Characterization of the Pyrroloquinoline Quinone Producing Rhodopseudomonas palustris as a Plant Growth-Promoting Bacterium under Photoautotrophic and Photoheterotrophic Culture Conditions
Abstract
1. Introduction
2. Results
2.1. PQQ Synthesis Genes in Rhodopseudomonas Palustris CGA009 Genome
2.2. Transcriptional Levels of PQQ Relative Genes
2.3. Confirmation of PQQ Production in R. palustris CGA009 under Different Culturing Conditions
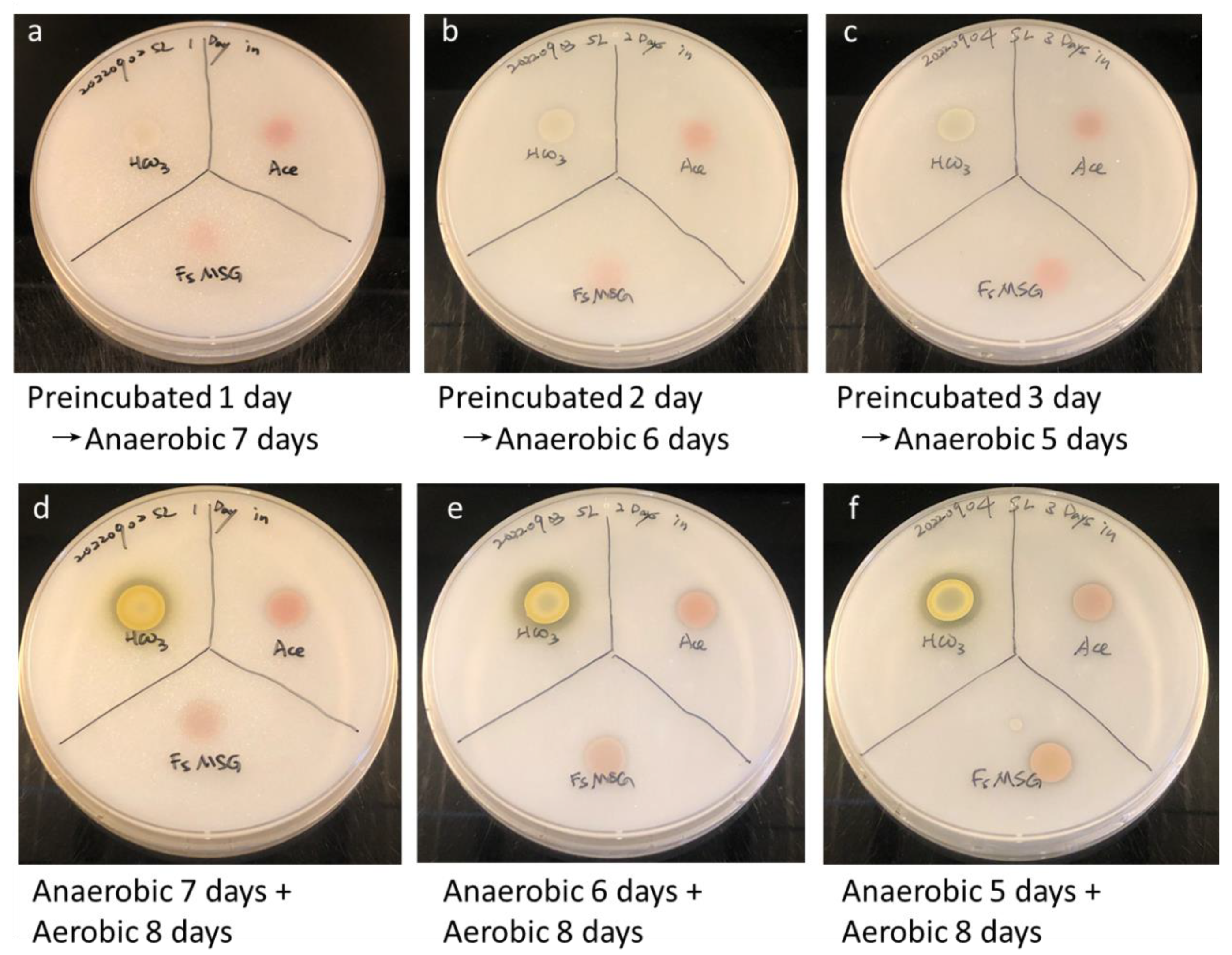
2.4. Phosphorus-Solubilizing Activity Assay
2.5. Estimated Siderophore Activity
2.6. R. palustris CGA009 Effect on Arabidopsis thaliana’s Growth Parameters
2.7. Identification of Diverse Endophytes in Arabidopsis following R. palustris CGA009 Inoculation
| Conditions | Pellets or Supernatant | Endophyte Colony | Phosphorus-Solubilizing Activity | The Best Match with 16S rRNA Gene in BLAST Results (Identity %) | Relative Researches |
|---|---|---|---|---|---|
| HCO3 medium, microaerobic, light, 30 °C, 17 days | R. palustris Pellets | HP1 | No | Stenotrophomonas maltophilia (99.51%) | Plant-growth-promoting rhizobacterium against stress conditions [34] |
| HP2 | Yes | Microbacterium proteolyticum (99.21%) | Endophytic bacterium isolated from roots of Halimione portulacoides [37] | ||
| HP3 | Yes | Paraburkholderia pallidirosea (97.70%) 1 | Belong to plant-beneficial environmental groups of bacterium [38] | ||
| Supernatant | HS1 | No | Rhodanobacter lindaniclasticus (99.17%) | A lindane-degrading bacterium [39] | |
| HS2 | Yes | Rhodanobacter fulvus (99.15%) | Biological control activity towards the root-rot plant pathogen Cylindrocladium spathiphylli [40] | ||
| HS3 | Yes | Rhodanobacter soli (93%) 1 | A soil bacterium from a ginseng field [41] | ||
| Acetate medium, microaerobic, light, 30 °C, 8 days | R. palustris Pellets | AP1 | Yes | Burkholderia anthina (99.79%) | Plant-growth-promoting bacteria of sugarcane [35] |
| Supernatant | AS1 | Yes | Pseudomonas citronellolis (95.17%) 1 | Multi-metal resistant [42] | |
| FsMSG medium, microaerobic, light, 30 °C, 8 days | R. palustris Pellets | FP1 | Yes | Achromobacter insuavis (94.45%) 1 | Could be isolated from cystic fibrosis patients [43] |
| FP2 | Yes | Achromobacter insuavis (99.56%) | |||
| FP3 | Yes | Achromobacter insuavis (99.86%) | |||
| Supernatant | FS1 | No | Paraburkholderia kururiensis (99.58) | A trichloroethylene-degrading bacterium [44] | |
| FS2 | No | Ferrovibrio xuzhouensis (99.49%) | A cyhalothrin-degrading bacterium [45] | ||
| FS3 | Yes | Amycolatopsis rhabdoformis (98.81%) | A soil bacterium from a tropical forest [46] |
3. Discussion
4. Materials and Methods
4.1. Culture Conditions
4.2. Transcriptome Analysis
4.3. Phosphorus-Solubilizing Activity Assay
4.4. Siderophore Estimation Assay
4.5. PQQ Extraction for LC-MS Analysis
4.6. LC-MS Method
4.7. Enzymatic PQQ Determination
4.7.1. Preparation of E. coli Membrane Fractions
4.7.2. PQQ Bioassays
4.8. Plant’s Growth Condition
4.9. Chlorophyll Content Measurements
4.10. Isolation and Identification of Endophytes
4.11. Statistic
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larimer, F.W.; Chain, P.; Hauser, L.; Lamerdin, J.; Malfatti, S.; Do, L.; Land, M.L.; Pelletier, D.A.; Beatty, J.T.; Lang, A.S.; et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 2004, 22, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Nookongbut, P.; Kantachote, D.; Khuong, N.Q.; Tantirungkij, M. The biocontrol potential of acid-resistant Rhodopseudomonas palustris KTSSR54 and its exopolymeric substances against rice fungal pathogens to enhance rice growth and yield. Biol. Control 2020, 150, 104354. [Google Scholar] [CrossRef]
- Luo, L.; Wang, P.; Zhai, Z.; Su, P.; Tan, X.; Zhang, D.; Zhang, Z.; Liu, Y. The effects of Rhodopseudomonas palustris PSB06 and CGA009 with different agricultural applications on rice growth and rhizosphere bacterial communities. AMB Express 2019, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-T.; Tseng, C.-H.; Hsu, S.-H.; Lur, H.-S.; Mo, C.-W.; Huang, C.-N.; Hsu, S.-C.; Lee, K.-T.; Liu, C.-T. Promoting effects of a single Rhodopseudomonas palustris inoculant on plant growth by Brassica rapa chinensis under low fertilizer input. Microbes Environ. 2014, 29, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Tan, X.; Li, C.; Zhang, D.; Cheng, J.e.; Zhang, S.; Zhou, X.; Yan, Q.; Peng, J.; Zhang, Z.; et al. Photosynthetic bacterium Rhodopseudomonas palustris GJ-22 induces systemic resistance against viruses. Microb. Biotechnol. 2017, 10, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Han, J. The influence of photosynthetic bacteria treatments on the crop yield, dry matter content, and protein content of the mushroom Agaricus bisporus. Sci. Hortic. 1999, 82, 171–178. [Google Scholar] [CrossRef]
- Yin, Z.P.; Shang, Z.W.; Wei, C.; Ren, J.; Song, X.S. Foliar sprays of photosynthetic bacteria improve the growth and anti-oxidative capability on Chinese dwarf cherry seedings. J. Plant Nutr. 2012, 35, 840–853. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Shen, M.-W.; Chen, J.-C.; Lur, H.-S.; Liu, C.-T. The photosynthetic bacterium Rhodopseudomonas palustris strain PS3 exerts plant growth-promoting effects by stimulating nitrogen uptake and elevating auxin levels in expanding leaves. Front. Plant Sci. 2021, 12, 573634. [Google Scholar] [CrossRef]
- Cordell, G.A.; Daley, S. Pyrroloquinoline quinone chemistry, biology, and biosynthesis. Chem. Res. Toxicol. 2022, 35, 355–377. [Google Scholar] [CrossRef]
- Choi, O.; Kim, J.; Kim, J.-G.; Jeong, Y.; Moon, J.S.; Park, C.S.; Hwang, I. Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol. 2008, 146, 657–668. [Google Scholar] [CrossRef]
- Crespo, J.M.; Boiardi, J.L.; Luna, M.F. Mineral phosphate solubilization activity of Gluconacetobacter diazotrophicus under P-limitation and plant root environment. Agric. Sci. 2011, 2, 16–22. [Google Scholar] [CrossRef][Green Version]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Sohail, Y.; Khalid, N.; Ahmed, I.; Mumtaz, A.S. Evaluation of glucose dehydrogenase and pyrroloquinoline quinine (pqq) mutagenesis that renders functional inadequacies in host plants. J. Microbiol. Biotechnol. 2015, 25, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.N.; Huang, C.C. Draft genome sequence of Burkholderia cenocepacia strain 869T2, a plant-beneficial endophytic bacterium. Genome Announc. 2015, 3, e01327-15. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiao, Z.; Hale, L.; Wu, W.; Guo, Y. Disruption of gene pqqA or pqqB reduces plant growth promotion activity and biocontrol of crown gall disease by Rahnella aquatilis HX2. PLoS ONE 2014, 9, e115010. [Google Scholar] [CrossRef] [PubMed]
- Toyama, H.; Lidstrom, M.E. pqqA is not required for biosynthesis of pyrroloquinoline quinone in Methylobacterium extorquens AM1. Microbiology 1998, 144, 183–191. [Google Scholar] [CrossRef]
- Velterop, J.; Sellink, E.; Meulenberg, J.; David, S.; Bulder, I.; Postma, P. Synthesis of pyrroloquinoline quinone in vivo and in vitro and detection of an intermediate in the biosynthetic pathway. J. Bacteriol. 1995, 177, 5088–5098. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, L.; Han, X.; Wang, J.; Zhang, W. Knockout and function analysis of pqqL gene in Escherichia coli. Wei Sheng Wu Xue Bao 2010, 50, 1380–1384. [Google Scholar]
- Hölscher, T.; Görisch, H. Knockout and overexpression of pyrroloquinoline quinone biosynthetic genes in Gluconobacter oxydans 621H. J. Bacteriol. 2006, 188, 7668–7676. [Google Scholar] [CrossRef]
- Martins, A.M.; Latham, J.A.; Martel, P.J.; Barr, I.; Iavarone, A.T.; Klinman, J.P. A two-component protease in Methylorubrum extorquens with high activity toward the peptide precursor of the redox cofactor pyrroloquinoline quinone. J. Biol. Chem. 2019, 294, 15025–15036. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Mazodier, P.; Biville, F.; Turlin, E.; Gasser, F. Localization of a pyrroloquinoline quinone biosynthesis gene near the methanol dehydrogenase structural gene in Methylobacterium organophilum DSM 760. Microbiology 1988, 134, 2513–2524. [Google Scholar] [CrossRef]
- Goosen, N.; Huinen, R.; Van de Putte, P. A 24-amino-acid polypeptide is essential for the biosynthesis of the coenzyme pyrrolo-quinoline-quinone. J. Bacteriol. 1992, 174, 1426–1427. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Kim, C.H.; Lee, J.H.; Park, J.Y.; Cho, S.M.; Park, S.K.; Kim, K.Y.; Krishnan, H.B.; Kim, Y.C. Inactivation of pqq genes of Enterobacter intermedium 60-2G reduces antifungal activity and induction of systemic resistance. FEMS Microbiol. Lett. 2008, 282, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.H.; Browne, P.; Prigent-Combaret, C.; Combes-Meynet, E.; Morrissey, J.P.; O’Gara, F. Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environ. Microbiol. Rep. 2010, 2, 403–411. [Google Scholar] [CrossRef]
- Gliese, N.; Khodaverdi, V.; Görisch, H. The PQQ biosynthetic operons and their transcriptional regulation in Pseudomonas aeruginosa. Arch. Microbiol. 2010, 192, 1–14. [Google Scholar] [CrossRef]
- Ke, C. Breeding of Hyphomicrobium denitrificans for high production of pyrroloquinoline quinone by adaptive directed domestication. Sheng Wu Gong Cheng Xue Bao 2020, 36, 152–161. [Google Scholar] [CrossRef]
- Adachi, O.; Okamoto, K.; Shinagawa, E.; Matsushita, K.; Ameyama, M. Adduct formation of pyrroloquinoline quinone and amino acid. Biofactors 1988, 1, 251–254. [Google Scholar]
- Stines-Chaumeil, C.; Mavré, F.; Kauffmann, B.; Mano, N.; Limoges, B. Mechanism of reconstitution/activation of the soluble PQQ-dependent glucose dehydrogenase from Acinetobacter calcoaceticus: A comprehensive study. ACS Omega 2020, 5, 2015–2026. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.C.; Lo, S.-C.; Mathew, G.M.; Chang, K.-H.; Huang, C.-C. Genomic sequence analysis of a plant-associated Photobacterium halotolerans MELD1: From marine to terrestrial environment? Stand. Genom. Sci. 2016, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Singh, V.K.; Mishra, A.; Jha, B. Plant growth promoting rhizobacterium Stenotrophomonas maltophilia BJ01 augments endurance against N2 starvation by modulating physiology and biochemical activities of Arachis hypogea. PLoS ONE 2019, 14, e0222405. [Google Scholar] [CrossRef]
- Malviya, M.K.; Li, C.-N.; Solanki, M.K.; Singh, R.K.; Htun, R.; Singh, P.; Verma, K.K.; Yang, L.-T.; Li, Y.-R. Comparative analysis of sugarcane root transcriptome in response to the plant growth-promoting Burkholderia anthina MYSP113. PLoS ONE 2020, 15, e0231206. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Feng, Y.; Wang, Y.; Lin, X. Effect of rhizobacterium Rhodopseudomonas palustris inoculation on Stevia rebaudiana plant growth and soil microbial community. Pedosphere 2018, 28, 793–803. [Google Scholar] [CrossRef]
- Alves, A.; Riesco, R.; Correia, A.; Trujillo, M.E. Microbacterium proteolyticum sp. nov. isolated from roots of Halimione portulacoides. Int. J. Syst. Evol. Microbiol. 2015, 65, 1794–1798. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Evidence of endophytic diazotrophic bacteria in lodgepole pine and hybrid white spruce trees growing in soils with different nutrient statuses in the West Chilcotin region of British Columbia, Canada. For. Ecol. Manag. 2018, 430, 558–565. [Google Scholar] [CrossRef]
- Nalin, R.; Simonet, P.; Vogel, T.M.; Normand, P. Rhodanobacter lindaniclasticus gen. nov., sp. nov., a lindane-degrading bacterium. Int. J. Syst. Evol. Microbiol. 1999, 49, 19–23. [Google Scholar] [CrossRef]
- De Clercq, D.; Van Trappen, S.; Cleenwerck, I.; Ceustermans, A.; Swings, J.; Coosemans, J.; Ryckeboer, J. Rhodanobacter spathiphylli sp. nov., a gammaproteobacterium isolated from the roots of Spathiphyllum plants grown in a compost-amended potting mix. Int. J. Syst. Evol. Microbiol. 2006, 56, 1755–1759. [Google Scholar] [CrossRef]
- Bui, T.P.N.; Kim, Y.J.; Kim, H.; Yang, D.C. Rhodanobacter soli sp. nov., isolated from soil of a ginseng field. Int. J. Syst. Evol. Microbiol. 2010, 60, 2935–2939. [Google Scholar] [CrossRef]
- Adhikary, A.; Kumar, R.; Pandir, R.; Bhardwaj, P.; Wusirika, R.; Kumar, S. Pseudomonas citronellolis; a multi-metal resistant and potential plant growth promoter against arsenic (V) stress in chickpea. Plant Physiol. Biochem. 2019, 142, 179–192. [Google Scholar] [CrossRef]
- Chalhoub, H.; Kampmeier, S.; Kahl, B.C.; Van Bambeke, F. Role of efflux in antibiotic resistance of Achromobacter xylosoxidans and Achromobacter insuavis isolates from patients with cystic fibrosis. Front. Microbiol. 2022, 13, 762307. [Google Scholar] [CrossRef]
- Zhang, H.; Hanada, S.; Shigematsu, T.; Shibuya, K.; Kamagata, Y.; Kanagawa, T.; Kurane, R. Burkholderia kururiensis sp. nov., a trichloroethylene (TCE)-degrading bacterium isolated from an aquifer polluted with TCE. Int. J. Syst. Evol. Microbiol. 2000, 50, 743–749. [Google Scholar] [CrossRef]
- Song, M.; Zhang, L.; Sun, B.; Zhang, H.; Ding, H.; Li, Q.; Guo, S.; Huang, X. Ferrovibrio xuzhouensis sp. nov., a cyhalothrin-degrading bacterium isolated from cyhalothrin contaminated wastewater. Antonie Van Leeuwenhoek 2015, 108, 377–382. [Google Scholar] [CrossRef]
- Souza, W.R.; Silva, R.E.; Goodfellow, M.; Busarakam, K.; Figueiro, F.S.; Ferreira, D.; Rodrigues-Filho, E.; Moraes, L.A.B.; Zucchi, T.D. Amycolatopsis rhabdoformis sp. nov., an actinomycete isolated from a tropical forest soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 1786–1793. [Google Scholar] [CrossRef]
- Nunkaew, T.; Kantachote, D.; Kanzaki, H.; Nitoda, T.; Ritchie, R.J. Effects of 5-aminolevulinic acid (ALA)-containing supernatants from selected Rhodopseudomonas palustris strains on rice growth under NaCl stress, with mediating effects on chlorophyll, photosynthetic electron transport and antioxidative enzymes. Electron. J. Biotechnol. 2014, 17, 19–26. [Google Scholar] [CrossRef]
- Lo, K.-J.; Lee, S.-K.; Liu, C.-T. Development of a low-cost culture medium for the rapid production of plant growth-promoting Rhodopseudomonas palustris strain PS3. PLoS ONE 2020, 15, e0236739. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Lur, H.-S.; Liu, C.-T. From lab to farm: Elucidating the beneficial roles of photosynthetic bacteria in sustainable agriculture. Microorganisms 2021, 9, 2453. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Koh, R.-H.; Song, H.-G. Enhancement of growth and yield of tomato by Rhodopseudomonas sp. under greenhouse conditions. J. Microbiol. 2008, 46, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Adhikari, A.; Khan, M.A.; Kwon, E.-H.; Park, Y.-S.; Lee, I.-J. Influence of the rhizobacterium Rhodobacter sphaeroides KE149 and biochar on waterlogging stress tolerance in Glycine max L. Environments 2021, 8, 94. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Lin, X. Purple phototrophic bacterium enhances stevioside yield by Stevia rebaudiana bertoni via foliar spray and rhizosphere Irrigation. PLoS ONE 2013, 8, e67644. [Google Scholar] [CrossRef]
- Lo, K.-J.; Lin, S.-S.; Lu, C.-W.; Kuo, C.-H.; Liu, C.-T. Whole-genome sequencing and comparative analysis of two plant-associated strains of Rhodopseudomonas palustris (PS3 and YSC3). Sci. Rep. 2018, 8, 12769. [Google Scholar] [CrossRef] [PubMed]
- Batool, K.; tuz Zahra, F.; Rehman, Y. Arsenic-redox transformation and plant growth promotion by purple nonsulfur bacteria Rhodopseudomonas palustris CS2 and Rhodopseudomonas faecalis SS5. BioMed Res. Int. 2017, 2017, 6250327. [Google Scholar] [CrossRef] [PubMed]
- Adachi, O.; Matsushita, K.; Ameyama, M. Biochemistry and physiology of pyrroloquinoline quinone and quinoprotein dehydrogenases. J. Nutr. Sci. Vitaminol. 1992, 38, 224–227. [Google Scholar] [CrossRef]
- Lo, S.-C.; Shih, S.-H.; Chang, J.-J.; Wang, C.-Y.; Huang, C.-C. Enhancement of photoheterotrophic biohydrogen production at elevated temperatures by the expression of a thermophilic clostridial hydrogenase. Appl. Microbiol. Biotechnol. 2012, 95, 969–977. [Google Scholar] [CrossRef]
- Lo, S.-C.; Chiang, E.-P.I.; Yang, Y.-T.; Li, S.-Y.; Peng, J.-H.; Tsai, S.-Y.; Wu, D.-Y.; Yu, C.-H.; Huang, C.-H.; Su, T.-T.; et al. Growth enhancement facilitated by gaseous CO2 through heterologous expression of reductive tricarboxylic acid cycle genes in Escherichia coli. Fermentation 2021, 7, 98. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wu, Z.-Z.; Wang, G.-L.; Yang, X.-P. Separation and purification of pyrroloquinoline quinone from Gluconobacter oxydans fermentation broth using supramolecular solvent complex extraction. Food Chem. 2021, 361, 130067. [Google Scholar] [CrossRef] [PubMed]
- Noji, N.; Nakamura, T.; Kitahata, N.; Taguchi, K.; Kudo, T.; Yoshida, S.; Tsujimoto, M.; Sugiyama, T.; Asami, T. Simple and sensitive method for pyrroloquinoline quinone (PQQ) analysis in various foods using liquid chromatography/electrospray-ionization tandem mass spectrometry. J. Agric. Food Chem. 2007, 55, 7258–7263. [Google Scholar] [CrossRef]
- Kato, C.; Kawai, E.; Shimizu, N.; Mikekado, T.; Kimura, F.; Miyazawa, T.; Nakagawa, K. Determination of pyrroloquinoline quinone by enzymatic and LC-MS/MS methods to clarify its levels in foods. PLoS ONE 2018, 13, e0209700. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Arents, J.C.; Bader, R.; Yamada, M.; Adachi, O.; Postma, P.W. Escherichia coli is unable to produce pyrroloquinoline quinone (PQQ). Microbiology 1997, 143 Pt 10, 3149–3156. [Google Scholar] [CrossRef] [PubMed]
- Geiger, O.; Görisch, H. Enzymatic determination of pyrroloquinoline quinone using crude membranes from Escherichia coli. Anal. Biochem. 1987, 164, 418–423. [Google Scholar] [CrossRef]
- An, R.; Moe, L.A. Regulation of pyrroloquinoline quinone-dependent glucose dehydrogenase activity in the model rhizosphere-dwelling bacterium Pseudomonas putida KT2440. Appl. Environ. Microbiol. 2016, 82, 4955–4964. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-H.; Chien, P.-R.; Huang, F.-C.; Yeh, P.-H.; Hung, S.-H.W.; Deng, W.-L.; Huang, C.-C. A plant endophytic bacterium Priestia megaterium strain BP-R2 isolated from the halophyte Bolboschoenus planiculmis enhances plant growth under salt and drought stresses. Microorganisms 2022, 10, 2047. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth stage–based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]



| PQQ Synthesis Related Genes | Conserved Protein Domain Family | References for PQQ Synthesis | Locus Tag in CGA009 (NCBI Reference Sequence: NC_005296.1) 1 |
|---|---|---|---|
| pqqA | PQQ_syn_pqqA (TIGR02107) | [17,22,23] | TX73_RS09945 |
| pqqB | PRK05184 | [17,24,25] | TX73_RS09950 |
| pqqC | PRK05157 | [17] | TX73_RS09955 |
| pqqD | PqqD Superfamily (cl05126) | [17] | TX73_RS09960 |
| pqqE | PRK05301 | [17,25] | TX73_RS09965 |
| Peptidases | |||
| pqqF | PQQ_syn_pqqF (TIGR02110) | [17] | None |
| pqqL | PqqL (COG0612) | [18] | TX73_RS04330, TX73_RS22305, TX73_RS22310 |
| pqqG | PqqL (COG0612) | [20] | TX73_RS04330, TX73_RS22305, TX73_RS22310 |
| pqqH | DAP2 (COG1506) 2 | [26] | none |
| pqqM | DAP2 (COG1506) 2 | [10] | none |
| TldD | PmbA_TldD Superfamily (cl19356) | [19] | TX73_RS04255, TX73_RS05895 |
| Gene Name 1 | Gene Description 2 | HCO3 (TPM) | Acetate (TPM) | log2 Ratio | p Value |
|---|---|---|---|---|---|
| PQQ-dependent enzyme genes | |||||
| TX73_RS03805 | PQQ_dependent_sugar_dehydrogenase | 136.1 | 3.3 | 5.33 | 0.007 |
| TX73_RS16265 | PQQ_dependent_dehydrogenase__methanol_ethanol_family | 298.7 | 2.5 | 6.81 | 0.006 |
| TX73_RS22090 | PQQ_dependent_sugar_dehydrogenase | 18.0 | 8.5 | 1.06 | 0.429 |
| PQQ synthesis genes | |||||
| pqqA | pyrroloquinoline_quinone_precursor_peptide_PqqA | 2523.6 | 160.9 | 3.97 | 0.039 |
| pqqB | pyrroloquinoline_quinone_biosynthesis_protein_PqqB | 159.7 | 6.9 | 4.51 | 0.016 |
| pqqC | pyrroloquinoline_quinone_synthase_PqqC | 69.3 | 5.0 | 3.78 | 0.016 |
| pqqD | pyrroloquinoline_quinone_biosynthesis_peptide_chaperone_PqqD | 30.5 | 1.7 | 4.12 | 0.004 |
| pqqE | pyrroloquinoline_quinone_biosynthesis_protein_PqqE | 20.9 | 1.6 | 3.65 | 0.013 |
| TX73_RS20405 | PqqD_family_protein | 38.3 | 7.3 | 2.39 | 0.066 |
| Peptidase genes 3 | |||||
| TX73_RS04330 | Predicted Zn-dependent peptidase (PqqL) | 20.7 | 35.2 | −0.76 | 0.608 |
| TX73_RS22305 | Predicted Zn-dependent peptidase (PqqL) | 12.1 | 18.2 | −0.59 | 0.669 |
| TX73_RS22310 | Predicted Zn-dependent peptidase (PqqL) | 13.3 | 21.6 | −0.69 | 0.620 |
| tldD | PmbA_TldD Superfamily | 116.8 | 149.9 | −0.35 | 0.847 |
| TX73_RS05895 | PmbA_TldD Superfamily | 15.0 | 39.7 | −1.39 | 0.357 |
| Medium | Culture Condition | Culture Period (Day) | Final OD650 | Percent Siderophore Unit |
|---|---|---|---|---|
| HCO3 | Photoautotrophic | 17 | 0.745 | 28.9 ± 4 |
| Acetate | Photoheterotrophic | 8 | 1.84 | 0 |
| FsMSG | photoheterotrophic | 8 | 4.19 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, S.-C.; Tsai, S.-Y.; Chang, W.-H.; Wu, I.-C.; Sou, N.-L.; Hung, S.-H.W.; Chiang, E.-P.I.; Huang, C.-C. Characterization of the Pyrroloquinoline Quinone Producing Rhodopseudomonas palustris as a Plant Growth-Promoting Bacterium under Photoautotrophic and Photoheterotrophic Culture Conditions. Int. J. Mol. Sci. 2023, 24, 14080. https://doi.org/10.3390/ijms241814080
Lo S-C, Tsai S-Y, Chang W-H, Wu I-C, Sou N-L, Hung S-HW, Chiang E-PI, Huang C-C. Characterization of the Pyrroloquinoline Quinone Producing Rhodopseudomonas palustris as a Plant Growth-Promoting Bacterium under Photoautotrophic and Photoheterotrophic Culture Conditions. International Journal of Molecular Sciences. 2023; 24(18):14080. https://doi.org/10.3390/ijms241814080
Chicago/Turabian StyleLo, Shou-Chen, Shang-Yieng Tsai, Wei-Hsiang Chang, I-Chen Wu, Nga-Lai Sou, Shih-Hsun Walter Hung, En-Pei Isabel Chiang, and Chieh-Chen Huang. 2023. "Characterization of the Pyrroloquinoline Quinone Producing Rhodopseudomonas palustris as a Plant Growth-Promoting Bacterium under Photoautotrophic and Photoheterotrophic Culture Conditions" International Journal of Molecular Sciences 24, no. 18: 14080. https://doi.org/10.3390/ijms241814080
APA StyleLo, S.-C., Tsai, S.-Y., Chang, W.-H., Wu, I.-C., Sou, N.-L., Hung, S.-H. W., Chiang, E.-P. I., & Huang, C.-C. (2023). Characterization of the Pyrroloquinoline Quinone Producing Rhodopseudomonas palustris as a Plant Growth-Promoting Bacterium under Photoautotrophic and Photoheterotrophic Culture Conditions. International Journal of Molecular Sciences, 24(18), 14080. https://doi.org/10.3390/ijms241814080






