doi.org/10.3390/ijms241814311
Pan X, Liu W, Du Q, Zhang H, Han D. Recent Advances in Bacterial Persistence Mechanisms. International Journal of Molecular Sciences. 2023; 24(18):14311. https://doi.org/10.3390/ijms241814311
Chicago/Turabian StylePan, Xiaozhou, Wenxin Liu, Qingqing Du, Hong Zhang, and Dingding Han. 2023. "Recent Advances in Bacterial Persistence Mechanisms" International Journal of Molecular Sciences 24, no. 18: 14311. https://doi.org/10.3390/ijms241814311
APA StylePan, X., Liu, W., Du, Q., Zhang, H., & Han, D. (2023). Recent Advances in Bacterial Persistence Mechanisms. International Journal of Molecular Sciences, 24(18), 14311. https://doi.org/10.3390/ijms241814311





