The P2X7 Receptor in Autoimmunity
Abstract
:1. Introduction
2. The P2X7 Receptor
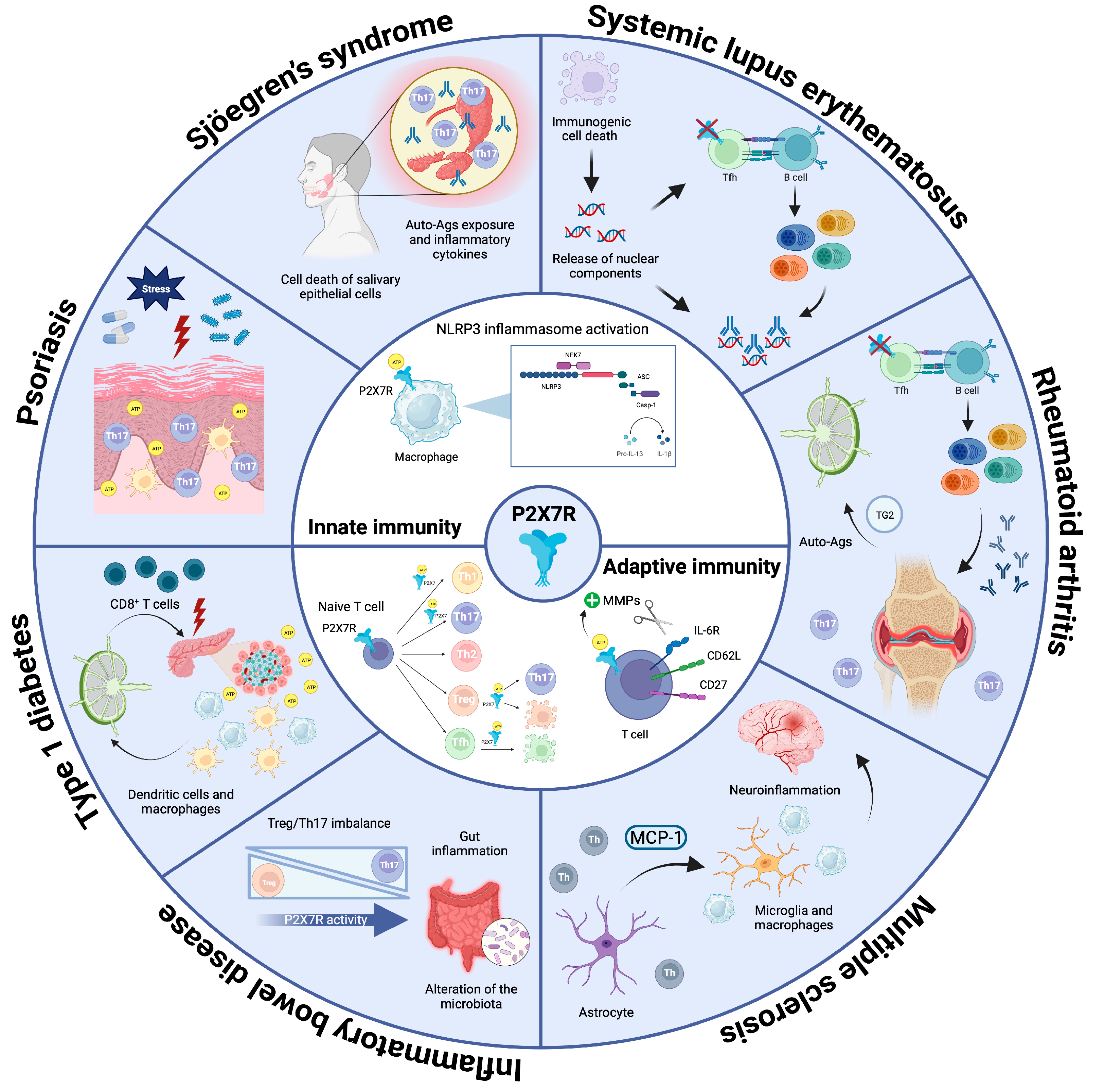
2.1. P2X7R in Innate Immunity
2.2. P2X7R in Adaptive Immunity
3. The P2X7R in Autoimmunity
3.1. Systemic Lupus Erythematosus (SLE)
3.2. Rheumatoid Arthritis (RA)
3.3. Multiple Sclerosis (MS)
3.4. Inflammatory Bowel Disease (IBD)
3.5. Type 1 Diabetes (T1D)
3.6. Psoriasis
3.7. Sjögren’s Syndrome (SS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnstock, G. Purinergic Signalling—An Overview. Novartis Found. Symp. 2006, 276, 26–48; discussion 48–57, 275–281. [Google Scholar] [PubMed]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Giuliani, A.L. Purinergic Signalling in Autoimmunity: A Role for the P2X7R in Systemic Lupus Erythematosus? Biomed. J. 2016, 39, 326–338. [Google Scholar] [CrossRef]
- Cao, F.; Hu, L.-Q.; Yao, S.-R.; Hu, Y.; Wang, D.-G.; Fan, Y.-G.; Pan, G.-X.; Tao, S.-S.; Zhang, Q.; Pan, H.-F.; et al. P2X7 Receptor: A Potential Therapeutic Target for Autoimmune Diseases. Autoimmun. Rev. 2019, 18, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Buell, G.N.; Talabot, F.; Gos, A.; Lorenz, J.; Lai, E.; Morris, M.A.; Antonarakis, S.E. Gene Structure and Chromosomal Localization of the Human P2X7 Receptor. Recept. Channels 1998, 5, 347–354. [Google Scholar]
- Pelegrin, P. P2X7 Receptor and the NLRP3 Inflammasome: Partners in Crime. Biochem. Pharmacol. 2021, 187, 114385. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cuesta, M.Á.; Blanch-Ruiz, M.A.; Ortega-Luna, R.; Sánchez-López, A.; Álvarez, Á. Structural and Functional Basis for Understanding the Biological Significance of P2X7 Receptor. Int. J. Mol. Sci. 2020, 21, 8454. [Google Scholar] [CrossRef]
- Khakh, B.S.; North, R.A. P2X Receptors as Cell-Surface ATP Sensors in Health and Disease. Nature 2006, 442, 527–532. [Google Scholar] [CrossRef]
- Khadra, A.; Tomić, M.; Yan, Z.; Zemkova, H.; Sherman, A.; Stojilkovic, S.S. Dual Gating Mechanism and Function of P2X7 Receptor Channels. Biophys. J. 2013, 104, 2612–2621. [Google Scholar] [CrossRef]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandonà, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic Activity of a Naturally Occurring Truncated Isoform of the P2X7 Receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Giuliani, A.L.; Vultaggio-Poma, V.; Falzoni, S.; Sarti, A.C. Non-Nucleotide Agonists Triggering P2X7 Receptor Activation and Pore Formation. Front. Pharmacol. 2018, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.; de Andrade Mello, P.; Da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Alves, L.A.; de Melo Reis, R.A.; de Souza, C.A.M.; de Freitas, M.S.; Teixeira, P.C.N.; Neto Moreira Ferreira, D.; Xavier, R.F. The P2X7 Receptor: Shifting from a Low- to a High-Conductance Channel—An Enigmatic Phenomenon? Biochim. Biophys. Acta 2014, 1838, 2578–2587. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in Immunity and Inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.J.; Chira, N.; Róg, J.; Al-Khalidi, R.; Benard, M.; Galas, L.; Chan, P.; Vaudry, D.; Zablocki, K.; Górecki, D.C. Sustained Activation of P2X7 Induces MMP-2-Evoked Cleavage and Functional Purinoceptor Inhibition. J. Mol. Cell Biol. 2018, 10, 229–242. [Google Scholar] [CrossRef]
- Mehta, V.B.; Hart, J.; Wewers, M.D. ATP-Stimulated Release of Interleukin (IL)-1beta and IL-18 Requires Priming by Lipopolysaccharide and Is Independent of Caspase-1 Cleavage. J. Biol. Chem. 2001, 276, 3820–3826. [Google Scholar] [CrossRef]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential Activation of the Inflammasome by Caspase-1 Adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef]
- Di Virgilio, F. Liaisons Dangereuses: P2X(7) and the Inflammasome. Trends Pharmacol. Sci. 2007, 28, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxid. Redox Signal 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangère, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rébé, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef]
- Franceschini, A.; Capece, M.; Chiozzi, P.; Falzoni, S.; Sanz, J.M.; Sarti, A.C.; Bonora, M.; Pinton, P.; Di Virgilio, F. The P2X7 Receptor Directly Interacts with the NLRP3 Inflammasome Scaffold Protein. FASEB J. 2015, 29, 2450–2461. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of ProIL-Beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Wiley, J.S.; Sluyter, R.; Gu, B.J.; Stokes, L.; Fuller, S.J. The Human P2X7 Receptor and Its Role in Innate Immunity. Tissue Antigens 2011, 78, 321–332. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid Secretion of Interleukin-1beta by Microvesicle Shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef]
- Dubyak, G.R. P2X7 Receptor Regulation of Non-Classical Secretion from Immune Effector Cells. Cell Microbiol. 2012, 14, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered Cytokine Production in Mice Lacking P2X(7) Receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef]
- Hanley, P.J.; Musset, B.; Renigunta, V.; Limberg, S.H.; Dalpke, A.H.; Sus, R.; Heeg, K.M.; Preisig-Müller, R.; Daut, J. Extracellular ATP Induces Oscillations of Intracellular Ca2+ and Membrane Potential and Promotes Transcription of IL-6 in Macrophages. Proc. Natl. Acad. Sci. USA 2004, 101, 9479–9484. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Rubini, P.; Tang, Y.; Engel, T.; Illes, P. Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. Int. J. Mol. Sci. 2021, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, S.; Wei, S.; Zhong, Y.; Yi, G.; Chen, H.; Liang, L.; Chen, H.; Lu, X. Extracellular ATP Activates P2X7R-NF-ΚB (P65) Pathway to Promote the Maturation of Bone Marrow-Derived Dendritic Cells of Mice. Cytokine 2019, 119, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz-Burkiewicz, M.; Kocbuch, K.; Grden, M.; Maciejewska, I.; Szutowicz, A.; Pawelczyk, T. High Glucose Concentration Impairs ATP Outflow and Immunoglobulin Production by Human Peripheral B Lymphocytes: Involvement of P2X7 Receptor. Immunobiology 2013, 218, 591–601. [Google Scholar] [CrossRef]
- Sengstake, S.; Boneberg, E.-M.; Illges, H. CD21 and CD62L Shedding Are Both Inducible via P2X7Rs. Int. Immunol. 2006, 18, 1171–1178. [Google Scholar] [CrossRef]
- Pupovac, A.; Geraghty, N.J.; Watson, D.; Sluyter, R. Activation of the P2X7 Receptor Induces the Rapid Shedding of CD23 from Human and Murine B Cells. Immunol. Cell Biol. 2015, 93, 77–85. [Google Scholar] [CrossRef]
- Engeroff, P.; Vogel, M. The Role of CD23 in the Regulation of Allergic Responses. Allergy 2021, 76, 1981–1989. [Google Scholar] [CrossRef]
- Kovács, K.G.; Mácsik-Valent, B.; Matkó, J.; Bajtay, Z.; Erdei, A. Revisiting the Coreceptor Function of Complement Receptor Type 2 (CR2, CD21); Coengagement with the B-Cell Receptor Inhibits the Activation, Proliferation, and Antibody Production of Human B Cells. Front. Immunol. 2021, 12, 620427. [Google Scholar] [CrossRef]
- Grassi, F. The P2X7 Receptor as Regulator of T Cell Development and Function. Front. Immunol. 2020, 11, 1179. [Google Scholar] [CrossRef] [PubMed]
- Frascoli, M.; Marcandalli, J.; Schenk, U.; Grassi, F. Purinergic P2X7 Receptor Drives T Cell Lineage Choice and Shapes Peripheral Γδ Cells. J. Immunol. 2012, 189, 174–180. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory Cytokines as a Third Signal for T Cell Activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic Control of T Cell Activation by ATP Released through Pannexin-1 Hemichannels. Sci. Signal. 2008, 1, ra6. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.; Woehrle, T.; Corriden, R.; Hirsh, M.; Chen, Y.; Inoue, Y.; Ferrari, V.; Insel, P.A.; Junger, W.G. Autocrine Regulation of T-Cell Activation by ATP Release and P2X7 Receptors. FASEB J. 2009, 23, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Sarti, A.C.; Grassi, F. Modulation of Innate and Adaptive Immunity by P2X Ion Channels. Curr. Opin. Immunol. 2018, 52, 51–59. [Google Scholar] [CrossRef]
- Hendriks, J.; Gravestein, L.A.; Tesselaar, K.; van Lier, R.A.; Schumacher, T.N.; Borst, J. CD27 Is Required for Generation and Long-Term Maintenance of T Cell Immunity. Nat. Immunol. 2000, 1, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.J.; Nüssing, S.; Sant, S.; Clemens, E.B.; Kedzierska, K. The Role of CD27 in Anti-Viral T-Cell Immunity. Curr. Opin. Virol. 2017, 22, 77–88. [Google Scholar] [CrossRef]
- Qu, Y.; Dubyak, G.R. P2X7 Receptors Regulate Multiple Types of Membrane Trafficking Responses and Non-Classical Secretion Pathways. Purinergic Signal. 2009, 5, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Schenk, U.; Frascoli, M.; Proietti, M.; Geffers, R.; Traggiai, E.; Buer, J.; Ricordi, C.; Westendorf, A.M.; Grassi, F. ATP Inhibits the Generation and Function of Regulatory T Cells through the Activation of Purinergic P2X Receptors. Sci. Signal. 2011, 4, ra12. [Google Scholar] [CrossRef]
- Taylor, S.R.J.; Alexander, D.R.; Cooper, J.C.; Higgins, C.F.; Elliott, J.I. Regulatory T Cells Are Resistant to Apoptosis via TCR but Not P2X7. J. Immunol. 2007, 178, 3474–3482. [Google Scholar] [CrossRef]
- Yang, Y.; Story, M.E.; Hao, X.; Sumpter, T.L.; Mathers, A.R. P2X7 Receptor Expression and Signaling on Dendritic Cells and CD4+ T Cells Is Not Required but Can Enhance Th17 Differentiation. Front. Cell Dev. Biol. 2022, 10, 687659. [Google Scholar] [CrossRef]
- Proietti, M.; Cornacchione, V.; Rezzonico Jost, T.; Romagnani, A.; Faliti, C.E.; Perruzza, L.; Rigoni, R.; Radaelli, E.; Caprioli, F.; Preziuso, S.; et al. ATP-Gated Ionotropic P2X7 Receptor Controls Follicular T Helper Cell Numbers in Peyer’s Patches to Promote Host-Microbiota Mutualism. Immunity 2014, 41, 789–801. [Google Scholar] [CrossRef]
- Perruzza, L.; Gargari, G.; Proietti, M.; Fosso, B.; D’Erchia, A.M.; Faliti, C.E.; Rezzonico-Jost, T.; Scribano, D.; Mauri, L.; Colombo, D.; et al. T Follicular Helper Cells Promote a Beneficial Gut Ecosystem for Host Metabolic Homeostasis by Sensing Microbiota-Derived Extracellular ATP. Cell Rep. 2017, 18, 2566–2575. [Google Scholar] [CrossRef]
- Stark, R.; Wesselink, T.H.; Behr, F.M.; Kragten, N.A.M.; Arens, R.; Koch-Nolte, F.; van Gisbergen, K.P.J.M.; van Lier, R.A.W. T RM Maintenance Is Regulated by Tissue Damage via P2RX7. Sci. Immunol. 2018, 3, eaau1022. [Google Scholar] [CrossRef]
- Romagnani, A.; Rottoli, E.; Mazza, E.M.C.; Rezzonico-Jost, T.; De Ponte Conti, B.; Proietti, M.; Perotti, M.; Civanelli, E.; Perruzza, L.; Catapano, A.L.; et al. P2X7 Receptor Activity Limits Accumulation of T Cells within Tumors. Cancer Res. 2020, 80, 3906–3919. [Google Scholar] [CrossRef]
- Faliti, C.E.; Gualtierotti, R.; Rottoli, E.; Gerosa, M.; Perruzza, L.; Romagnani, A.; Pellegrini, G.; De Ponte Conti, B.; Rossi, R.L.; Idzko, M.; et al. P2X7 Receptor Restrains Pathogenic Tfh Cell Generation in Systemic Lupus Erythematosus. J. Exp. Med. 2019, 216, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Su, M.A. AIRE Expands: New Roles in Immune Tolerance and Beyond. Nat. Rev. Immunol. 2016, 16, 247–258. [Google Scholar] [CrossRef]
- Bluestone, J.A. Mechanisms of Tolerance. Immunol. Rev. 2011, 241, 5–19. [Google Scholar] [CrossRef]
- Yang, S.; Fujikado, N.; Kolodin, D.; Benoist, C.; Mathis, D. Immune Tolerance. Regulatory T Cells Generated Early in Life Play a Distinct Role in Maintaining Self-Tolerance. Science 2015, 348, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Oftedal, B.E.; Wolff, A.B.; Husebye, E.S. AIRE-Mutations and Autoimmune Disease. Curr. Opin. Immunol. 2016, 43, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-A.; Chiang, B.-L. Inflammasomes and Human Autoimmunity: A Comprehensive Review. J. Autoimmun. 2015, 61, 1–8. [Google Scholar] [CrossRef]
- Podolska, M.J.; Biermann, M.H.; Maueröder, C.; Hahn, J.; Herrmann, M. Inflammatory Etiopathogenesis of Systemic Lupus Erythematosus: An Update. J. Inflamm. Res. 2015, 8, 161–171. [Google Scholar] [CrossRef]
- You, R.; He, X.; Zeng, Z.; Zhan, Y.; Xiao, Y.; Xiao, R. Pyroptosis and Its Role in Autoimmune Disease: A Potential Therapeutic Target. Front. Immunol. 2022, 13, 841732. [Google Scholar] [CrossRef]
- Perry, D.; Sang, A.; Yin, Y.; Zheng, Y.-Y.; Morel, L. Murine Models of Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2011, 2011, 271694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, H.; Dai, C.; Wang, H.; Zhang, H.; Huang, Y.; Wang, S.; Gaskin, F.; Yang, N.; Fu, S.M. P2X7 Blockade Attenuates Murine Lupus Nephritis by Inhibiting Activation of the NLRP3/ASC/Caspase 1 Pathway. Arthritis Rheum. 2013, 65, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.J.; Turner, C.M.; Elliott, J.I.; McDaid, J.; Hewitt, R.; Smith, J.; Pickering, M.C.; Whitehouse, D.L.; Cook, H.T.; Burnstock, G.; et al. P2X7 Deficiency Attenuates Renal Injury in Experimental Glomerulonephritis. J. Am. Soc. Nephrol. 2009, 20, 1275–1281. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Yalavarthi, S.; Zhao, W.; Hodgin, J.B.; Reed, T.J.; Tsuji, N.M.; Kaplan, M.J. An Essential Role of Caspase 1 in the Induction of Murine Lupus and Its Associated Vascular Damage. Arthritis Rheumatol. 2014, 66, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Dayan, M.; Zinger, H.; Gayvoronsky, L.; Lin, J.-P.; Iwakura, Y.; Apte, R.N.; Mozes, E. IL-1 Beta-Deficient Mice Are Resistant to Induction of Experimental SLE. Eur. Cytokine Netw. 2006, 17, 109–116. [Google Scholar] [PubMed]
- Nath, S.K.; Quintero-Del-Rio, A.I.; Kilpatrick, J.; Feo, L.; Ballesteros, M.; Harley, J.B. Linkage at 12q24 with Systemic Lupus Erythematosus (SLE) Is Established and Confirmed in Hispanic and European American Families. Am. J. Hum. Genet. 2004, 74, 73–82. [Google Scholar] [CrossRef]
- Chen, G.-M.; Feng, C.-C.; Ye, Q.-L.; Tao, J.; Li, R.; Peng, H.; Zhou, M.; Leng, R.-X.; Li, J.; Cen, H.; et al. Association of P2X7R Gene Polymorphisms with Systemic Lupus Erythematosus in a Chinese Population. Mutagenesis 2013, 28, 351–355. [Google Scholar] [CrossRef]
- Al-Shukaili, A.; Al-Kaabi, J.; Hassan, B.; Al-Araimi, T.; Al-Tobi, M.; Al-Kindi, M.; Al-Maniri, A.; Al-Gheilani, A.; Al-Ansari, A. P2X7 Receptor Gene Polymorphism Analysis in Rheumatoid Arthritis. Int. J. Immunogenet. 2011, 38, 389–396. [Google Scholar] [CrossRef]
- Guerini, F.R.; Agliardi, C.; Bolognesi, E.; Zanzottera, M.; Caputo, D.; Pasanisi, M.B.; Rovaris, M.; Clerici, M. Two Single Nucleotide Polymorphisms in the Purinergic Receptor P2X7 Gene Are Associated with Disease Severity in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 15381. [Google Scholar] [CrossRef]
- Tezza, S.; Ben Nasr, M.; D’Addio, F.; Vergani, A.; Usuelli, V.; Falzoni, S.; Bassi, R.; Dellepiane, S.; Fotino, C.; Rossi, C.; et al. Islet-Derived EATP Fuels Autoreactive CD8+ T Cells and Facilitates the Onset of Type 1 Diabetes. Diabetes 2018, 67, 2038–2053. [Google Scholar] [CrossRef]
- Oyanguren-Desez, O.; Rodríguez-Antigüedad, A.; Villoslada, P.; Domercq, M.; Alberdi, E.; Matute, C. Gain-of-Function of P2X7 Receptor Gene Variants in Multiple Sclerosis. Cell Calcium 2011, 50, 468–472. [Google Scholar] [CrossRef]
- Hu, S.; Yu, F.; Ye, C.; Huang, X.; Lei, X.; Dai, Y.; Xu, H.; Wang, Y.; Yu, Y. The Presence of P2RX7 Single Nuclear Polymorphism Is Associated with a Gain of Function in P2X7 Receptor and Inflammasome Activation in SLE Complicated with Pericarditis. Clin. Exp. Rheumatol. 2020, 38, 442–449. [Google Scholar]
- Portales-Cervantes, L.; Niño-Moreno, P.; Salgado-Bustamante, M.; García-Hernández, M.H.; Baranda-Candido, L.; Reynaga-Hernández, E.; Barajas-López, C.; González-Amaro, R.; Portales-Pérez, D.P. The His155Tyr (489C > T) Single Nucleotide Polymorphism of P2RX7 Gene Confers an Enhanced Function of P2X7 Receptor in Immune Cells from Patients with Rheumatoid Arthritis. Cell. Immunol. 2012, 276, 168–175. [Google Scholar] [CrossRef]
- Gu, B.J.; Field, J.; Dutertre, S.; Ou, A.; Kilpatrick, T.J.; Lechner-Scott, J.; Scott, R.; Lea, R.; Taylor, B.V.; Stankovich, J.; et al. A Rare P2X7 Variant Arg307Gln with Absent Pore Formation Function Protects against Neuroinflammation in Multiple Sclerosis. Hum. Mol. Genet. 2015, 24, 5644–5654. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.; Stokes, L.; Skarratt, K.K.; Gu, B.J.; Sivils, K.L.; Lessard, C.J.; Wiley, J.S.; Rischmueller, M. Epistasis with HLA DR3 Implicates the P2X7 Receptor in the Pathogenesis of Primary Sjögren’s Syndrome. Arthritis Res. Ther. 2013, 15, R71. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.M.; Tam, F.W.K.; Lai, P.-C.; Tarzi, R.M.; Burnstock, G.; Pusey, C.D.; Cook, H.T.; Unwin, R.J. Increased Expression of the Pro-Apoptotic ATP-Sensitive P2X7 Receptor in Experimental and Human Glomerulonephritis. Nephrol. Dial. Transplant. 2007, 22, 386–395. [Google Scholar] [CrossRef]
- Cigni, A.; Pileri, P.V.; Faedda, R.; Gallo, P.; Sini, A.; Satta, A.E.; Marras, R.; Carta, E.; Argiolas, D.; Rum, I.; et al. Interleukin 1, Interleukin 6, Interleukin 10, and Tumor Necrosis Factor α in Active and Quiescent Systemic Lupus Erythematosus. J. Investig. Med. 2014, 62, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Calvani, N.; Richards, H.B.; Tucci, M.; Pannarale, G.; Silvestris, F. Up-Regulation of IL-18 and Predominance of a Th1 Immune Response Is a Hallmark of Lupus Nephritis. Clin. Exp. Immunol. 2004, 138, 171–178. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Chen, Y.-M.; Wen, M.-C.; Hsieh, T.-Y.; Hung, W.-T.; Lan, J.-L. The Potential Role of Th17 Cells and Th17-Related Cytokines in the Pathogenesis of Lupus Nephritis. Lupus 2012, 21, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, C.; Wang, Y.; Song, W.; Jia, L.; Peng, X.; Zhao, R. The Expression of P2X7 Receptor on Th1, Th17, and Regulatory T Cells in Patients with Systemic Lupus Erythematosus or Rheumatoid Arthritis and Its Correlations with Active Disease. J. Immunol. 2020, 205, 1752–1762. [Google Scholar] [CrossRef]
- Mellouk, A.; Hutteau-Hamel, T.; Legrand, J.; Safya, H.; Benbijja, M.; Mercier-Nomé, F.; Benihoud, K.; Kanellopoulos, J.M.; Bobé, P. P2X7 Purinergic Receptor Plays a Critical Role in Maintaining T-Cell Homeostasis and Preventing Lupus Pathogenesis. Front. Immunol. 2022, 13, 957008. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.P.; Kurreeman, F.; Li, G.; Duclos, G.; Murphy, S.; Raul Guzman, P.; Cai, T.; Gupta, N.; Gainer, V.; Schur, P.; et al. Autoantibodies, Autoimmune Risk Alleles and Clinical Associations in Rheumatoid Arthritis Cases and Non-RA Controls in the Electronic Medical Records. Arthritis Rheum. 2013, 65, 571–581. [Google Scholar] [CrossRef]
- Yi, Y.-S. Role of Inflammasomes in Inflammatory Autoimmune Rheumatic Diseases. Korean J. Physiol. Pharmacol. 2018, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Cruwys, S.; Bowers, K.; Braddock, M. Targeting the P2X7 Receptor in Rheumatoid Arthritis: Biological Rationale for P2X7 Antagonism. Clin. Exp. Rheumatol. 2014, 32, 878–882. [Google Scholar]
- Fan, Z.-D.; Zhang, Y.-Y.; Guo, Y.-H.; Huang, N.; Ma, H.-H.; Huang, H.; Yu, H.-G. Involvement of P2X7 Receptor Signaling on Regulating the Differentiation of Th17 Cells and Type II Collagen-Induced Arthritis in Mice. Sci. Rep. 2016, 6, 35804. [Google Scholar] [CrossRef]
- Ardissone, V.; Radaelli, E.; Zaratin, P.; Ardizzone, M.; Ladel, C.; Gattorno, M.; Martini, A.; Grassi, F.; Traggiai, E. Pharmacologic P2X Purinergic Receptor Antagonism in the Treatment of Collagen-Induced Arthritis. Arthritis Rheum. 2011, 63, 3323–3332. [Google Scholar] [CrossRef] [PubMed]
- Portales-Cervantes, L.; Niño-Moreno, P.; Doníz-Padilla, L.; Baranda-Candido, L.; García-Hernández, M.; Salgado-Bustamante, M.; González-Amaro, R.; Portales-Pérez, D. Expression and Function of the P2X(7) Purinergic Receptor in Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis. Hum. Immunol. 2010, 71, 818–825. [Google Scholar] [CrossRef]
- Felix, K.M.; Teng, F.; Bates, N.A.; Ma, H.; Jaimez, I.A.; Sleiman, K.C.; Tran, N.L.; Wu, H.-J.J. P2RX7 Deletion in T Cells Promotes Autoimmune Arthritis by Unleashing the Tfh Cell Response. Front. Immunol. 2019, 10, 411. [Google Scholar] [CrossRef]
- Aeschlimann, D.; Knäuper, V. P2X7 Receptor-Mediated TG2 Externalization: A Link to Inflammatory Arthritis? Amino Acids 2017, 49, 453–460. [Google Scholar] [CrossRef]
- Dzhambazov, B.; Lindh, I.; Engström, A.; Holmdahl, R. Tissue Transglutaminase Enhances Collagen Type II-Induced Arthritis and Modifies the Immunodominant T-Cell Epitope CII260-270. Eur. J. Immunol. 2009, 39, 2412–2423. [Google Scholar] [CrossRef]
- Lauzier, A.; Charbonneau, M.; Paquette, M.; Harper, K.; Dubois, C.M. Transglutaminase 2 Cross-Linking Activity Is Linked to Invadopodia Formation and Cartilage Breakdown in Arthritis. Arthritis Res. Ther. 2012, 14, R159. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Theaker, J.; Pelegrin, P.; Clifton, A.D.; Braddock, M.; Surprenant, A. P2X(7) Receptor-Mediated Release of Cathepsins from Macrophages Is a Cytokine-Independent Mechanism Potentially Involved in Joint Diseases. J. Immunol. 2010, 185, 2611–2619. [Google Scholar] [CrossRef]
- Behl, T.; Chadha, S.; Sehgal, A.; Singh, S.; Sharma, N.; Kaur, R.; Bhatia, S.; Al-Harrasi, A.; Chigurupati, S.; Alhowail, A.; et al. Exploring the Role of Cathepsin in Rheumatoid Arthritis. Saudi J. Biol. Sci. 2022, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Yao, P.; Zhao, H. P2X7, a Critical Regulator and Potential Target for Bone and Joint Diseases. J. Cell. Physiol. 2019, 234, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Caporali, F.; Capecchi, P.L.; Gamberucci, A.; Lazzerini, P.E.; Pompella, G.; Natale, M.; Lorenzini, S.; Selvi, E.; Galeazzi, M.; Laghi Pasini, F. Human Rheumatoid Synoviocytes Express Functional P2X7 Receptors. J. Mol. Med. 2008, 86, 937–949. [Google Scholar] [CrossRef]
- Panupinthu, N.; Rogers, J.T.; Zhao, L.; Solano-Flores, L.P.; Possmayer, F.; Sims, S.M.; Dixon, S.J. P2X7 Receptors on Osteoblasts Couple to Production of Lysophosphatidic Acid: A Signaling Axis Promoting Osteogenesis. J. Cell Biol. 2008, 181, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B.; D1520C00001 Study Team. Clinical Evaluation of the Efficacy of the P2X7 Purinergic Receptor Antagonist AZD9056 on the Signs and Symptoms of Rheumatoid Arthritis in Patients with Active Disease despite Treatment with Methotrexate or Sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef]
- Stock, T.C.; Bloom, B.J.; Wei, N.; Ishaq, S.; Park, W.; Wang, X.; Gupta, P.; Mebus, C.A. Efficacy and Safety of CE-224,535, an Antagonist of P2X7 Receptor, in Treatment of Patients with Rheumatoid Arthritis Inadequately Controlled by Methotrexate. J. Rheumatol. 2012, 39, 720–727. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS Myelin—From Mechanisms to Experimental Medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Amadio, S.; Parisi, C.; Piras, E.; Fabbrizio, P.; Apolloni, S.; Montilli, C.; Luchetti, S.; Ruggieri, S.; Gasperini, C.; Laghi-Pasini, F.; et al. Modulation of P2X7 Receptor during Inflammation in Multiple Sclerosis. Front. Immunol. 2017, 8, 1529. [Google Scholar] [CrossRef]
- Sidoryk-Węgrzynowicz, M.; Strużyńska, L. Astroglial and Microglial Purinergic P2X7 Receptor as a Major Contributor to Neuroinflammation during the Course of Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 8404. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Durrenberger, P.; Chessell, I.P.; Naylor, A.; Bountra, C.; Banati, R.R.; Anand, P. COX-2, CB2 and P2X7-Immunoreactivities Are Increased in Activated Microglial Cells/Macrophages of Multiple Sclerosis and Amyotrophic Lateral Sclerosis Spinal Cord. BMC Neurol. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Matute, C.; Torre, I.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Alberdi, E.; Etxebarria, E.; Arranz, A.M.; Ravid, R.; Rodríguez-Antigüedad, A.; Sánchez-Gómez, M.; et al. P2X(7) Receptor Blockade Prevents ATP Excitotoxicity in Oligodendrocytes and Ameliorates Experimental Autoimmune Encephalomyelitis. J. Neurosci. 2007, 27, 9525–9533. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.J.; Polak, P.E.; Simonini, V.; Lin, S.X.; Richardson, J.C.; Bongarzone, E.R.; Feinstein, D.L. P2x7 Deficiency Suppresses Development of Experimental Autoimmune Encephalomyelitis. J. Neuroinflammation 2008, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Brosnan, C.F. Exacerbation of Experimental Autoimmune Encephalomyelitis in P2X7R−/− Mice: Evidence for Loss of Apoptotic Activity in Lymphocytes. J. Immunol. 2006, 176, 3115–3126. [Google Scholar] [CrossRef]
- Bijelić, D.D.; Milićević, K.D.; Lazarević, M.N.; Miljković, D.M.; Bogdanović Pristov, J.J.; Savić, D.Z.; Petković, B.B.; Andjus, P.R.; Momčilović, M.B.; Nikolić, L.M. Central Nervous System-Infiltrated Immune Cells Induce Calcium Increase in Astrocytes via Astroglial Purinergic Signaling. J. Neurosci. Res. 2020, 98, 2317–2332. [Google Scholar] [CrossRef]
- Bernal-Chico, A.; Manterola, A.; Cipriani, R.; Katona, I.; Matute, C.; Mato, S. P2x7 Receptors Control Demyelination and Inflammation in the Cuprizone Model. Brain Behav. Immun. Health 2020, 4, 100062. [Google Scholar] [CrossRef]
- Hiltensperger, M.; Beltrán, E.; Kant, R.; Tyystjärvi, S.; Lepennetier, G.; Domínguez Moreno, H.; Bauer, I.J.; Grassmann, S.; Jarosch, S.; Schober, K.; et al. Skin and Gut Imprinted Helper T Cell Subsets Exhibit Distinct Functional Phenotypes in Central Nervous System Autoimmunity. Nat. Immunol. 2021, 22, 880–892. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Carding, S.R. Inflammatory Bowel Disease: Cause and Immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.E.; Cho, M.-L. Immunological Pathogenesis of Inflammatory Bowel Disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef]
- Haas, S.L.; Ruether, A.; Singer, M.V.; Schreiber, S.; Böcker, U. Functional P2X7 Receptor Polymorphisms (His155Tyr, Arg307Gln, Glu496Ala) in Patients with Crohn’s Disease. Scand. J. Immunol. 2007, 65, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X. Research Progress of P2X7 Receptor in Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2019, 54, 521–527. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Stahl, L.; Cheung, K.-K.; de Campos, N.E.; de Oliveira Souza, C.; Ojcius, D.M.; Burnstock, G. P2X and P2Y Purinergic Receptors on Human Intestinal Epithelial Carcinoma Cells: Effects of Extracellular Nucleotides on Apoptosis and Cell Proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1024–G1035. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP Mediates Mast Cell-Dependent Intestinal Inflammation through P2X7 Purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Marques, C.C.; Castelo-Branco, M.T.; Pacheco, R.G.; Buongusto, F.; do Rosário, A.; Schanaider, A.; Coutinho-Silva, R.; de Souza, H.S.P. Prophylactic Systemic P2X7 Receptor Blockade Prevents Experimental Colitis. Biochim. Biophys. Acta 2014, 1842, 65–78. [Google Scholar] [CrossRef]
- Neves, A.R.; Castelo-Branco, M.T.L.; Figliuolo, V.R.; Bernardazzi, C.; Buongusto, F.; Yoshimoto, A.; Nanini, H.F.; Coutinho, C.M.L.M.; Carneiro, A.J.V.; Coutinho-Silva, R.; et al. Overexpression of ATP-Activated P2X7 Receptors in the Intestinal Mucosa Is Implicated in the Pathogenesis of Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Liu, X.; Xiong, Y.; Ren, Y.; Chen, J.; Lu, N.; Guo, Y.; Bai, A. Extracellular ATP Mediates Inflammatory Responses in Colitis via P2 × 7 Receptor Signaling. Sci. Rep. 2016, 6, 19108. [Google Scholar] [CrossRef]
- Figliuolo, V.R.; Savio, L.E.B.; Safya, H.; Nanini, H.; Bernardazzi, C.; Abalo, A.; de Souza, H.S.P.; Kanellopoulos, J.; Bobé, P.; Coutinho, C.M.L.M.; et al. P2X7 Receptor Promotes Intestinal Inflammation in Chemically Induced Colitis and Triggers Death of Mucosal Regulatory T Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1183–1194. [Google Scholar] [CrossRef]
- Hofman, P.; Cherfils-Vicini, J.; Bazin, M.; Ilie, M.; Juhel, T.; Hébuterne, X.; Gilson, E.; Schmid-Alliana, A.; Boyer, O.; Adriouch, S.; et al. Genetic and Pharmacological Inactivation of the Purinergic P2RX7 Receptor Dampens Inflammation but Increases Tumor Incidence in a Mouse Model of Colitis-Associated Cancer. Cancer Res. 2015, 75, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Bernardazzi, C.; Castelo-Branco, M.T.L.; Pêgo, B.; Ribeiro, B.E.; Rosas, S.L.B.; Santana, P.T.; Machado, J.C.; Leal, C.; Thompson, F.; Coutinho-Silva, R.; et al. The P2X7 Receptor Promotes Colorectal Inflammation and Tumorigenesis by Modulating Gut Microbiota and the Inflammasome. Int. J. Mol. Sci. 2022, 23, 4616. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 Diabetes: New Perspectives on Disease Pathogenesis and Treatment. Lancet 2001, 358, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Onaca, N.; Klintmalm, G.B.; Levy, M.F. Pancreatic Islet Cell Transplantation: A Treatment Strategy for Type I Diabetes Mellitus. Nutr. Clin. Pract. 2004, 19, 154–164. [Google Scholar] [CrossRef]
- Novak, I. Purinergic Receptors in the Endocrine and Exocrine Pancreas. Purinergic Signal. 2008, 4, 237–253. [Google Scholar] [CrossRef]
- Glas, R.; Sauter, N.S.; Schulthess, F.T.; Shu, L.; Oberholzer, J.; Maedler, K. Purinergic P2X7 Receptors Regulate Secretion of Interleukin-1 Receptor Antagonist and Beta Cell Function and Survival. Diabetologia 2009, 52, 1579–1588. [Google Scholar] [CrossRef]
- Chen, D.; Thayer, T.C.; Wen, L.; Wong, F.S. Mouse Models of Autoimmune Diabetes: The Nonobese Diabetic (NOD) Mouse. Methods Mol. Biol. 2020, 2128, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Robson, T.; Beales, P.E.; Burnstock, G. Changes in Expression of P2X7 Receptors in NOD Mouse Pancreas during the Development of Diabetes. Autoimmunity 2007, 40, 108–116. [Google Scholar] [CrossRef]
- Vieira, F.S.; Nanini, H.F.; Takiya, C.M.; Coutinho-Silva, R. P2X7 Receptor Knockout Prevents Streptozotocin-Induced Type 1 Diabetes in Mice. Mol. Cell. Endocrinol. 2016, 419, 148–157. [Google Scholar] [CrossRef]
- Pearl-Yafe, M.; Iskovich, S.; Kaminitz, A.; Stein, J.; Yaniv, I.; Askenasy, N. Does Physiological Beta Cell Turnover Initiate Autoimmune Diabetes in the Regional Lymph Nodes? Autoimmun. Rev. 2006, 5, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ding, H.; Li, Y.; Pearson, J.A.; Zhang, X.; Flavell, R.A.; Wong, F.S.; Wen, L. NLRP3 Deficiency Protects from Type 1 Diabetes through the Regulation of Chemotaxis into the Pancreatic Islets. Proc. Natl. Acad. Sci. USA 2015, 112, 11318–11323. [Google Scholar] [CrossRef] [PubMed]
- Sticherling, M. Psoriasis and Autoimmunity. Autoimmun. Rev. 2016, 15, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Casciano, F.; Secchiero, P.; Reali, E. Purinergic Signaling and Inflammasome Activation in Psoriasis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9449. [Google Scholar] [CrossRef]
- Killeen, M.E.; Ferris, L.; Kupetsky, E.A.; Falo, L.; Mathers, A.R. Signaling through Purinergic Receptors for ATP Induces Human Cutaneous Innate and Adaptive Th17 Responses: Implications in the Pathogenesis of Psoriasis. J. Immunol. 2013, 190, 4324–4336. [Google Scholar] [CrossRef]
- Geraghty, N.J.; Mansfield, K.J.; Fuller, S.J.; Watson, D.; Sluyter, R. The P2X7 Receptor Is Not Essential for Development of Imiquimod-Induced Psoriasis-like Inflammation in Mice. Purinergic Signal. 2017, 13, 405–415. [Google Scholar] [CrossRef]
- Elias, P.M.; Arbiser, J.; Brown, B.E.; Rossiter, H.; Man, M.-Q.; Cerimele, F.; Crumrine, D.; Gunathilake, R.; Choi, E.H.; Uchida, Y.; et al. Epidermal Vascular Endothelial Growth Factor Production Is Required for Permeability Barrier Homeostasis, Dermal Angiogenesis, and the Development of Epidermal Hyperplasia: Implications for the Pathogenesis of Psoriasis. Am. J. Pathol. 2008, 173, 689–699. [Google Scholar] [CrossRef]
- Ameglio, F.; Bonifati, C.; Pietravalle, M.; Fazio, M. Interleukin-6 and Tumour Necrosis Factor Levels Decrease in the Suction Blister Fluids of Psoriatic Patients during Effective Therapy. Dermatology 1994, 189, 359–363. [Google Scholar] [CrossRef]
- Nikolov, N.P.; Illei, G.G. Pathogenesis of Sjögren’s Syndrome. Curr. Opin. Rheumatol. 2009, 21, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Beer, R.G.; Rischmueller, M.; Coates, T.; Purcell, A.W.; Keech, C.L.; McCluskey, J.; Gordon, T.P. Nonprecipitating Anti-La(SS-B) Autoantibodies in Primary Sjögren’s Syndrome. Clin. Immunol. Immunopathol. 1996, 79, 314–318. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Li, M.; Gao, Q.; Peng, Y.; Gong, Q.; Zhang, Z.; Wu, X. Paeoniflorin Down-Regulates ATP-Induced Inflammatory Cytokine Production and P2X7R Expression on Peripheral Blood Mononuclear Cells from Patients with Primary Sjögren’s Syndrome. Int. Immunopharmacol. 2015, 28, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Rossi, C.; Ferro, F.; Santini, E.; Seccia, V.; Donati, V.; Solini, A. The P2X7 Receptor-Inflammasome Complex Has a Role in Modulating the Inflammatory Response in Primary Sjögren’s Syndrome. J. Intern. Med. 2013, 274, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, M.G.; Woods, L.T.; Jasmer, K.J.; Forti, K.M.; Camden, J.M.; Jensen, J.L.; Limesand, K.H.; Galtung, H.K.; Weisman, G.A. P2 Receptors as Therapeutic Targets in the Salivary Gland: From Physiology to Dysfunction. Front. Pharmacol. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.T.; Camden, J.M.; Batek, J.M.; Petris, M.J.; Erb, L.; Weisman, G.A. P2X7 Receptor Activation Induces Inflammatory Responses in Salivary Gland Epithelium. Am. J. Physiol. Cell Physiol. 2012, 303, C790–C801. [Google Scholar] [CrossRef]
- Khalafalla, M.G.; Woods, L.T.; Camden, J.M.; Khan, A.A.; Limesand, K.H.; Petris, M.J.; Erb, L.; Weisman, G.A. P2X7 Receptor Antagonism Prevents IL-1β Release from Salivary Epithelial Cells and Reduces Inflammation in a Mouse Model of Autoimmune Exocrinopathy. J. Biol. Chem. 2017, 292, 16626–16637. [Google Scholar] [CrossRef]
- Eser, A.; Colombel, J.-F.; Rutgeerts, P.; Vermeire, S.; Vogelsang, H.; Braddock, M.; Persson, T.; Reinisch, W. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn’s Disease: A Randomized Placebo-Controlled, Double-Blind, Phase IIa Study. Inflamm. Bowel Dis. 2015, 21, 2247–2253. [Google Scholar] [CrossRef]
| Polymorphism | Effect on Function | Implicated Conditions |
|---|---|---|
| rs1718119 A348T | SLE [67] RA [68] MS [69] | |
| Gain | ||
| rs3752243 E496A | Loss | RA [68] Protective against T1D [70] |
| rs22390912 G464R | Gain | MS [69] |
| rs17525809 A76V | Gain | MS [71] |
| rs208294 H155Y | Gain | SLE [72] RA [73] MS [71] |
| rs7958311 R270H | Loss | Protective against T1D [70] |
| rs28360457 R307Q | Loss | MS [74] |
| rs2230912 A1405G | Gain | pSS [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, F.; Salina, G. The P2X7 Receptor in Autoimmunity. Int. J. Mol. Sci. 2023, 24, 14116. https://doi.org/10.3390/ijms241814116
Grassi F, Salina G. The P2X7 Receptor in Autoimmunity. International Journal of Molecular Sciences. 2023; 24(18):14116. https://doi.org/10.3390/ijms241814116
Chicago/Turabian StyleGrassi, Fabio, and Gaia Salina. 2023. "The P2X7 Receptor in Autoimmunity" International Journal of Molecular Sciences 24, no. 18: 14116. https://doi.org/10.3390/ijms241814116
APA StyleGrassi, F., & Salina, G. (2023). The P2X7 Receptor in Autoimmunity. International Journal of Molecular Sciences, 24(18), 14116. https://doi.org/10.3390/ijms241814116






