Associations between Inflammation, Hemoglobin Levels, and Coronary Artery Disease in Non-Albuminuric Subjects with and without Type 2 Diabetes Mellitus
Abstract
:1. Introduction
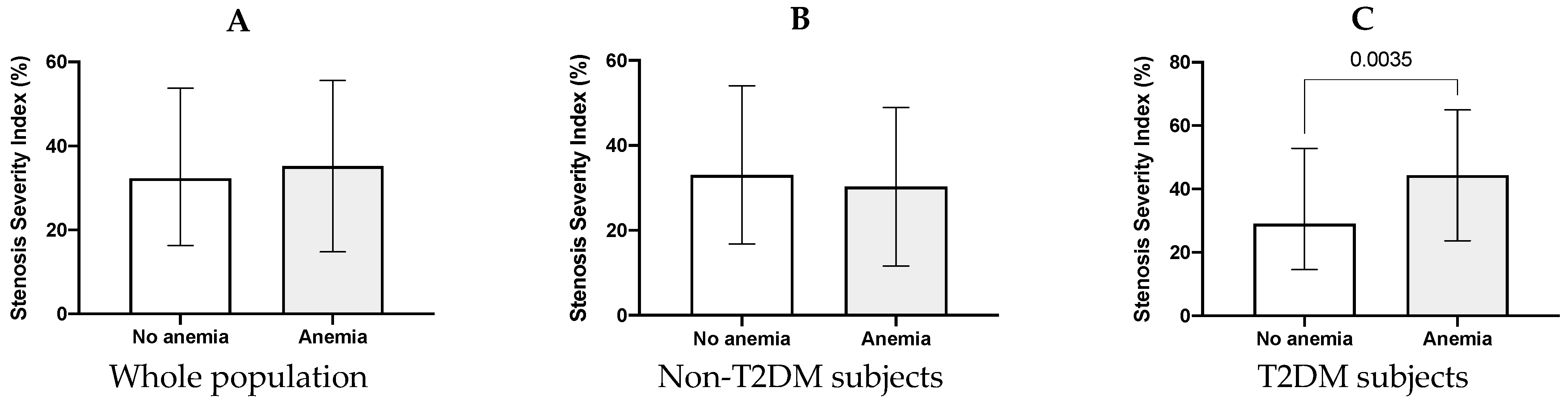
2. Results
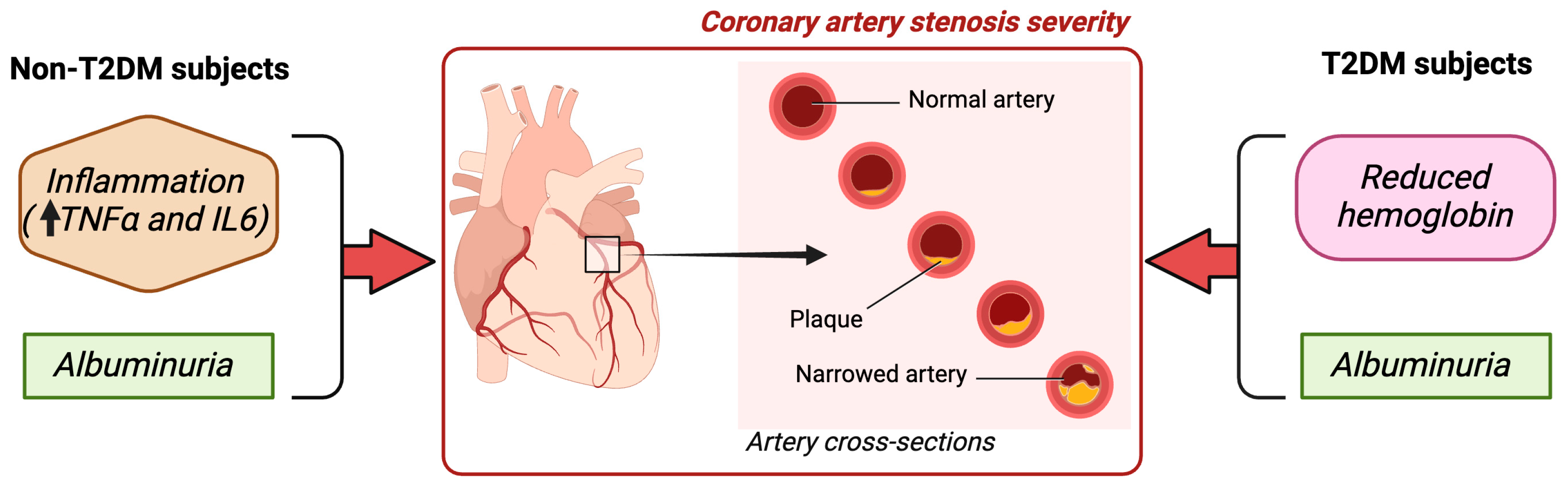
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Coronary Angiography
4.3. Clinical and Biochemical Variables
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (IDF). Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Boccara, F. Interplay of Diabetes and Coronary Heart Disease on Cardiovascular Mortality. Heart 2004, 90, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and without Prior Myocardial Infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Gisterå, A.; Hansson, G.K. The Immunology of Atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Muhlestein, J.B.; Anderson, J.L.; Horne, B.D.; Lavasani, F.; Allen Maycock, C.A.; Bair, T.L.; Pearson, R.R.; Carlquist, J.F. Effect of Fasting Glucose Levels on Mortality Rate in Patients with and without Diabetes Mellitus and Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Am. Heart J. 2003, 146, 351–358. [Google Scholar] [CrossRef]
- Katsiki, N.; Banach, M.; Mikhailidis, D. Is Type 2 Diabetes Mellitus a Coronary Heart Disease Equivalent or Not? Do Not Just Enjoy the Debate and Forget the Patient! Arch. Med. Sci. 2019, 15, 1357–1364. [Google Scholar] [CrossRef]
- Evans, J.M.M. Comparison of Cardiovascular Risk between Patients with Type 2 Diabetes and Those Who Had Had a Myocardial Infarction: Cross Sectional and Cohort Studies. BMJ 2002, 324, 939. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Kengne, A.P. On the Importance of Global Cardiovascular Risk Assessment in People with Type 2 Diabetes. Prim. Care Diabetes 2013, 7, 95–102. [Google Scholar] [CrossRef]
- Jouven, X.; Lemaître, R.N.; Rea, T.D.; Sotoodehnia, N.; Empana, J.-P.; Siscovick, D.S. Diabetes, Glucose Level, and Risk of Sudden Cardiac Death. Eur. Heart J. 2005, 26, 2142–2147. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma Concentration of Interleukin-6 and the Risk of Future Myocardial Infarction Among Apparently Healthy Men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Caselli, C.; De Graaf, M.A.; Lorenzoni, V.; Rovai, D.; Marinelli, M.; Del Ry, S.; Giannessi, D.; Bax, J.J.; Neglia, D.; Scholte, A.J. HDL Cholesterol, Leptin and Interleukin-6 Predict High Risk Coronary Anatomy Assessed by CT Angiography in Patients with Stable Chest Pain. Atherosclerosis 2015, 241, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Nazarewicz, R.R.; Wallis, B.B.; Yanes, R.E.; Watanabe, R.; Hilhorst, M.; Tian, L.; Harrison, D.G.; Giacomini, J.C.; Assimes, T.L.; et al. The Glycolytic Enzyme PKM2 Bridges Metabolic and Inflammatory Dysfunction in Coronary Artery Disease. J. Exp. Med. 2016, 213, 337–354. [Google Scholar] [CrossRef]
- Calabro, P.; Golia, E.; Yeh, E.T.H. Role of C-Reactive Protein in Acute Myocardial Infarction and Stroke: Possible Therapeutic Approaches. Curr. Pharm. Biotechnol. 2012, 13, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Pu, L.J.; Xu, X.W.; Zhang, Q.; Zhang, R.Y.; Zhang, J.S.; Hu, J.; Yang, Z.K.; Lu, A.K.; Ding, F.H.; et al. Association of Serum Levels of Glycated Albumin, C-Reactive Protein and Tumor Necrosis Factor-α with the Severity of Coronary Artery Disease and Renal Impairment in Patients with Type 2 Diabetes Mellitus. Clin. Biochem. 2007, 40, 810–816. [Google Scholar] [CrossRef]
- Khatana, S.A.M.; Taveira, T.H.; Choudhary, G.; Eaton, C.B.; Wu, W.-C. Change in Hemoglobin A1c and C-Reactive Protein Levels in Patients with Diabetes Mellitus. J. CardioMetabolic Syndr. 2009, 4, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; MacIsaac, R.J.; Tsalamandris, C.; Power, D.; Jerums, G. Unrecognized Anemia in Patients with Diabetes. Diabetes Care 2003, 26, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- McClellan, W.M.; Flanders, W.D.; Langston, R.D.; Jurkovitz, C.; Presley, R. Anemia and Renal Insufficiency Are Independent Risk Factors for Death among Patients with Congestive Heart Failure Admitted to Community Hospitals. J. Am. Soc. Nephrol. 2002, 13, 1928–1936. [Google Scholar] [CrossRef]
- Silverberg, D.S.; Wexler, D.; Blum, M.; Wollman, Y.; Schwartz, D.; Sheps, D.; Keren, G.; Iaina, A. The Interaction between Heart Failure, Renal Failure and Anemia—The Cardio-Renal Anemia Syndrome. Blood Purif. 2004, 22, 277–284. [Google Scholar] [CrossRef]
- Silverberg, D.S.; Wexler, D.; Blum, M.; Keren, G.; Sheps, D.; Leibovitch, E.; Brosh, D.; Laniado, S.; Schwartz, D.; Yachnin, T.; et al. The Use of Subcutaneous Erythropoietin and Intravenous Iron for the Treatment of the Anemia of Severe, Resistant Congestive Heart Failure Improves Cardiac and Renal Function and Functional Cardiac Class, and Markedly Reduces Hospitalizations. J. Am. Coll. Cardiol. 2000, 35, 1737–1744. [Google Scholar] [CrossRef]
- Craig, K.J.; Williams, J.D.; Riley, S.G.; Smith, H.; Owens, D.R.; Worthing, D.; Cavill, I.; Phillips, A.O. Anemia and Diabetes in the Absence of Nephropathy. Diabetes Care 2005, 28, 1118–1123. [Google Scholar] [CrossRef]
- Adetunji, O.; Mani, H.; Olujohungbe, A.; Ronald, J.; Morgan, C.; Gill, G. Prevalence and Characteristics of Anaemia in Diabetes. Pract. Diabetes Int. 2008, 25, 110–113. [Google Scholar] [CrossRef]
- Dikow, R.; Schwenger, V.; Schömig, M.; Ritz, E. How Should We Manage Anaemia in Patients with Diabetes? Nephrol. Dial. Transplant. 2002, 17, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, O.R.; Mani, H.; Olujohungbe, A.; Abraham, K.A.; Gill, G.V. ‘Microalbuminuric Anaemia’—The Relationship between Haemoglobin Levels and Albuminuria in Diabetes. Diabetes Res. Clin. Pract. 2009, 85, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wachtell, K. Albuminuria and Cardiovascular Risk in Hypertensive Patients with Left Ventricular Hypertrophy: The LIFE Study. Ann. Intern. Med. 2003, 139, 901. [Google Scholar] [CrossRef]
- Dinneen, S.F. The Association of Microalbuminuria and Mortality in Non—Insulin-Dependent Diabetes Mellitus. Arch. Intern. Med. 1997, 157, 1413. [Google Scholar] [CrossRef]
- Gianotti, L.; Belcastro, S.; D’Agnano, S.; Tassone, F. The Stress Axis in Obesity and Diabetes Mellitus: An Update. Endocrines 2021, 2, 334–347. [Google Scholar] [CrossRef]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The Tryptophan-Kynurenine Metabolic System Is Suppressed in Cuprizone-Induced Model of Demyelination Simulating Progressive Multiple Sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-Induced Bradycardia in Mental Disorders: Foundations, Current Advances, Future Perspectives. Neurosci. Biobehav. Rev. 2023, 149, 105163. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Integrating Armchair, Bench, and Bedside Research for Behavioral Neurology and Neuropsychiatry: Editorial. Biomedicines 2022, 10, 2999. [Google Scholar] [CrossRef]
- Tanaka, M.; Diano, M.; Battaglia, S. Editorial: Insights into Structural and Functional Organization of the Brain: Evidence from Neuroimaging and Non-Invasive Brain Stimulation Techniques. Front. Psychiatry 2023, 14, 1225755. [Google Scholar] [CrossRef] [PubMed]
- Gæde, P.; Vedel, P.; Larsen, N.; Jensen, G.V.H.; Parving, H.-H.; Pedersen, O. Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2003, 348, 383–393. [Google Scholar] [CrossRef] [PubMed]
- del Cañizo Gómez, F.J.; Moreira Andrés, M.N. Strict Control of Modifiable Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus. Med. Clin. 2008, 130, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.A. Risk Factors for Coronary Heart Disease in Diabetes. Ann. Intern. Med. 2000, 133, 154. [Google Scholar] [CrossRef] [PubMed]
- Saito, I. Nontraditional Risk Factors for Coronary Heart Disease Incidence among Persons with Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Ann. Intern. Med. 2000, 133, 81. [Google Scholar] [CrossRef]
- Martín-Timón, I. Type 2 Diabetes and Cardiovascular Disease: Have All Risk Factors the Same Strength? World J. Diabetes 2014, 5, 444. [Google Scholar] [CrossRef]
- Holland, D.C.; Lam, M. Predictors of Hospitalization and Death among Pre-dialysis Patients: A Retrospective Cohort Study. Nephrol. Dial. Transplant. 2000, 15, 650–658. [Google Scholar] [CrossRef]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of Kidney Function With Anemia. Arch. Intern. Med. 2002, 162, 1401. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Tighiouart, H.; Manjunath, G.; MacLeod, B.; Griffith, J.; Salem, D.; Levey, A.S. Anemia as a Risk Factor for Cardiovascular Disease in the Atherosclerosis Risk in Communities (Aric) Study. J. Am. Coll. Cardiol. 2002, 40, 27–33. [Google Scholar] [CrossRef]
- Vlagopoulos, P.T.; Tighiouart, H.; Weiner, D.E.; Griffith, J.; Pettitt, D.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Anemia as a Risk Factor for Cardiovascular Disease and All-Cause Mortality in Diabetes. J. Am. Soc. Nephrol. 2005, 16, 3403–3410. [Google Scholar] [CrossRef]
- Kazmi, W.H.; Kausz, A.T.; Khan, S.; Abichandani, R.; Ruthazer, R.; Obrador, G.T.; Pereira, B.J.G. Anemia: An Early Complication of Chronic Renal Insufficiency. Am. J. Kidney Dis. 2001, 38, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Obrador, G.T.; Roberts, T.; St. Peter, W.L.; Frazier, E.; Pereira, B.J.G.; Collins, A.J. Trends in Anemia at Initiation of Dialysis in the United States. Kidney Int. 2001, 60, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Li, S.; Gilbertson, D.T.; Liu, J.; Chen, S.-C.; Herzog, C.A. Chronic Kidney Disease and Cardiovascular Disease in the Medicare Population. Kidney Int. 2003, 64, S24–S31. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; Ohmit, S.E.; Mccullough, P.A.; Crook, E.D.; Brown, W.W.; Grimm, R.; Bakris, G.L.; Keane, W.F.; Flack, J.M. Higher Prevalence of Anemia with Diabetes Mellitus in Moderate Kidney Insufficiency: The Kidney Early Evaluation Program. Kidney Int. 2005, 67, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Chonchol, M.; Nielson, C. Hemoglobin Levels and Coronary Artery Disease. Am. Heart J. 2008, 155, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Coresh, J.; Levey, A.S.; Tonelli, M.; Muntner, P. Estimated GFR, Albuminuria, and Complications of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 2322–2331. [Google Scholar] [CrossRef]
- Waikar, S.S.; Sabbisetti, V.; Ärnlöv, J.; Carlsson, A.C.; Coresh, J.; Feldman, H.I.; Foster, M.C.; Fufaa, G.D.; Helmersson-Karlqvist, J.; Hsu, C.; et al. Relationship of Proximal Tubular Injury to Chronic Kidney Disease as Assessed by Urinary Kidney Injury Molecule-1 in Five Cohort Studies. Nephrol. Dial. Transplant. 2016, 31, 1460–1470. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between Albuminuria, Kidney Function, and Inflammatory Biomarker Profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of Chronic Disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin Resistance: The Role of Inflammation and Pro-inflammatory Cytokines. Nephrol. Dial. Transplant. 2002, 17, 39–43. [Google Scholar] [CrossRef]
- Li Vecchi, M.; Fuiano, G.; Francesco, M.; Mancuso, D.; Faga, T.; Sponton, A.; Provenzano, R.; Andreucci, M.; Tozzo, C. Prevalence and Severity of Anaemia in Patients with Type 2 Diabetic Nephropathy and Different Degrees of Chronic Renal Insufficiency. Nephron Clin. Pract. 2006, 105, c62–c67. [Google Scholar] [CrossRef] [PubMed]
| Non-T2DM | T2DM | p | All Subjects | |
|---|---|---|---|---|
| Characteristics | ||||
| N | 384 | 254 | 638 | |
| Age (years) | 66 (56–75) | 65.5 (58–73) | 0.99 | 65.2 ± 11.3 |
| Sex (% male) | 276 (71.9) | 170 (66.9) | 0.15 | 446 (69.9) |
| BMI (kg/m2) | 24.8 ± 3.1 | 27.2 ± 5.2 | 0.1 | 26.5 ± 5.1 |
| SBP (mm Hg) | 124 ± 12.1 | 129 ± 10.1 | 0.21 | 126 ± 10.1 |
| DBP (mm Hg) | 74.6 ± 7.2 | 75.7 ± 8.6 | 0.17 | 74.9 ± 7.2 |
| Comorbidities | ||||
| Anemia (%) | 65 (16.9) | 58 (22.7) | 0.08 | 123 (19.3) |
| Significant CAD (%) | 178 (46.6) | 117 (45.9) | 0.11 | 305 (47.8) |
| Obesity (%) | 57 (14.8) | 46 (18.1) | 0.11 | 83 (13) |
| Hypertension (%) | 107 (27.9) | 73 (28.6) | 0.48 | 180 (28.2) |
| Former smokers (%) | 83 (21.6) | 53 (20.1) | 0.24 | 114 (21.3) |
| Current smokers (%) | 118 (30.7) | 59 (23.4) | 0.14 | 177 (27.7) |
| Dyslipidemia (%) | 178 (46.4) | 107 (42.2) | 0.22 | 285 (44.7) |
| Laboratory data | ||||
| SSI (%) | 32 (16.3–51.4) | 34.4 (16.1–55) | 0.64 | 32.4 (16.3–53.8) |
| Hb (g/dL) | 14 (13.1–15) | 13.8 (12.4–14.9) | 0.039 | 13.9 (12.9–15) |
| T-cholesterol (mg/dL) | 173.3 (149–200) | 168 (139.8–189) | 0.04 | 173.3 (145–197.3) |
| HDL-C (mg/dL) | 41 (33–46.8) | 37 (30–43.3) | 0.02 | 39 (32–46) |
| LDL-C (mg/dL) | 100.9 (83–125) | 95 (75–114.3) | <0.01 | 100.9 (80–122) |
| TG (mg/dL) | 140 (102–167) | 140 (111.8–198.5) | 0.17 | 140 (105.5–173.5) |
| FG (mg/dL) | 98 (89–112.8) | 148 (133–183.3) | <0.001 | 119.7 ± 47 |
| Hb1ac (%) | 5.6 ± 0.97 | 7.8 ± 0.89 | <0.001 | 6.37 ± 1.62 |
| TyG index | 4.77 (4.63–4.93) | 4.93 (4.76–5.17) | <0.001 | 4.82 (4.65–4.99) |
| eGFR (mL/min/1.73 m2) | 99.1 ± 7.1 | 96.3 ± 9.2 | 0.11 | 97.3 ± 6.2 |
| Creatinine (mg/dL) | 0.91 (0.75–1.04) | 0.87 (0.71–1.03) | 0.13 | 0.9 (0.74–1.04) |
| ACR (mg/g) | 6.4 (3.6–14.6) | 8.37 (4.1–16) | 0.08 | 6.9 (3.6–15) |
| Uric acid (mg/dL) | 5.8 (4.7–6.7) | 5.4 (4.5–6.4) | 0.23 | 5.7 (4.6–6.7) |
| Leukocytes | 8 (6.6–9.4) | 7.8 (6.5–9.5) | 0.97 | 7.9 (6.5–9.4) |
| Monocytes | 5.9 (0.7–8.8) | 5.5 (0.7–8.4) | 0.35 | 5.8 (0.7–8.8) |
| Lymphocytes | 15.6 (2.1–26.3) | 13.7 (2.1–26.8) | 0.88 | 14.7 (2.1–26.3) |
| Neutrophyles | 49 (5.1–63) | 44.1 (4.9–64.4) | 0.92 | 48.3 (5–63.2) |
| NLR (/mL) | 2.46 (1.75–3.36) | 2.29 (1.74–3.41) | 0.85 | 2.42 (1.74–3.27) |
| hs-CRP (mg/L) | 3.5 (2–6.8) | 3.8 (2–6.72) | 0.77 | 3.6 (2–6.8) |
| TNFα (pg/mL) | 1.99 (1.38–2.67) | 2.2 (1.59–2.99) | <0.01 | 2.05 (1.45–2.78) |
| IL6 (pg/mL) | 6.55 (3.5–11.5) | 6.5 (3.5–12.9) | 0.43 | 6.54 (3.55–11.93) |
| Medication | ||||
| Statin (%) | 91 (23.7) | 116 (45.7) | 0.03 | 207 (32.5) |
| ACEI/ARB (%) | 78 (20.3) | 81 (31.9) | 0.12 | 159 (24.9) |
| Non-T2DM (n = 384) | T2DM (n = 254) | |||
|---|---|---|---|---|
| r | p | r | p | |
| ACR (mg/g) | −0.103 | 0.043 | −0.224 | 0.005 |
| FG (mg/dL) | −0.036 | 0.48 | −0.199 | 0.013 |
| hs-CRP (mg/L) | −0.004 | 0.943 | −0.167 | 0.038 |
| TNFα (pg/mL) | −0.13 | 0.8 | −0.01 | 0.98 |
| IL6 (pg/mL) | −0.078 | 0.13 | −0.069 | 0.39 |
| NLR | −0.041 | 0.426 | −0.181 | 0.25 |
| SSI | −0.03 | 0.948 | −0.19 | 0.018 |
| Obs LCA (%) | 0.009 | 0.863 | −0.055 | 0.5 |
| Obs RCA (%) | −0.331 | 0.551 | −0.167 | 0.039 |
| Obs LAD (%) | 0.017 | 0.734 | −0.175 | 0.036 |
| Obs CA (%) | 0.06 | 0.913 | −0.014 | 0.861 |
| Non-CAD | CAD | p | |
|---|---|---|---|
| Characteristics | |||
| N | 137 | 117 | |
| Age (years) | 61 (54–73) | 66 (59–74) | 0.22 |
| Sex (% male) | 90 (65.7) | 80 (68.4) | 0.39 |
| BMI (kg/m2) | 26.8 ± 2.2 | 27.3 ± 2.9 | 0.13 |
| SBP (mm Hg) | 129 ± 10.1 | 131 ± 11.1 | 0.04 |
| DBP (mm Hg) | 75.3 ± 7.7 | 75.9 ± 9.1 | 0.47 |
| Comorbidities | |||
| Anemia (%) | 18 (13.1) | 40 (34.2) | 0.03 |
| Obesity (%) | 24 (17.5) | 22 (18.8) | 0.74 |
| Hypertension (%) | 33 (24.1) | 40 (34.2) | 0.51 |
| Former smokers (%) | 25 (18.3) | 28 (23.9) | 0.62 |
| Current smokers (%) | 32 (23.4) | 27 (23.1) | 0.47 |
| Dyslipidemia (%) | 51 (37.2) | 56 (47.9) | 0.69 |
| Laboratory data | |||
| Hb (g/dL) | 14.4 (13.6–15.1) | 13.6 (12.2–14.8) | 0.03 |
| T-cholesterol (mg/dL) | 162 (136–192) | 168 (140–188) | 0.96 |
| HDL-C (mg/dL) | 33 (25–41.7) | 37 (31–44) | 0.17 |
| LDL-C (mg/dL) | 96 (82–120) | 94 (74–111) | 0.44 |
| TG (mg/dL) | 129 (107–198) | 141 (112–200) | 0.63 |
| FG (mg/dL) | 135 (127–165) | 153 (134–188) | 0.09 |
| Hb1ac (%) | 7.42 ± 0.2 | 7.58 ± 0.21 | 0.11 |
| TyG index | 5 (4.74–5.34) | 4.93 (4.77.5.14) | 0.36 |
| eGFR (ml/min/1.73 m2) | 99.8 ± 10.3 | 96.9 ± 9.3 | 0.16 |
| Creatinine (mg/dL) | 0.9 (0.79–1.05) | 0.86 (0.71–1.02) | 0.28 |
| ACR (mg/g) | 4.73 (3.25–12.6) | 9 (4.4–18.8) | 0.04 |
| Uric acid (mg/dL) | 5.54 (4.46–6.79) | 5.42 (4.51–6.23) | 0.84 |
| Leukocytes | 7.7 (6.4–9.3) | 7.8 (6.5–9.6) | 0.98 |
| Monocytes | 7.8 (0.8–9) | 7.2 (0.6–8.4) | 0.04 |
| Lymphocytes | 18.8 (2.9–27.2) | 3.3 (2–25.9) | 0.11 |
| Neutrophyles | 52.9 (6.8–67.1) | 49.3 (4.7–62.1) | 0.44 |
| NLR | 2.51 (1.75–3.68) | 2.19 (1.74–3.38) | 0.46 |
| hs-CRP (mg/L) | 3.14 (1.3–6.1) | 4.6 (2.1–6.8) | 0.02 |
| TNFα (pg/mL) | 2 (1.59–2.78) | 2.22 (1.59–3.1) | 0.19 |
| IL6 (pg/mL) | 5.43 (3–8.89) | 7.47 (3.51–14) | 0.21 |
| Medication | |||
| Statin (%) | 61 (44.5) | 55 (47.2) | 0.24 |
| ACEI/ARB (%) | 37 (27) | 44 (37.4) | 0.31 |
| Stenosis Severity Index | Adjusted R2 | ß | SE | t | p |
|---|---|---|---|---|---|
| Non-T2DM subjects | 0.038 | <0.001 | |||
| ACR (mg/g) | 0.447 | 0.138 | 3.234 | 0.001 | |
| IL6 (pg/mL) | 0.094 | 0.052 | 1.815 | 0.03 | |
| TNFα (pg/mL) | 0.349 | 0.205 | 1.702 | 0.04 | |
| T2DM subjects | 0.081 | <0.001 | |||
| Hb (g/dL) | −1.705 | 0.939 | −1.816 | 0.01 | |
| ACR (mg/g) | 0.695 | 0.215 | 3.238 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donate-Correa, J.; Martín-Núñez, E.; Mora-Fernández, C.; González-Luis, A.; Martín-Olivera, A.; Navarro-González, J.F. Associations between Inflammation, Hemoglobin Levels, and Coronary Artery Disease in Non-Albuminuric Subjects with and without Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 14131. https://doi.org/10.3390/ijms241814131
Donate-Correa J, Martín-Núñez E, Mora-Fernández C, González-Luis A, Martín-Olivera A, Navarro-González JF. Associations between Inflammation, Hemoglobin Levels, and Coronary Artery Disease in Non-Albuminuric Subjects with and without Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2023; 24(18):14131. https://doi.org/10.3390/ijms241814131
Chicago/Turabian StyleDonate-Correa, Javier, Ernesto Martín-Núñez, Carmen Mora-Fernández, Ainhoa González-Luis, Alberto Martín-Olivera, and Juan F. Navarro-González. 2023. "Associations between Inflammation, Hemoglobin Levels, and Coronary Artery Disease in Non-Albuminuric Subjects with and without Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 24, no. 18: 14131. https://doi.org/10.3390/ijms241814131
APA StyleDonate-Correa, J., Martín-Núñez, E., Mora-Fernández, C., González-Luis, A., Martín-Olivera, A., & Navarro-González, J. F. (2023). Associations between Inflammation, Hemoglobin Levels, and Coronary Artery Disease in Non-Albuminuric Subjects with and without Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 24(18), 14131. https://doi.org/10.3390/ijms241814131







