Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy
Abstract
1. Introduction
2. Results
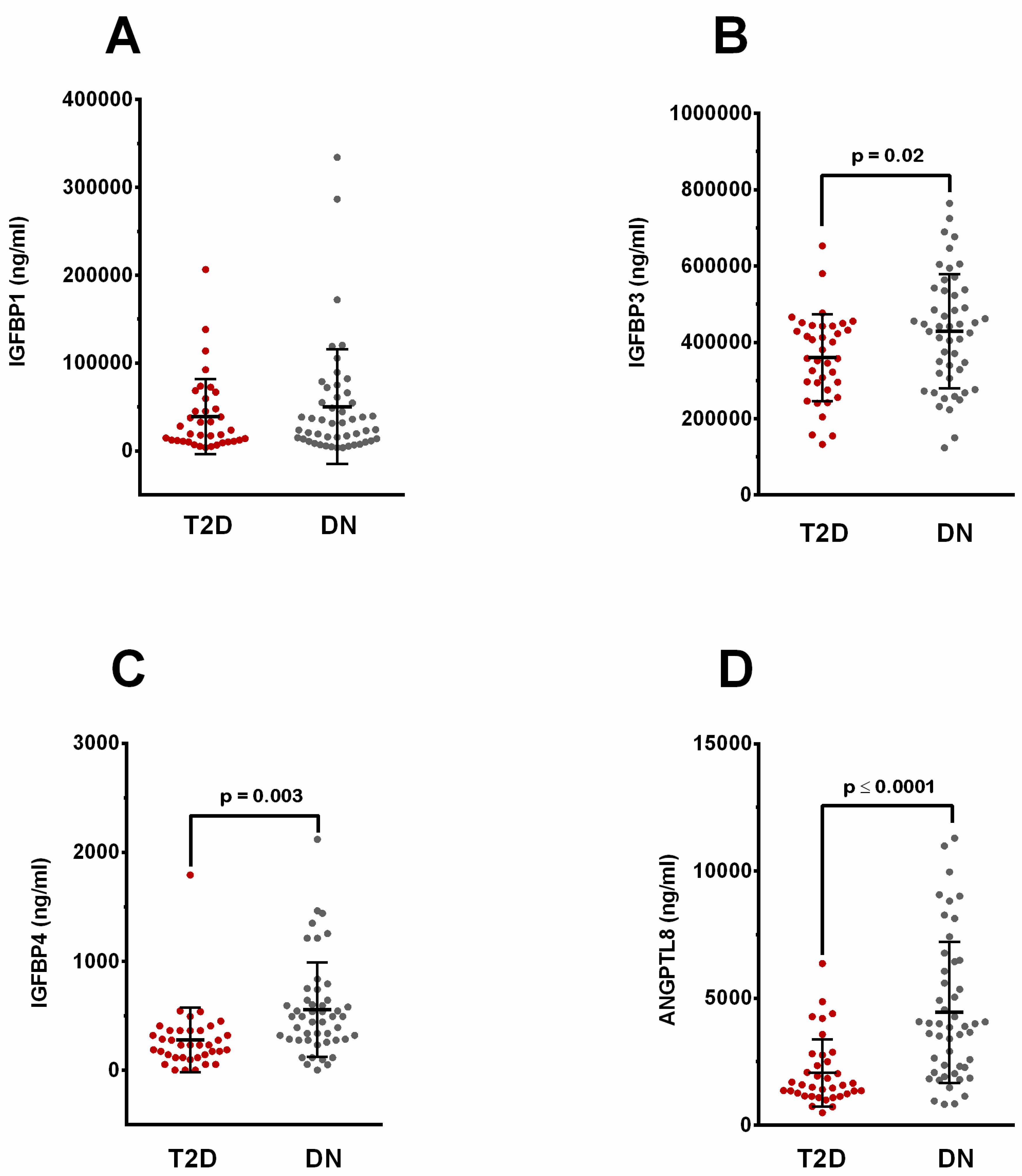
2.1. Elevated Levels of Circulating IGFBP-1, -3, -4, and ANGPTL8 in People with Diabetic Nephropathy
2.2. Elevated IGFBP-4 and ANGPTL8 Are Correlated with Clinical Parameters of Nephropathy
2.3. ANGPTL8 Is Correlated with IGFBP-4 in Nephropathy
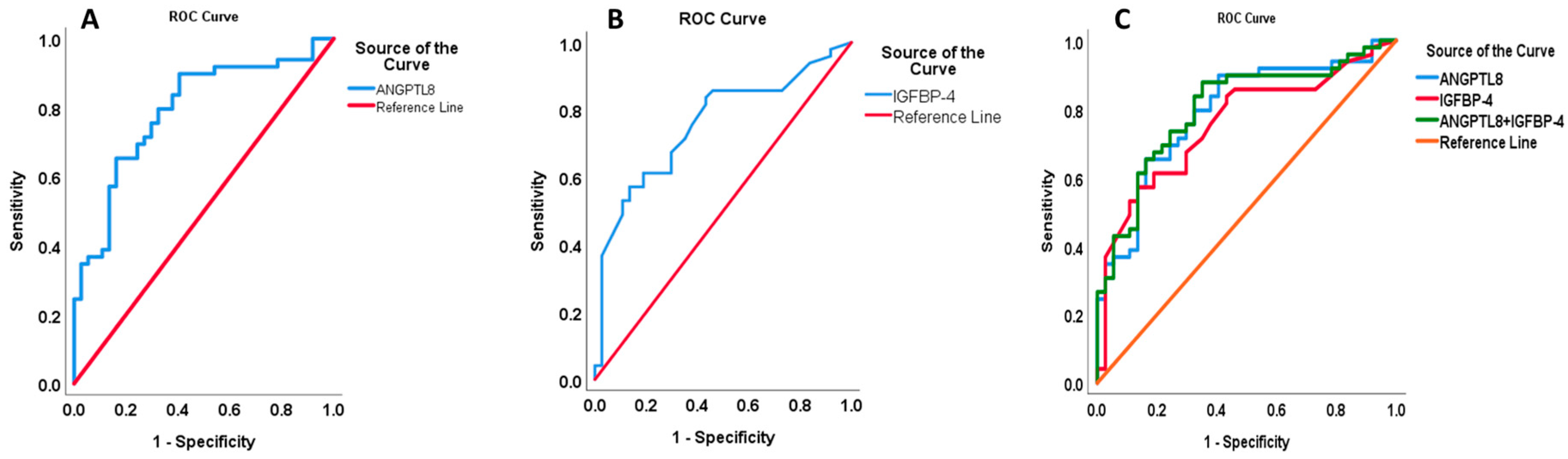
2.4. Predictive Parameters of ANGPTL8 Increase in Nephropathy
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Groups Definitions
4.3. Anthropometric and Biochemical Measurements
4.4. Levels of Serum and Urinary Creatinine and Urinary Protein
4.5. ANGPTL8 Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Quantifying IGFBP-1, -3, and -4
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Metascreen Writing, Committee; Bonadonna, R.; Cucinotta, D.; Fedele, D.; Riccardi, G.; Tiengo, A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: Results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care 2006, 29, 2701–2707. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2011, 29, 116–122. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Castoldi, G.; di Gioia, C.R.; Bombardi, C.; Maestroni, S.; Carletti, R.; Steckelings, U.M.; Dahlof, B.; Unger, T.; Zerbini, G.; Stella, A. Prevention of diabetic nephropathy by compound 21, selective agonist of angiotensin type 2 receptors, in Zucker diabetic fatty rats. Am. J. Physiol. Ren. Physiol. 2014, 307, F1123–F1131. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C. The Role of Inflammatory Cytokines in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef]
- Sai Varsha, M.K.; Raman, T.; Manikandan, R.; Dhanasekaran, G. Hypoglycemic action of vitamin K1 protects against early-onset diabetic nephropathy in streptozotocin-induced rats. Nutrition 2015, 31, 1284–1292. [Google Scholar] [CrossRef]
- Quagliarini, F.; Wang, Y.; Kozlitina, J.; Grishin, N.V.; Hyde, R.; Boerwinkle, E.; Valenzuela, D.M.; Murphy, A.J.; Cohen, J.C.; Hobbs, H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 2012, 109, 19751–19756. [Google Scholar] [CrossRef]
- Ren, G.; Kim, J.Y.; Smas, C.M. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E334–E351. [Google Scholar] [CrossRef]
- Zhang, R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012, 424, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quagliarini, F.; Gusarova, V.; Gromada, J.; Valenzuela, D.M.; Cohen, J.C.; Hobbs, H.H. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, 16109–16114. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Abubaker, J.; Al-Khairi, I.; Cherian, P.; Noronha, F.; Hu, F.B.; Behbehani, K.; Elkum, N. Higher plasma betatrophin/ANGPTL8 level in Type 2 Diabetes subjects does not correlate with blood glucose or insulin resistance. Sci. Rep. 2015, 5, 10949. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Al Madhoun, A.; Abubaker, J. The Rise and the Fall of Betatrophin/ANGPTL8 as an Inducer of beta-Cell Proliferation. J. Diabetes Res. 2016, 2016, 4860595. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Abubaker, J.; Tuomilehto, J. ANGPTL8 (betatrophin) role in diabetes and metabolic diseases. Diabetes/Metab. Res. Rev. 2017, 33, e2919. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Abubaker, J.; Al-Khairi, I.; Cherian, P.; Noronha, F.; Kavalakatt, S.; Khadir, A.; Behbehani, K.; Alarouj, M.; Bennakhi, A. Circulating angiopoietin-like protein 8 (betatrophin) association with HsCRP and metabolic syndrome. Cardiovasc. Diabetol. 2016, 15, 25. [Google Scholar] [CrossRef]
- Zou, H.; Xu, Y.; Chen, X.; Yin, P.; Li, D.; Li, W.; Xie, J.; Shao, S.; Liu, L.; Yu, X. Predictive values of ANGPTL8 on risk of all-cause mortality in diabetic patients: Results from the REACTION Study. Cardiovasc. Diabetol. 2020, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, J.; Zhang, X.; Xiao, L.; Hu, X.; Pan, H.; Qin, L.; Liu, H.; Ge, B.; Zheng, T. Association of Serum Angiopoietin-Like Protein 8 With Albuminuria in Type 2 Diabetic Patients: Results from the GDMD Study in China. Front. Endocrinol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Vasylyeva, T.L.; Ferry, R.J., Jr. Novel roles of the IGF-IGFBP axis in etiopathophysiology of diabetic nephropathy. Diabetes Res. Clin. Pract. 2007, 76, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A.; Hale, L.J. Insulin-like growth factors and kidney disease. Am. J. Kidney Dis. 2015, 65, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Gama Axelsson, T.; Heimburger, O.; Barany, P.; Lindholm, B.; Stenvinkel, P.; Qureshi, A.R. IGF-1 and survival in ESRD. Clin. J. Am. Soc. Nephrol. 2014, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Gunter, M.J.; Wylie-Rosett, J.; Ho, G.Y.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Strickler, H.D. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab. Res. Rev. 2009, 25, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Aneke-Nash, C.S.; Xue, X.; Qi, Q.; Biggs, M.L.; Cappola, A.; Kuller, L.; Pollak, M.; Psaty, B.M.; Siscovick, D.; Mukamal, K.; et al. The Association between IGF-I and IGFBP-3 and Incident Diabetes in an Older Population of Men and Women in the Cardiovascular Health Study. J. Clin. Endocrinol. Metab. 2017, 102, 4541–4547. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Brismar, K.; Wang, J.; Wivall-Helleryd, I.L.; Sindelar, P.; Gonzalez, F.J.; Bergman, T.; Bobek, G.A. Responses of insulin-like growth factor (IGF)-I and IGF-binding proteins to nutritional status in peroxisome proliferator-activated receptor-alpha knockout mice. Growth Horm. IGF Res. 2001, 11, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, A.; Chiarelli, F.; Vorwerk, P.; Boscherini, B.; Rosenfeld, R.G. Evaluation of the components of insulin-like growth factor (IGF)-IGF binding protein (IGFBP) system in adolescents with type 1 diabetes and persistent microalbuminuria: Relationship with increased urinary excretion of IGFBP-3 18 kD N-terminal fragment. Clin. Endocrinol. 1999, 51, 587–596. [Google Scholar] [CrossRef]
- Wheatcroft, S.B.; Kearney, M.T.; Shah, A.M.; Grieve, D.J.; Williams, I.L.; Miell, J.P.; Crossey, P.A. Vascular endothelial function and blood pressure homeostasis in mice overexpressing IGF binding protein-1. Diabetes 2003, 52, 2075–2082. [Google Scholar] [CrossRef]
- Meza Letelier, C.E.; San Martin Ojeda, C.A.; Ruiz Provoste, J.J.; Frugone Zaror, C.J. Pathophysiology of diabetic nephropathy: A literature review. Medwave 2017, 17, e6839. [Google Scholar] [CrossRef]
- Papadopoulou-Marketou, N.; Kanaka-Gantenbein, C.; Marketos, N.; Chrousos, G.P.; Papassotiriou, I. Biomarkers of diabetic nephropathy: A 2017 update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 326–342. [Google Scholar] [CrossRef]
- Sharma, D.; Bhattacharya, P.; Kalia, K.; Tiwari, V. Diabetic nephropathy: New insights into established therapeutic paradigms and novel molecular targets. Diabetes Res. Clin. Pract. 2017, 128, 91–108. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N. The role of novel biomarkers in predicting diabetic nephropathy: A review. Int. J. Nephrol. Renovasc. Dis. 2017, 10, 221–231. [Google Scholar] [CrossRef]
- Van Krieken, R.; Krepinsky, J.C. Caveolin-1 in the Pathogenesis of Diabetic Nephropathy: Potential Therapeutic Target? Curr. Diabetes Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y. The insulin-like growth factor system in chronic kidney disease: Pathophysiology and therapeutic opportunities. Kidney Res. Clin. Pract. 2012, 31, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, T. Insulin-Like Growth Factor Binding Proteins in Autoimmune Diseases. Front. Endocrinol. 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Kaskel, F. Growth hormone axis in chronic kidney disease. Pediatr. Nephrol. 2008, 23, 41–48. [Google Scholar] [CrossRef]
- Song, C.; Wang, S.; Fu, Z.; Chi, K.; Geng, X.; Liu, C.; Cai, G.; Chen, X.; Wu, D.; Hong, Q. IGFBP5 promotes diabetic kidney disease progression by enhancing PFKFB3-mediated endothelial glycolysis. Cell Death Dis. 2022, 13, 340. [Google Scholar] [CrossRef]
- Zhang, R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol. 2016, 6, 150272. [Google Scholar] [CrossRef]
- Al-Shawaf, E.; Al-Ozairi, E.; Al-Asfar, F.; Al-Beloushi, S.; Kumari, S.; Tuomilehto, J.; Arefanian, H. Biphasic changes in angiopoietin-like 8 level after laparoscopic sleeve gastrectomy and type 2 diabetes remission during a 1-year follow-up. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2018, 14, 1284–1294. [Google Scholar] [CrossRef]
- Chen, X.; Lu, P.; He, W.; Zhang, J.; Liu, L.; Yang, Y.; Liu, Z.; Xie, J.; Shao, S.; Du, T.; et al. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J. Clin. Endocrinol. Metab. 2015, 100, E96–E100. [Google Scholar] [CrossRef]
- Fu, Z.; Berhane, F.; Fite, A.; Seyoum, B.; Abou-Samra, A.B.; Zhang, R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci. Rep. 2014, 4, 5013. [Google Scholar] [CrossRef]
- Gao, T.; Jin, K.; Chen, P.; Jin, H.; Yang, L.; Xie, X.; Yang, M.; Hu, C.; Yu, X. Circulating Betatrophin Correlates with Triglycerides and Postprandial Glucose among Different Glucose Tolerance Statuses—A Case-Control Study. PLoS ONE 2015, 10, e0133640. [Google Scholar] [CrossRef]
- Yamada, H.; Saito, T.; Aoki, A.; Asano, T.; Yoshida, M.; Ikoma, A.; Kusaka, I.; Toyoshima, H.; Kakei, M.; Ishikawa, S.E. Circulating betatrophin is elevated in patients with type 1 and type 2 diabetes. Endocr. J. 2015, 62, 417–421. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Li, L.; Li, Y.; Li, Q.; An, Z.; Sun, X.; Tian, H. Circulating Betatrophin in Patients with Type 2 Diabetes: A Meta-Analysis. J. Diabetes Res. 2016, 2016, 6194750. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Susanto, H.; Chuang, W.H.; Liu, T.Y.; Wang, C.H. Higher serum betatrophin level in type 2 diabetes subjects is associated with urinary albumin excretion and renal function. Cardiovasc. Diabetol. 2016, 15, 3. [Google Scholar] [CrossRef][Green Version]
- Issa, Y.A.; Abd ElHafeez, S.S.; Amin, N.G. The potential role of angiopoietin-like protein-8 in type 2 diabetes mellitus: A possibility for predictive diagnosis and targeted preventive measures? EPMA J. 2019, 10, 239–248. [Google Scholar] [CrossRef]
- Ebert, T.; Kralisch, S.; Hoffmann, A.; Bachmann, A.; Lössner, U.; Kratzsch, J.; Blüher, M.; Stumvoll, M.; Tönjes, A.; Fasshauer, M. Circulating angiopoietin-like protein 8 is independently associated with fasting plasma glucose and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2014, 99, E2510–E2517. [Google Scholar] [CrossRef] [PubMed]
- Levitt Katz, L.E.; Satin-Smith, M.S.; Collett-Solberg, P.; Thornton, P.S.; Baker, L.; Stanley, C.A.; Cohen, P. Insulin-like growth factor binding protein-1 levels in the diagnosis of hypoglycemia caused by hyperinsulinism. J. Pediatr. 1997, 131, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, K.C.; Schulz, N.; Karampelias, C.; Charbord, J.; Hilding, A.; Rautio, L.; Bertolino, P.; Ostenson, C.G.; Brismar, K.; et al. IGFBP1 increases beta-cell regeneration by promoting alpha- to beta-cell transdifferentiation. EMBO J. 2016, 35, 2026–2044. [Google Scholar] [CrossRef] [PubMed]
- Rajwani, A.; Ezzat, V.; Smith, J.; Yuldasheva, N.Y.; Duncan, E.R.; Gage, M.; Cubbon, R.M.; Kahn, M.B.; Imrie, H.; Abbas, A.; et al. Increasing circulating IGFBP1 levels improves insulin sensitivity, promotes nitric oxide production, lowers blood pressure, and protects against atherosclerosis. Diabetes 2012, 61, 915–924. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, P.R.; Logtenberg, S.J.; Groenier, K.H.; Kleefstra, N.; Bilo, H.J.; Arnqvist, H.J. Effect of i.p. insulin administration on IGF1 and IGFBP1 in type 1 diabetes. Endocr. Connect. 2014, 3, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Dent, C.L.; Ma, Q.; Dastrala, S.; Grenier, F.; Workman, R.; Syed, H.; Ali, S.; Barasch, J.; Devarajan, P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin. J. Am. Soc. Nephrol. 2008, 3, 665–673. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Abubaker, J.; Noronha, F.; Al-Khairi, I.; Cherian, P.; Alarouj, M.; Bennakhi, A.; Elkum, N. Lack of associations between betatrophin/ANGPTL8 level and C-peptide in type 2 diabetic subjects. Cardiovasc. Diabetol. 2015, 14, 112. [Google Scholar] [CrossRef] [PubMed]


| Parameters | T2D n = 37 | DN n = 49 | p |
|---|---|---|---|
| Gender (M/F) | 16/21 | 34/15 | 0.015 |
| Age (years) | 57.27 ± 1.11 | 59.0 ± 1.32 | 0.319 |
| BMI (kg/m2) | 31.56 ± 0.60 | 31.88 ± 0.64 | 0.712 |
| SBP (mmHg) | 131.6 ± 2.46 | 130.98 ± 5.36 | 0.975 |
| DBP (mmHg) | 72.46 ± 1.78 | 68.51 ± 3.01 | 0.262 |
| Fasting Glucose (mmol/L) | 7.86 ± 0.41 | 8.67 ± 0.44 | 0.177 |
| HbA1C (%) | 7.59 ± 0.19 | 7.87 ± 0.25 | 0.371 |
| TC (mmol/L) | 4.10 ± 0.16 | 3.87 ± 0.12 | 0.258 |
| TGL (mmol/L) | 1.39 ± 0.20 | 1.70 ± 0.13 | 0.211 |
| HDL (mmol/L) | 1.21 ± 0.6 | 1.10 ± 0.04 | 0.084 |
| LDL (mmol/L) | 2.28 ± 0.13 | 2.01 ± 0.11 | 0.099 |
| VLDL (mmol/L) | 0.56 ± 0.08 | 0.68 ± 0.05 | 0.216 |
| C Peptide (pg/mL) | 0.67 ± 0.05 | 0.66 ± 0.06 | 0.955 |
| Serum Creatinine (mg/L) | 73.08 ± 3.14 | 109.57 ± 6.28 | <0.001 |
| eGFR (mL/min/1.73 m2) | 86.64 ± 3.03 | 64.08 ± 3.48 | <0.001 |
| Albumin (mcg/L) | 39.11 ± 0.50 | 37.51 ± 0.53 | 0.031 |
| CRP (μg/mL) | 0.38 ± 0.07 | 0.46 ± 0.09 | 0.505 |
| ACR (mg/L) | 10.24 ± 1.12 | 707.07 ± 217.78 | 0.002 |
| Urine Creatinine (mg/day) | 12.62 ± 1.07 | 8.78 ± 0.84 | 0.006 |
| Microalbumin (mg/day) | 13.57 ± 1.56 | 592.87 ± 226.96 | 0.014 |
| IGFBP-4 | ||||
|---|---|---|---|---|
| Parameters | T2D | DN | ||
| r | p | r | p | |
| Age (years) | 0.117 | 0.49 | 0.3 | 0.037 |
| BMI (kg/m2) | 0.156 | 0.355 | −0.072 | 0.629 |
| SBP (mmHg) | 0.019 | 0.913 | 0.067 | 0.646 |
| DBP (mmHg) | −0.278 | 0.096 | 0.058 | 0.691 |
| Fasting Glucose (mmol/L) | −0.118 | 0.488 | 0.394 | 0.005 |
| HbA1C (%) | −0.035 | 0.836 | −0.159 | 0.275 |
| T. Chol (mmol/L) | −0.001 | 0.996 | −0.334 | 0.019 |
| TGL (mmol/L) | 0.001 | 0.994 | 0.197 | 0.175 |
| HDL (mmol/L) | −0.039 | 0.818 | −0.096 | 0.511 |
| LDL (mmol/L) | 0.009 | 0.958 | −0.482 | 0.001 |
| VLDL (mmol/L) | 0.001 | 0.994 | 0.197 | 0.175 |
| C Peptide (pg/mL) | −0.098 | 0.562 | −0.039 | 0.789 |
| Serum Creatinine (mg/L) | 0.017 | 0.92 | 0.703 | <0.001 |
| eGFR (mL/min/1.73 m2) | −0.148 | 0.388 | −0.551 | <0.001 |
| Albumin(mcg/L) | −0.177 | 0.294 | −0.259 | 0.073 |
| ACR (mg/g) | 0.07 | 0.679 | 0.211 | 0.146 |
| Urine Creatinine (mg/day) | 0.217 | 0.196 | −0.079 | 0.591 |
| Microalbumin (mg/day) | 0.288 | 0.084 | 0.05 | 0.731 |
| IGFBP1 | 0.058 | 0.732 | 0.08 | 0.586 |
| IGFBP3 | −0.214 | 0.204 | 0.187 | 0.198 |
| ANGPTL8 | 0.258 | 0.124 | 0.468 | 0.001 |
| ANGPTL8 | ||||
|---|---|---|---|---|
| Parameters | T2D | DN | ||
| r | p | r | p | |
| Age (years) | 0.333 | 0.044 | 0.378 | 0.007 |
| BMI (kg/m2) | 0.068 | 0.687 | −0.137 | 0.358 |
| SBP (mmHg) | −0.041 | 0.808 | −0.134 | 0.357 |
| DBP (mmHg) | −0.331 | 0.046 | −0.246 | 0.088 |
| Fasting Glucose (mmol/L) | −0.091 | 0.593 | 0.016 | 0.915 |
| HbA1C (%) | −0.145 | 0.391 | −0.223 | 0.123 |
| T. Chol (mmol/L) | −0.22 | 0.191 | −0.228 | 0.115 |
| TGL (mmol/L) | 0.075 | 0.66 | 0.022 | 0.879 |
| HDL (mmol/L) | −0.086 | 0.614 | −0.114 | 0.436 |
| LDL (mmol/L) | −0.323 | 0.054 | −0.242 | 0.098 |
| VLDL (mmol/L) | 0.077 | 0.652 | 0.023 | 0.878 |
| C Peptide (pg/mL) | 0.232 | 0.167 | 0.085 | 0.559 |
| Serum Creatinine (mg/L) | 0.228 | 0.176 | 0.378 | 0.007 |
| eGFR (mL/min/1.73 m2) | −0.372 | 0.026 | −0.44 | 0.002 |
| Albumin(mcg/L) | −0.03 | 0.86 | −0.182 | 0.21 |
| ACR (mg/g) | 0.007 | 0.969 | 0.075 | 0.609 |
| Urine Creatinine (mg/day) | 0.07 | 0.682 | −0.407 | 0.004 |
| Microalbumin (mg/day) | 0.116 | 0.495 | −0.06 | 0.684 |
| IGFBP1 | 0.325 | 0.049 | 0.069 | 0.636 |
| IGFBP3 | 0.203 | 0.227 | 0.211 | 0.145 |
| IGFBP4 | 0.258 | 0.124 | 0.468 | 0.001 |
| Parameters | T2D | DN | ||
|---|---|---|---|---|
| β | p-Value | β | p-Value | |
| VLDL | 0.413 | 0.009 | −0.037 | 0.982 |
| LDL | −0.355 | 0.023 | −0.004 | 0.775 |
| HDL | −0.022 | 0.740 | −0.07 | 0.991 |
| IGFBP4 | 0.624 | 0.006 | 0.435 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMajed, H.T.; Abu-Farha, M.; Alshawaf, E.; Devarajan, S.; Alsairafi, Z.; Elhelaly, A.; Cherian, P.; Al-Khairi, I.; Ali, H.; Jose, R.M.; et al. Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 14244. https://doi.org/10.3390/ijms241814244
AlMajed HT, Abu-Farha M, Alshawaf E, Devarajan S, Alsairafi Z, Elhelaly A, Cherian P, Al-Khairi I, Ali H, Jose RM, et al. Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy. International Journal of Molecular Sciences. 2023; 24(18):14244. https://doi.org/10.3390/ijms241814244
Chicago/Turabian StyleAlMajed, Hana Th., Mohamed Abu-Farha, Eman Alshawaf, Sriraman Devarajan, Zahra Alsairafi, Ashraf Elhelaly, Preethi Cherian, Irina Al-Khairi, Hamad Ali, Rose Mol Jose, and et al. 2023. "Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy" International Journal of Molecular Sciences 24, no. 18: 14244. https://doi.org/10.3390/ijms241814244
APA StyleAlMajed, H. T., Abu-Farha, M., Alshawaf, E., Devarajan, S., Alsairafi, Z., Elhelaly, A., Cherian, P., Al-Khairi, I., Ali, H., Jose, R. M., Thanaraj, T. A., Al-Ozairi, E., Al-Mulla, F., Al Attar, A., & Abubaker, J. (2023). Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy. International Journal of Molecular Sciences, 24(18), 14244. https://doi.org/10.3390/ijms241814244









