Abstract
Cardiovascular diseases, particularly coronary artery disease (CAD), remain the leading cause of death worldwide in recent years, with myocardial infarction (MI) being the most common form of CAD. Atherosclerosis has been highlighted as one of the drivers of CAD, and much research has been carried out to understand and treat this disease. However, there remains much to be better understood and developed in treating this disease. Genome editing technologies have been widely used to establish models of disease as well as to treat various genetic disorders at their root. In this review, we aim to highlight the various ways genome editing technologies can be applied to establish models of atherosclerosis, as well as their therapeutic roles in both atherosclerosis and the clinical implications of CAD.
1. Introduction
Despite advances in medicine, cardiovascular diseases (CVDs) remain one of the leading causes of death worldwide. As of 2019, CVDs account for almost one-third of deaths globally [1,2] with cases increasing significantly since 1990 [3]. CVD is an umbrella term for a variety of diseases that affect the heart such as stroke, coronary artery disease, and hypertensive heart disease [4,5]. Often, these diseases can result in heart failure (HF), with coronary artery diseases (CADs), in particular, being indicated as one of the leading forms of CVD related mortality and disease burden, contributing to 49.2% of all CVD related deaths [3,4,5].
CAD mainly results from plaque formation in the intima of arteries, resulting in the narrowing and eventual occlusion of the vessel, a disease known as atherosclerosis [6]. In the clinic, CADs manifest mainly as myocardial infarctions (MIs) or ischemic cardiomyopathies [7]. While reperfusion is often the main therapy for instances of MIs, they may sometimes result in ischemia/reperfusion injury, leading to myocardial dysfunction and eventual heart failure [8]. It is, thus, unsurprising that the study of atherosclerosis is highly important in not only the understanding but also the potential treatment of CAD.
Much effort has been made to understand the genetic risk factors underlying CAD and, consequently, potential areas for intervention. CAD is a complex disease influenced by both the environment and genetics. In recent years, advances in sequencing technologies have allowed for the identification of various gene loci that are significantly associated with CAD [9]. Genome-wide association studies (GWAS) on various cohorts have identified various genetic risk loci that are associated with CAD. Many of the genetic loci identified lie near genes with roles in the metabolism of cholesterol and lipoproteins such as PCSK9, APOB, and ANGPTL4 [10].
Many population studies on atherosclerosis have also identified elevation of low-density lipoprotein (LDL) cholesterol and apolipoprotein B (APOB) 100 to be associated with risk of atherosclerosis related cardiovascular events. In particular, LDL cholesterol has been identified as a causal factor in the development of atherosclerotic cardiovascular disease [11]. GWAS studies have further identified several risk loci associated with both CAD and MI [12,13] in genes known to affect plasma LDL levels. Conditions such as familial hypercholesterolemia have been linked to increased cholesterol levels and increased risk for CAD. This condition is characterised by mutations in LDLR, PCSK9, and APOB. Unsurprisingly, many therapeutics aimed at managing CAD have also been aimed at lowering the levels of LDL cholesterol (e.g., statins).
In this review, we will briefly cover the pathophysiology of CAD, and how genome editing technologies such as CRISPR can assist in the further understanding of and treatment for CAD.
2. Pathophysiology of Atherosclerosis
Atherosclerosis is an inflammatory disease [14,15] characterised by the formation of plaques that result from the accumulation of lipids and inflammation of the arteries. It can clinically manifest with either acute or chronic presentations in a variety of ways, depending on the vascular territory involved, and have both acute and chronic presentations [16]. For example, plaques that form in the coronary arteries result in CAD, while those that form in the legs and/or arms result in peripheral artery disease (PAD), and those that form in the carotid and cerebral arteries can present as ischemic stroke [17]. Low-density lipoprotein (LDL), in particular, has been implicated as having a causal effect on the initiation and progression of atherosclerosis [11], though it is still unclear how exactly excessive LDL cholesterol results in atherosclerosis. The accumulation of LDL and other lipoproteins in the intima of arteries activates the endothelium and, in turn, triggers a series of events that lead to inflammation and eventual uptake of modified LDL cholesterol by macrophages, forming what is known as “foam cells”. Over time, these plaques continue to grow through the accumulation of lipids and foam cells derived from both macrophages and smooth muscle cells (SMCs) [18,19]. Impaired efferocytosis due to plaque development eventually leads to an accumulation of apoptotic cells in the plaque, driving the formation of a necrotic core [20]. This, in turn, results in the formation of a fibrous cap and calcification.
Eventually, “vulnerable” plaques rupture, exposing the necrotic core to the circulation. This, in turn, results in the activation of tissue factor [21], which subsequently triggers a cascade of events such as the recruitment of platelets and inflammatory cells, eventually forming a thrombus. Occlusion of a vessel by a thrombus eventually leads to ischemia (low oxygen) and ultimately infarct (tissue death). If these occur in the coronary arteries, they could result in clinical manifestations of myocardial infarction and angina. If a thrombus occludes cerebral arteries, it could result in a stroke [22,23]. On the other hand, a thrombus may dislodge and block a distal vessel elsewhere in a process known as thromboembolism, which could lead to ischemia and potential infarction of neighbouring tissue.
Lipid-lowering drugs have been the primary therapeutic strategy for managing atherosclerosis. Statins, 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoAR) inhibitors, have long been used to lower LDL cholesterol levels, and large meta-analyses of statin trials have shown that more intensive statin therapy was significantly associated with further reductions in major vascular events. More importantly, these trials showed that reductions in LDL cholesterol levels result in reductions in the rates of major vascular events such as strokes and nonfatal myocardial infarctions [24]. Nonstatin lipid-lowering drugs such as Ezetimibe have also been used in combination with statins to further reduce LDL cholesterol levels by 15–20% [25].
On the other hand, the discovery and understanding of the function of proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme in lipid metabolism has led to the development of PCSK9 inhibitors to treat hypercholesterolemia and atherosclerotic cardiovascular disease. PCSK9 regulates cholesterol homeostasis by binding to the LDL receptor (LDLR) and promoting its degradation [26,27]. Loss of function mutations in PCSK9 have been linked to reduced levels of LDL cholesterol and lower incidences of coronary heart disease [28,29,30,31,32]. On the other hand, gain of function mutations such as the PCSK9-rs562556 variant in the STANISLAS cohort [33] have been linked to higher PCSK9 levels and are associated with carotid arterial plaques. PCSK9 inhibitors such as evolocumab [34,35,36] and alirocumab [37] have been evaluated in clinical trials and have shown significant efficacy in reducing LDL cholesterol levels and incidences of cardiovascular events. Additionally, nucleic-acid-based therapies such as inclisiran, a small interfering RNA (siRNA) therapeutic targeting PCSK9 mRNA, have also been shown to be effective in lowering PCSK9 and LDL cholesterol levels in patients with elevated LDL cholesterol levels [38,39].
3. Genome Editing Approaches
In recent years, many gene editing systems have been identified and developed for various purposes. Gene editors not only allow us to understand disease mechanisms and gene function, but also harbour potential for therapeutic applications. However, issues of safety and efficiency must be answered before widespread adaptation. A summary of the following approaches and their pros and cons can be found in Table 1.

Table 1.
Summary of genome editing approaches.
3.1. Zinc-Finger Nucleases
Zinc-finger nucleases (ZFNs) are chimeric restriction endonucleases comprising a DNA binding zinc-finger protein domain and a FokI catalytic domain [40]. The dimerization of the FokI catalytic domain is necessary for its function; thus, two sets of zinc fingers must bind to their target sequences in close enough proximity with the correct orientations [41]. Upon successful binding, DNA cleavage occurs, generating a double-stranded break (DSB) at the target site. DSBs can be repaired by nonhomologous end joining (NHEJ) or by homology-directed repair (HDR) (Figure 1).
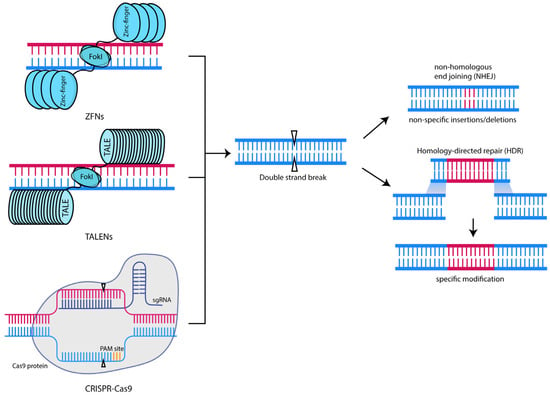
Figure 1.
Genome editing technologies and their mechanism of action. Except for base editors, most of the major genome editing technologies utilised today function by recognising their target site and inducing a double-stranded break. These DSBs can be repaired by either NHEJ or, if a donor DNA template is provided, HDR.
The zinc-finger protein domain contains a tandem array of Cys2-His2 fingers that recognizes approximately 3 bp each. Depending on the target, the number of fingers per zinc-finger protein can range from three to six, corresponding to a recognition site of approximately 9–18 bp. ZFNs have been successfully used to generate knockout cell lines such as a DHFR−/− Chinese hamster ovary (CHO) cell line [42], as well as insert corrective transgenes [43].
Additionally, ZFNs have also been utilised to generate animal models to further the understanding of diseases. For instance, Yan et al. utilised ZFN-mediated gene editing to specifically knockout apolipoprotein C-III (apoCIII) in rabbits to understand its role in atherosclerosis [44]. Similarly, LDLR knockout rats [45,46] have been generated to provide animal models of hypercholesterolemia and atherosclerosis.
3.2. Transcription-Activator-like Effector Nucleases
Similar to ZFNs, transcription-activator-like effector nucleases (TALENs) carry out their genome editing activity by recognition and cleavage of the target sequence via a double-stranded break. These nucleases consist of transcription-activator-like effectors (TALEs), derived from the plant bacteria in the genus Xanthomonas, linked to a FokI catalytic domain. TALEs contain a central repeat domain that allows for DNA recognition, with a repeat unit of 33–35 amino acids specifying one target base [47]. DNA recognition specificity is encoded by the highly variable amino acids at positions 12 and 13, referred to as the “repeat variable di-residue” (RVD) [48]. Much like ZFNs, a pair of TALENs is required for the successful cleavage of the target site, generating a double-stranded break.
The foundations of these TALEN modifications were first laid out in the 2011 study by Miller et al. [49]. Since then, multiple modifications have been made to TALENs to enable greater gene editing efficiency as well as to improve their specificity. Numerous methods have also been developed to enable cost-effective assembly of TALE repeats, allowing for efficient production [50,51,52]. While TALEN-mediated gene editing is often characterised as being less efficient than CRISPR, TALENs have the benefit of inducing less off-target editing [53] as well as a greater editing efficiency in heterochromatin target sites [54]. Notably, a TALEN-based knockout library has been utilized to develop human-induced pluripotent stem cell-based (hIPSC) models of cardiovascular disease. Karakikes et al. designed a collection of TALENs that knocked out 88 human genes associated with cardiovascular diseases by inducing induced double-stranded breaks near their associated start codon [55]. More recently, preclinical data from Cellectis showed successful gene editing of HBB, a gene linked to sickle cell disease, utilising TALENs [56].
3.3. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-Associated (Cas) Systems
CRISPR was first discovered in E. coli and other bacterial species [57]. In bacteria and archaea, the CRISPR/Cas system acts as a defence and regulatory system to protect the host from external insult [58,59,60,61] via the integration of the foreign DNA into the host CRISPR locus. Unlike ZFNs and TALENs which rely on protein–DNA interactions for targeting, Cas nucleases rely on short RNA sequences (termed guides) that recognise the target DNA sequence.
The most commonly used Cas protein derives from Streptococcus pyogenes (SpCas9), a type II CRISPR/Cas system. Unlike type I and type III CRISPR/Cas systems, type II systems rely on a single Cas protein for DNA cleavage [59]. In type II CRISPR systems, the CRISPR locus is transcribed to produce pre-CRISPR RNA (crRNA). Concurrently, trans-activating RNA (tracrRNA), involved in processing of pre-crRNA to crRNA [62], is also transcribed from a region upstream of the CRISPR locus. Seminal work by Jinek et al. found that both tracrRNA and crRNA are required for the cleavage of target DNA. Additionally, they found that it was possible to target the Cas9 endonuclease to specific DNA sequences by engineering guide RNAs (gRNAs) or single guide RNAs (sgRNAs) that contained both the target recognition sequence and the tracrRNA [63].
However, there are limitations in the regions that CRISPR/Cas systems can target for editing. The Cas nuclease first looks for its cognate protospacer adjacent motif (PAM) before assessing guide–target complementarity. Thus, targetable sequences in the genome are limited by the presence of a PAM motif downstream. Fortunately, there are a wide range of PAM motifs recognised by different Cas nucleases, and efforts have been made to characterise and engineer Cas nucleases to expand the range of targetable sequences [64,65,66].
Following successful targeting of the Cas nuclease by gRNA(s), the nuclease creates a DSB that can be repaired by nonhomologous end joining NHEJ or HDR (Figure 1). NHEJ can proceed either by the canonical or alternative pathways, resulting in the formation of insertions and/or deletions (indels) [67,68], leading to a loss of function or “knockout” of the target gene. On the other hand, HDR requires a donor DNA template with sequences homologous to the ends of DSBs. HDR allows for the precise replacement of the target gene, consequently generating a more precise edit. However, HDR has a lower efficiency than NHEJ, and various strategies, such as timed delivery of Cas9 ribonucleoprotein complexes [69] and delivery of a donor gRNA with a target region flanked by two sgRNA homology arms [70], have been explored to increase the rates of HDR.
The creation of DSBs in the Cas9 protein occurs through the HNH nuclease domain and the RuvC-like nuclease domain. The former cleaves the guide-bound target DNA strand, while the latter cleaves the PAM-containing complementary strand. Mutating the RuvC catalytic domain results in its inactivation, resulting in the cleavage of only one strand of DNA by the HNH domain [63]. This variant, termed nickase Cas9 (nCas9), has been used widely in base editing.
CRISPR base editing allows for the generation of site-specific point mutations without production of DSBs [71]. In general, CRISPR base editors consist of an nCas9 or catalytically inactive “dead” Cas protein, also known as dCas9, linked to a deaminase that can convert one target DNA base to another [71]. The Cas nuclease is directed by sgRNA to the target, where the formation of the Cas–sgRNA–DNA complex results in denaturation of the double helix, exposing a region of single-stranded DNA that can be modified by the linked deaminase (e.g., conversion of cytosine to uracil with cytosine base editors) [72,73]. The resulting base mismatch can then be repaired by cellular repair mechanisms (e.g., conversion of uracil to thymine post base editing).
Similarly, CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi), known collectively as CRISPRmod, consist of dCas9 fused to a transcriptional effector. CRISPR interference (CRISPRi) involves fusing dCas9 to transcriptional repressor(s). Early work suggests that CRISPRi works by blocking transcription, being more efficient in bacteria as compared to mammalian cells [74]. Later work further improved the efficiency of CRISPRi in human cells by fusing dCas9 to known repressive chromatin-modifying domains such as the KRAB domain [75]. In addition, activating effectors such as VP16 can also be fused to dCas9, allowing for activation of the target gene [76]. CRISPRmod has mainly been used in human cell lines such as HEK293 [77]; however, more recent work has sought to expand its repertoire to cultured primary cells such as T cells [78] and hematopoietic stem cells [79].
CRISPRi has been utilised to silence Pcsk9 as a proof of concept in vivo. Delivery of a dSaCas9-based repressor (dSaCas9KRAB) utilising a dual AAV8 system into the liver of adult mice resulted in significant decreases in Pcsk9 protein, as well as serum LDL levels, for up to 24 weeks [80].
4. Genome Editing Approaches for Coronary Artery Disease
Genome editing tools have been utilised to both understand and treat the drivers and consequences of CAD. In the following sections, the role of genome editing in these areas will be further elaborated on, and is summarised in Table 2.

Table 2.
Summary of various applications of genome editing technologies in vivo.
4.1. PCSK9
4.1.1. CRISPR-Based Editing of PCSK9
Numerous studies have pointed to the role of the PCSK9 gene in maintaining cholesterol homeostasis. Additionally, loss-of-function PCSK9 variants have been associated with a decrease in risk of coronary heart disease as well as extracoronary atherosclerotic phenotypes [31,116,117]. Consequently, many of the gene editing studies have focused on this gene. PCSK9 is mainly expressed in the liver, an easily targetable site by a variety of gene delivery vehicles such as adenoviral vectors and lipid nanoparticles.
In a 2014 study, Ding et al. explored the utility of CRISPR/Cas9 to disrupt mouse Pcsk9 as a proof of principle. Utilising an adenoviral vector, they delivered the gene encoding SpCas9 and an sgRNA-targeting Pcsk9 to C57BL/6 mice. Within a few days of the NHEJ-mediated Pcsk9 disruption, they observed a ~35–40% decrease in plasma cholesterol levels in addition to a decrease in plasma PSCK9 levels and an increase in LDLR levels [96].
A later study utilised a liver-specific human PCSK9 knock-in mouse model. In this hypercholesteraemic model, the CRISPR/Cas9-mediated disruption of human PCSK9 resulted in reduced plasma levels of human PSCK9, but not mouse, and a concurrent decrease in plasma cholesterol levels. In the same study, the authors utilised third-generation base editor BE3 and sgRNAs to target both mouse and human PCSK9. Similarly, significant reductions in the levels of mouse and human PCSK9 were observed. Interestingly, mice injected with the BE3 constructs exhibited more precise gene disruptions as compared to mice injected with CRISPR/Cas9 constructs, with no off-target editing or chromosomal translocations observed [94]. These observations were in concordance with an earlier study similarly utilising an adenoviral vector to deliver BE3 and an sgRNA-targeting Pcsk9 in a mouse model. Base editing resulted in a median rate of 25% base-edited alleles in the liver, while a low (~1%) of indels were detected. Similarly, the authors observed a greater than 50% reduction in plasma PCSK9 protein levels as well as a ~30% reduction in plasma cholesterol levels [110].
4.1.2. Cas9 Variants
One potential reason for the use of the adenoviral vector is the large size of the gene encoding SpCas9 (~4.2 kb), which prevents the usage of the less immunogenic adeno-associated virus (AAV). Utilising a Cas9 from Staphylococcus aureus (SaCas9), Ran et al. were able to package both the SaCas9 gene and an sgRNA-targeting Pcsk9 into a single AAV8 vector. Similarly, they observed a decrease in serum PCSK9 levels and a ~40% decrease in total cholesterol one week after administration, which was sustained till the end of the study at 4 weeks [100]. Other alternatives to SpCas9 also include Neisseria meningitidis Cas9 (NmeCas9). In a proof-of-concept study, Ibraheim et al. delivered NmeCas9 with an sgRNA-targeting Pcsk9 in a single AAV vector, resulting in significantly decreased cholesterol levels 50 days after systematic administration of the vector. While a humoral response to NmeCas9 was elicited, the authors did not report signs of liver damage [98]. Furthermore, they observed that this Cas9 variant had minimal off-target edits, similar to their previous observations [118]. These SpCas9 alternatives not only expand the potential sequences that can be targeted with CRISPR, but also broaden the choice of vectors available for therapeutic purposes.
In addition to utilising CRISPR/Cas9 systems to target PCSK9, an engineered meganuclease delivered by an AAV8 vector has been used as well. This resulted in up to 60% reduction in serum PCSK9 levels and up to 84% reduction in serum LDL cholesterol levels with a first-generation engineered meganuclease. However, multiple off-target cleavages were detected with this first-generation meganuclease. A second-generation meganuclease was also generated in an attempt to reduce off-target editing [111]. A 3-year follow-up showed that on-target editing of liver PCSK9 was sustained with a stable reduction in serum PCSK9 and LDL cholesterol levels, highlighting the potential of this method of gene editing. However, concerns remain over AAV integration at the cut sites as well as the potential immunotoxicity of this approach [111,112]. More recently, reductions in off-target activity of the meganuclease were observed after delivery with a modified self-targeting vector and a shorter promoter [119].
4.1.3. Nonviral Delivery Methods
CRISPR/Cas9 systems have also been delivered by nonviral vectors such as virus-like particles (VLPs) as well as lipid nanoparticles (LNPs). Banskota et al. utilised a VLP system to deliver an adenine base editor 8e (ABE8e) ribonucleoprotein (RNP) that can target and disrupt the Pcsk9 gene in mice. Systematic administration of the VLP resulted in 63% editing in bulk liver, which was comparable to editing efficiencies obtained by CRISPR delivered by other delivery systems such as AAVs and LNPs, as well as a 78% decrease in serum Pcsk9 protein levels 1 week post injection. Furthermore, they observed that base editing was highly specific to the liver, and minimal off-target editing was observed above background in potential off-target loci identified by CIRCLE-seq, an in vitro screening method for identifying genome-wide off-target mutations of CRISPR/Cas9 [102]. While this approach is relatively new, it appears to hold great promise as a delivery platform.
LNPs have also been used as an alternative delivery platform. Rothgangl et al. utilised LNPs encapsulating an ABEmax mRNA and an sgRNA-targeting Pcsk9 to disrupt the canonical Pcsk9 splice site. They induced ~61% base editing of the Pcsk9 gene in mice, resulting in a stable reduction of 95% plasma PCSK9 and 58% LDL cholesterol levels. Additionally, administration of this LNP construct in macaques resulted in ~26% base editing coupled with a 32% decrease in serum PCSK9 and a 14% reduction in LDL cholesterol levels. A subset of macaques was given a second dose 2 weeks later; however, no increase in editing rates was observed. Additionally, they detected antibodies against SpCas9 in this subgroup, indicating that there was a possibility of an ABE-specific T cell response. In general, off-target delivery of the LNP complex remained low, though the authors observed editing rates of 6–12% in the spleen. However, the study endpoint of 29 days was too short to fully understand the long-term safety of this approach [108].
In a separate study involving cynomolgus monkeys, Musunuru et al. similarly delivered a CRISPR adenine base editor with LNPs to introduce a single-nucleotide loss-of-function mutation in PCSK9. Unlike the Rothgangl et al. study, Musunuru et al. employed the ABE8.8 m base editor, which has increased editing efficiency as compared to older-generation ABE base editors [120]. Initial studies in mice showed that 1 week post intravenous administration of LNPs, there was ~70% base editing in the liver. Similarly, in cynomolgus monkeys, the liver remains the main editing site, though some PCSK9 editing in the spleen remains. However, minimal off-target editing was detected in both monkey liver samples and primary cynomolgus monkey hepatocytes. In a longer-term study of up to 8 months, base editing frequencies were ~66%, with a stable reduction of 90% of serum PCKS9. Similarly, persistent reductions of LDL cholesterol (60%) and lipoprotein(a) (35%) were observed. Similar to the Rothgangl et al. study, moderate increases in blood transaminases attributed to LNP treatment were observed that resolved within two weeks [101].
Taken together, these studies highlight the various methods that different groups have taken to safely deliver and knock down PCSK9. LNPs appear to be a viable method of delivering genome editing technologies such as base editors to the liver, and, indeed, VERVE-101, a single-dose CRISPR base editing therapeutic targeting PCSK9 has recently begun phase 1 b study in patients with heterozygous familial hypercholesterolemia and cardiovascular disease (NCT05398029). Early studies in nonhuman primates have shown good tolerance to the LNP-delivered therapeutic, with sustained reductions in blood PCSK9 and LDL cholesterol up to 476 days after dosing [106]. If similar successes can be observed in human trials, it has the potential to be a convenient one-dose therapeutic as compared to current monoclonal antibody-based treatments, which require more frequent dosing.
4.2. ANGPTL3
Angiopoietin-like 3 (ANGPTL3) is a protein primarily expressed in the liver that regulates lipoprotein levels in blood. It functions by inhibiting lipoprotein lipase, a key enzyme that is involved in the intravascular lipolysis of certain triglycerides. In homozygous, for a loss-of-function mutation in Angptl3 resulting in the production of a truncated Angptl3, hypolipidemia was observed, associated with an increase in lipoprotein lipase activity and increased lipolysis of VLDL triglycerides [121]. Loss-of-function mutations in ANGPTL3 have also been linked to lower blood triglycerides and LDL cholesterol levels, as well as a lower risk of CAD in human cohorts [122].
In line with this, Chadwick et al. utilised an adenoviral vector to deliver a cytosine-to-thymine base editor (BE3) with a guide RNA-targeting Angptl3 to 5-week-old C57BL/6J mice aimed at generating nonsense mutations at codon Gln-135. Deep sequencing performed at the day 7 time point showed that the mice injected with the BE3-Angptl3 construct had a median base editing rate of 35% with no evidence of off-target editing. Administration of this construct into a hyperlipidaemic Ldlr-knockout mice model that phenocopies patients with homozygous familial hypercholesterolemia resulted in a substantial (>50%) reduction in triglycerides and cholesterol in comparison with the BE3 control [109]. Considering that the 35% base editing rate already results in substantial reductions in cholesterol levels, it is likely that improvements in base editing efficiency can further improve the efficacy of this targeting method.
A recent study by Zuo et al. utilised a dual AAV construct to specifically deliver AncBE4max, a cytosine base editor, targeting mouse Angptl3, to generate a premature stop codon (Q135X) in the coding sequence of the gene. In this study, a higher base editing efficiency of ~63% was observed in the liver, with a near complete knockout of ANGPTL3 protein expression 2–4 weeks post AAV administration. Similarly, Angptl3 base editing also resulted in >50% reductions in triglyceride and total cholesterol levels in serum [103].
More recently, Verve Therapeutics published preclinical data on the administration of an LNP-based adenine base editor targeting ANGPTL3 aimed at disrupting the gene (VERVE-201) via generation of a premature stop codon. In a nonhuman primate (NHP) model of homozygous familial hypercholesterolemia and in wildtype monkeys, administration of the NHP homologue (VERVE-201cyno) resulted in reductions of ~88% in circulating ANGPTL3 90 days post administration [123]. Later publications showed that administering the murine surrogate (VERVE-201mu) in an Ldlr KO model of homozygous familial hypercholesterolemia led to a mean 47% decrease in LDL cholesterol after administration of VERVE-201, and an accompanying decrease in triglyceride concentrations in blood. Treatment of NHPs with VERVE-201cyno was also observed to induce mean liver ANGPTL3 editing of up to 63%, with a 95% reduction in ANGPTL3 protein levels 7 days after receiving the higher dose of 3.0 mg/kg. More importantly, NHPs treated with the LNP construct did not exhibit any signs of liver damage after administration [124]. These results suggest that ANGPTL3 base editing could be potentially safe and efficacious in future human trials.
In a separate study, a novel LNP was used to deliver Cas9 mRNA and an sgRNA-targeting Angptl3 for knockdown of liver Angptl3. The 306-O12B LNP construct was delivered specifically to the liver, with a median editing rate of 38.5% and a 65.2% reduction in ANGPTL3 in serum. This delivery method did not result in detectable off-target editing or significant changes to blood transaminases (ALT and AST) levels. Specifically, the authors compared their LNP construct to the current FDA-approved construct of MC-3. Seven days post administration, 306-O12B LNP-mediated delivery of the Cas9 components resulted in higher editing rates as compared to MC-3-mediated delivery of the same, with serum ANGPTL3, LDL cholesterol, and triglyceride levels being lower in the 306-O12B LNP-treated mice. Taken together, results of this study support a compelling argument for more detailed preclinical studies utilising this unique LNP construct [81].
4.3. APOC3 and Other Lipid-Related Genes
4.3.1. APOC3
Apolipoprotein C3 (APOC3) is another potential therapeutic target for coronary heart disease treatment. ApoC-III is an apolipoprotein that is synthesised mainly in the liver, and it has roles in inhibiting lipoprotein lipase-mediated lipolysis of triglyceride-rich lipoproteins [125]. Elevations in circulating ApoC-III levels have been correlated with increased triglyceride levels and an increase in risk for myocardial infarctions and CAD [126]. Loss-of-function mutations in APOC3 have similarly been associated with lower levels of plasma triglycerides and a lower risk of CADs [127]. In a clinical trial evaluating an antisense oligonucleotide (ASO) targeted at APOC3 mRNA, patients administered this ASO exhibited reduced apoC-III and triglycerides [128].
CRISPR/Cas9 systems have been used to generate APOC3 knockout rabbits [83], as well as hamsters [84]. In both animal models, ApoC3 knockout results in the animals exhibiting fewer atherosclerotic lesions as well as a less atherogenic lipid profile on a high-fat diet. Similarly, ZFNs have been used to generate apoCIII knockout rabbits. Under a high-cholesterol diet, these rabbits similarly exhibited reduced atherosclerosis as well as lower total cholesterol levels compared to wild-type rabbits [44]. Recent work from Xu et al. on ApoC3 deficiency in LDLR-deficient hamsters suggests that loss of ApoC3 may paradoxically accelerate the atherosclerotic phenotype observed in LDLR-deficient hamsters on a high-cholesterol diet [129]. Further investigation on the effects and benefits of APOC3 inhibition in different animal models of atherosclerosis need to be carried out.
4.3.2. ApoE
Apolipoprotein (apo) E is an important protein in the suppression of atherosclerosis expressed not only in the liver but also in the brain and adrenal gland [130]. It is a key regulator of lipid levels in the body by way of mediating the clearance of lipoproteins from the circulation [131]. It is thus unsurprising that ApoE knockout mice have often been used in the study of atherosclerosis. Gene editing technologies have been applied to generate such animal models in recent years. For instance, TALEN-mediated gene editing was used to knockout ApoE in rats, resulting in a phenotype of dyslipidaemia after a high-cholesterol diet, which was not observed in wild-type rats. Furthermore, partial ligation of the carotid artery resulted in formation in atherosclerotic plaques in knockout mice after a high-cholesterol diet [113]. Similar observations were detected in Bama miniature pigs after CRISPR/Cas9-mediated knockout of ApoE. Pigs with frameshift mutations developed severe hypercholesterolemia and atherosclerotic lesions after 6 months of a high-fat, high-cholesterol diet, recapitulating a human-like phenotype [85]. Additionally, double knockouts of both ApoE and LDLR were also explored in Bama minipigs [86] and rabbits [91] to generate animal models of atherosclerosis.
4.3.3. LDLR
The low-density lipoprotein receptor (LDLR) is a key regulator of cholesterol homeostasis responsible for the internalisation of cholesterol-laden lipoprotein particles [132]. Loss-of-function mutations in LDLR have been linked to increased LDL cholesterol levels in serum and atherosclerosis. Utilising CRISPR/Cas9 gene editing, LDLR-KO New Zealand white rabbits were generated to study the spontaneous development of hypercholesterolemia and atherosclerosis [90], which may be a useful model for human familial hypercholesterolemia.
While most animal models have been generated via CRISPR/Cas9-mediated germline genome editing, somatic gene editing can also be used to generate models of diseases. In 2017, Jarrett et al. utilised AAVs to deliver gRNAs, targeting Ldlr and Apob. Singular delivery of the gRNAs resulted in efficient reductions of >50% in mRNA expressions of both Ldlr and Apob in a Cas9-expressing transgenic mouse model. Expectedly, Ldlr disruption resulted in hypercholesterolemia and atherosclerosis. However, the concomitant disruption of Apob resulted in a lowering of blood cholesterol and atherosclerosis protection [82]. A later work similarly utilised AAVs to disrupt Ldlr, though instead of SpCas9, Jarrett et al. utilised SaCas9, a smaller Cas9 ortholog. SaCas9 and a guide RNA that targets Ldlr (AAV-CRISPR) were packaged into AAVs and intraperitoneally injected into wild-type C57BL/6J mice. Mice placed on a “standard Western diet” for 20 weeks were noted to exhibit severe hypercholesterolemia and develop atherosclerotic lesions. While mice injected with the AAV-CRISPR constructs had no detectable off-target edits, insertions of the AAV genome were detected at on-target cut sites, similar to the 2017 study [99]. While this may not be a concern for the purpose of model development, more attention must be placed on it when developing AAV-based genome editing therapeutics for gene therapy in humans.
Similarly, Zhao et al. utilised a dual AAV system to deliver SpCas9 (AAV-Cas9) and an sgRNA-Donor construct to neonatal mice harbouring a LdlrE208X mutation. The E208X mutation in mice is equivalent to the E207X mutation in human LDLR, a nonsense point mutation found in a patient with familial hypercholesterolemia [133]. These mutant mice lack a functional LDLR protein; thus, after 12 weeks of high-fat diet feeding, mutant mice had atherosclerotic lesions present in the aorta, unlike WT mice. These mice also exhibited higher total plasma cholesterol, recapitulating many of the features of familial hypercholesterolemia in humans. Post AAV-CRISPR/Cas9 treatment, these mice exhibited partial restoration of LDLR levels (~18%). Additionally, indels were detected in ~24% of the Ldlr alleles, and the HDR-mediated correction was observed in 6.7% of the LDLR alleles. Mice treated with the AAV-CRISPR/Cas9 system exhibited lower plasma cholesterol, triglycerides, and LDL-C, and a less severe atherosclerotic phenotype, suggesting that this could be a potential therapeutic approach for treatment of familial hypercholesterolemia [89].
While classical studies using Ldlr-KO mice cannot be replaced, somatic gene editing can allow for the simultaneous targeting of multiple genes, as well as avoid the issues of embryonically lethal knockouts. One can imagine that with this method, we can better understand the interactions of different genes on a disease background.
4.4. Genome Editing in the Understanding of the Complications Arising from CAD
Genome editing can also assist in the understanding the complications arising from CAD, such as myocardial infarction. Post MI, various complications can arise, such as ischemia-perfusion injury. Understanding the mechanisms and cellular consequences of myocardial infarction could provide new insights into potential strategies to minimise the damaging effects of the disease progression. In the following sections, we will briefly cover some of the potential genome editing targets that may ameliorate the impact of CADs (Figure 2).
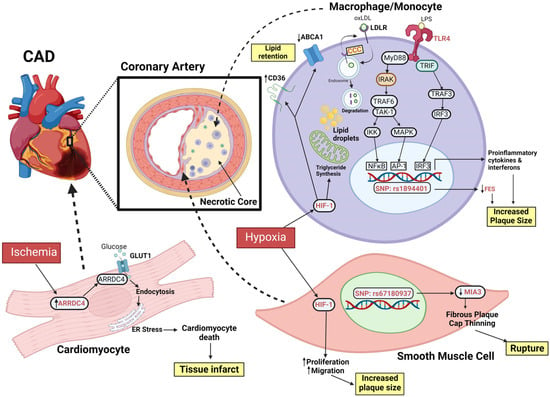
Figure 2.
Schematic of key molecular targets for genome editing in the complications arising from CAD. In summary, the three groups of cells involved are monocytes/macrophages, smooth muscle cells, and cardiomyocytes. In macrophages, the CCC complex plays an important role in the recycling of surface proteins such as LDLR. TLR4 plays an important role in the proinflammatory response. Its activation results in expression of key transcription factors that promotes the expression of proinflammatory cytokines and interferons that promote plaque progression. In the presence of hypoxia, HIF-1 enhances triglyceride synthesis and lipid droplet formation in foam cells and promotes lipid retention. FES has been shown to have a protective role against atherosclerosis, and the rs1894401 and rs1751486 SNPs (latter not shown) located at the same locus both decreased FES expression. In the ischemic heart, the alpha arrestin ARRDC4 is upregulated and plays an important role in interacting with GLUT1 and causing ER stress, and subsequently promoting cardiomyocyte death. Abbreviations: ABCA1, ATP Binding Cassette Subfamily A Member 1; AP-1, activator protein 1; ARRDC4, arrestin domain containing 4; CAD, coronary artery disease; CCC, COMMD-CCDC-CCDC93 complex; ER, endoplasmic reticulum; FES, FES proto-oncogene; HIF-1, hypoxia-inducible factor-1; GLUT1, glucose transporter 1; IKK, IκB kinase; IRAK, interleukin-1 receptor associated kinase; IRF3, interferon regulatory factor 3; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MIA3, MIA SH3 domain ER export factor 3; NFκB, nuclear factor kappa light chain enhancer of activated B cells; oxLDL, oxidated low-density lipoprotein; SNP, single nucleotide polymorphism; TAK-1, transforming growth factor-activated kinase 1; TLR4, toll-like receptor 4; TRAF3/6, TNF receptor associated factor 3/6; TRIF, TIR-domain-containing adapter protein inducing interferon beta; created with BioRender.com.
4.4.1. CCC Complex
The COMMD/CCDC22/CCDC93 (CCC) complex is a large protein complex comprising CCDC22, CCDC93, and the COMMD proteins. In macrophages, the CCC complex has been found to be responsible for the recycling of surface proteins and has been found to associate closely with a cargo recognition complex called retriever [134]. The CCC complex is crucial for the function and localisation of the low-density lipoprotein receptor (LDLR). Ablation in Commd1 expression results in increases in plasma LDL cholesterol levels as well as a mislocalisation of LDLR. [135]. CRISPR/Cas9-mediated deletion of Ccdc22 in mouse livers resulted in decreased expression of all COMMD proteins except COMMD6, as well as elevation in plasma LDL cholesterol levels. Taken together, these indicate the importance of the CCC complex in the regulation of plasma cholesterol levels in a mouse model [87].
4.4.2. HIF1
One of the major and early responses to myocardial infarctions by the body is an increase in the hypoxia-inducible factor 1 (HIF-1) transcriptional factor [136], where close to 200 genes are transcriptionally activated [137]. In the presence of hypoxia, HIF-1 enhances triglyceride synthesis and lipid droplet formation in foam cells. Additionally, it promotes lipid retention by upregulation of the expression of scavenger receptors such as CD36 and LOX1, and downregulation of the lipid exporter ABCA1. Additionally, HIF-1 can promote the proliferation and migration of smooth muscle cells, leading to plaque progression. Geng et al. discovered that UCHL1 is upregulated in the infarct and border zones of hearts following myocardial infarction. Utilising a birA-based proximity labelling system, HIF-1a, part of the HIF-1 complex, was discovered to interact with UCHL1. To investigate the function of UCHL1 in the regulation of HIF-1a, CRISPR/Cas9 genome editing was used to establish a UCHL−/− hiPSC line. After hypoxia treatment, UCHL1−/− hiPSC-cardiomyocytes exhibited lower levels of HIF-1a and its target genes as well as mislocalisation of HIF-1a to the cytosol. Furthermore, KO of UCHL1 resulted in enhanced ubiquitination of HIF-1a. Taken together, these suggest that the localisation and stability of HIF-1a is linked to UCHL1 [138].
4.4.3. Alpha-Arrestins
Alpha-arrestins have been known to respond to glucose availability in yeast [139], and recently, human homologs have been studied to better understand their function. ARRDC4 is a mammalian alpha-arrestin whose role in vivo has yet to be clearly understood. Utilising CRISPR/Cas9, Nakayama et al. generated a novel Arrdc4-KO mouse model aimed at better understanding the role of ARRDC4 in vivo. Ultimately, they discovered a new mechanism in which ARRDC4 plays a role in glucose deprivation and endoplasmic reticulum stress during ischemia, which may provide a potential therapeutic target for the ischemic heart [140].
4.4.4. Atherosclerosis and Monocyte Activation
In a 2022 study, Karamanavi et al. utilised CRISPR genome editing to study the impact of the rs17514846 and rs1894401 SNPs on FES (FES proto-oncogene, tyrosine kinase) expression in monocytes. FES is postulated to have a protective role against atherosclerosis. The two SNPs of interest are located near FURIN and FES and have been shown to have an expression quantitative trait loci effect on FES expression in atherogenic cell types of monocytes and vascular smooth muscle cells. THP-1 monocytes were edited with CRISPR to generate isogenic cells that only differ in either of the two SNPs. Cells containing the CAD risk genotype of rs17514846 A/A exhibited lower FES levels than cells containing the C/C genotype, while cells with the rs1894401 G/G genotype, which has been found to be in high linkage disequilibrium with rs17514846 A/A genotype, similarly exhibited lower FES levels. These suggest that both SNPs have a role in modulating FES expression in monocytes [141]. However, it is still unclear if base editing of the rs17514846 and rs1894401 SNPs to a nonrisk genotype has a beneficial effect on FES activity and, by extension, atherosclerosis.
4.4.5. MIA3
GWAS studies have recently identified rs67180937 at the 1q41 locus as an SNP associated with lower VSMC proliferation and MIA SH3 Domain ER Export Factor 3 (MIA3) expression. MIA3, also known as TANGO1, is a ubiquitously expressed protein that is involved in the export of collagen from the endoplasmic reticulum as well as angiogenesis and leukocyte migration, a phenomenon that has been linked to atherosclerosis [142]. While preliminary data have suggested that lower MIA3 may be linked to the formation of a thin fibrous plaque cap resulting from lower VSMC proliferation, it is still not understood what role MIA3 plays [143]. It may be possible to utilise base editing on a mouse model of atherosclerosis to insert the rs67180937 SNP and examine its impact on the atherosclerotic phenotype.
4.4.6. CaMKIIδ
Calcium calmodulin-dependent protein kinase IIδ (CaMKIIδ) is multifunctional Ser/Thr kinase, a member of the CaMKII family. CaMKIIδ has been found to be the predominant member of the CaMKII family expressed in the heart, and has been linked to mediating various signalling processes in cardiomyocytes [144]. Chronic overactivation has been linked to various cardiac diseases such as ischemia/reperfusion injury and heart failure. In particular, two methionine residues on the regulatory domain of CaMKIIδ have been identified to promote hyperactivation of the kinase. In a 2023 study by Lebek et al., the Abe8e base editor fused to a Cas9 variant, SpRY, was used to ablate the oxidation of the methionine residues. Delivery of the AAV9 constructs expressing the CRISPR/Cas9 gene editor immediately post cardiac ischemia/reperfusion injury resulted in improvements in cardiac function [104]. Results from this study suggest that CaMKIIδ gene editing can indeed be a viable therapeutic approach for heart disease.
4.4.7. TLR4
Interestingly, CRISPR/Cas9 editing has also been used to disrupt toll-like receptor 4 (TLR4) to improve the outcome of cell therapy for CVDs. TLR4 is a regulator of inflammation and is expressed in many cell types, including cardiomyocytes. Activation of TLR4 leads to the activation of several key transcription factors such as NFκB, AP-1, and IRF3, which promote the expression of proinflammatory cytokines and interferons that promote plaque progression. Cardiac ischemia/reperfusion often results in cellular damage, which in turns results in the activation of TLR4 and proinflammatory responses [145]. Human mesenchymal stromal cells (hMSCs) have been cited as a viable form of cardiac cell therapy; however, it has been hypothesised that the environment of the diseased heart could drive these hMSCs to a proinflammatory phenotype mediated by TLR4. Schary et al. utilised CRISPR/Cas9 to edit hMSCs to lower TLR4 expression. Mice administered with the edited hMSCs post MI exhibited improved survival, infarct healing, and remodelling [146]. While results of the study are promising, concerns regarding immune responses to hMSC implantation at the site of injury remain. Furthermore, more work must be carried out in generating a more heterogenous population of edited and unedited cells; however, this approach appears to be promising for translation into human therapy.
Taken together, these studies show a role of genome editing technologies in not only understanding the mechanisms driving the deleterious effects of MI, but also highlighting potential ways we can target them therapeutically. However, more work in larger animal models must be carried out to assess the safety and efficacy of these therapeutic approaches before translation to human therapy is possible.
5. Potential Challenges Facing Genome-Editing-Based Therapies
While genome editing is indeed a promising therapeutic approach, there are still concerns regarding its potential side effects. Undesirable side effects of ZFNs include insertional mutagenesis, toxicity, and low efficacy [147]. Off-target effects are lower in CRISPR/Cas9 technology compared to ZFNs, though prevalence of unpredictable off-target effects, which include off-target mutations, can still be high (up to >50%). CRISPR-induced DSBs can cause apoptosis, leading to DNA damage and cellular toxicity. Off-target effects are far rarer in TALEN-mediated systems, and their high degree of specificity and low cytotoxicity have been shown in diverse cell types, though it comes at the cost of them being more expensive and labour-intensive than their fellow genome editing counterpart, CRISPR/Cas9 [148]. Often, off-target effects for the CRISPR/Cas9 system can results from a mismatch between the guide RNA and double-stranded DNA [149,150]. As alluded to in the preceding sections, off-target editing remains a concern. Many bioinformatic tools have been developed to design gRNAs as well as to detect potential off-targets (e.g., CRISPRseek [151], COSMID [152]) to overcome this challenge. Other strategies to reduce off-target modification by CRISPR/Cas9 include the use of Cas9 nickase, which breaks down only one strand of the DNA and thereby reduces further damage of the target DNA, and anti-CRISPR proteins (ie. Acr), which deactivate the Cas9 protein after targeting its site [153]. The risk of off-target effects of CRISPR/dCas9 is much lower than Cas9, and tools such as CRISPRa and CRISPRi that use dCas9 are reversible, which reduces the number of unknown problems associated with off-target effects [150]. Additionally, depending on the choice of delivery vector, there are concerns surrounding potential integration of vector DNA into the genome. Other potential issues that may arise from such therapies are their persistence in the tissue of interest. While work in animal models has shown that effects of genome editing can persist for up to 476 days after dosing [106], it is unclear if similar observations will be made in humans.
6. Conclusions
Genome editing technologies have indeed been greatly utilised over the years not only in the development of disease models, both in animals and cells, but also in the treatment of disease. Various methods ranging from nonviral lipid nanoparticles to adenoviral vectors have been explored to deliver these gene editors to animal models. However, questions of immunogenicity and efficacy of gene editing remain. Currently, lipid nanoparticles appear to be one of the safer means of delivering gene editors in vivo with minimal risk of unwanted integration into the genome. Additionally, gene editing of PCKS9 and ANGPTL3 appears to be a promising avenue of therapy, with two LNP-delivered therapeutics already in preclinical study. It will be exciting to see if this approach will prove to be efficacious in human trials.
Author Contributions
Conceptualization, M.C.E.M. and R.G.; investigation, M.C.E.M.; writing—original draft preparation, M.C.E.M. and R.G.; writing—review and editing, M.C.E.M., R.G. and R.S.Y.F.; visualization, M.C.E.M. and R.G.; supervision, R.G. and R.S.Y.F.; project administration, R.G. and R.S.Y.F.; funding acquisition, R.S.Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAV | Adeno-associated virus |
| ABE | Adenine base editor |
| ALT | Alanine transaminase |
| ANGPTL4 | Angiopoietin-like 4 |
| apoAII | Apolipoprotein A-II |
| APOB | Apolipoprotein B |
| Apoc3/apoC-III | Apolipoprotein C-III |
| ApoE | Apolipoprotein E |
| ARRDC4 | Arrestin domain containing 4 |
| AST | Aspartate aminotransferase |
| BE3 | Base editor 3 |
| CAD | Coronary artery disease |
| CaMKIIδ | Calcium/calmodulin-dependent protein kinase IIδ |
| CCDC | Coiled-coil domain containing protein |
| CHO | Chinese hamster ovary |
| CIRCLE-seq | Circularization for in vitro reporting of cleavage effects by sequencing |
| COMMD | Copper metabolism gene MURR1 |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CRISPRa | CRISPR activation |
| CRISPRi | CRISPR interference |
| CRISPRmod | CRISPR modulation |
| crRNA | CRISPR RNA |
| CVD | Cardiovascular disease |
| dCas9 | Catalytically dead Cas9 |
| DNA | Deoxyribonucleic acid |
| dSaCas9 | Staphylococcus aureus Cas9 |
| DSB | Double-stranded break |
| E. coli | Escherichia coli |
| FES | FES proto-oncogene |
| gRNA | Guide RNA |
| GWAS | Genome-wide association studies |
| HBB | Haemoglobin subunit beta |
| HDR | Homology directed repair |
| HEK293 | Human embryonic kidney 293 |
| HF | Heart failure |
| HIF-1 | Hypoxia-inducible factor-1 |
| hIPSC | Human induced pluripotent stem cell |
| HMG-CoAR | 3-hydroxy-3-methylglutaryl coenzyme-A reductase |
| hMSCs | Human mesenchymal stromal cells |
| KO | Knockout |
| KRAB | Krüppel associated box domain |
| LCAT | Lecithin-cholesterol acylltransferase |
| LDL | low-density lipoprotein |
| LDLR | LDL receptor |
| LNP | Lipid nanoparticles |
| MC-3 | D-Lin-MC3-DMA |
| MI | Myocardial infarction |
| MIA3 | MIA SH3 domain ER export factor 3 |
| miR | microRNA |
| nCas9 | Nickase cas9 |
| NHEJ | Nonhomologous end joining |
| NHP | Nonhuman primate |
| NmeCas9 | Neisseria meningitidis Cas9 |
| PAM | Protospacer adjacent motif |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| RNP | Ribonucleoprotein |
| RVD | Repeat variable di-residue |
| SCNT | Somatic Cell Nuclear Transfer |
| sgRNA | Single guide RNA |
| siRNA | Small interfering RNA |
| SMC | Smooth muscle cell |
| SNP | Single nucleotide polymorphism |
| spCas9 | Streptococcus pyogenes |
| TALEN | Transcription activator-like effector nuclease |
| TLR4 | Toll-like receptor 4 |
| tracrRNA | Trans-activating CRISPR RNA |
| UCHL1 | Ubiquitin C-terminal hydrolase L1 |
| VLP | Virus-like particles |
| WT | Wild type |
| ZFN | Zinc finger nuclease |
References
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 18 July 2023).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and Aetiology of Heart Failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef]
- Vedin, O.; Lam, C.S.P.; Koh, A.S.; Benson, L.; Teng, T.H.K.; Tay, W.T.; Braun, O.Ö.; Savarese, G.; Dahlström, U.; Lund, L.H. Significance of Ischemic Heart Disease in Patients with Heart Failure and Preserved, Midrange, and Reduced Ejection Fraction: A Nationwide Cohort Study. Circ. Heart Fail. 2017, 10, e003875. [Google Scholar] [CrossRef] [PubMed]
- Grech, E.D. Pathophysiology and Investigation of Coronary Artery Disease. BMJ 2003, 326, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Suwaidi, S.K.B.M.A.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Darmaki, R.S.A.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury Following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, e7018393. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Tybjaerg-Hansen, A. Genetics of Coronary Artery Disease. Circ. Res. 2016, 118, 564–578. [Google Scholar] [CrossRef]
- Khera, A.V.; Kathiresan, S. Genetics of Coronary Artery Disease: Discovery, Biology and Clinical Translation. Nat. Rev. Genet. 2017, 18, 331–344. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Schunkert, H.; König, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.R.; Barbalic, M.; Gieger, C.; et al. Large-Scale Association Analysis Identifies 13 New Susceptibility Loci for Coronary Artery Disease. Nat. Genet. 2011, 43, 333–338. [Google Scholar] [CrossRef]
- Erdmann, J.; Kessler, T.; Munoz Venegas, L.; Schunkert, H. A Decade of Genome-Wide Association Studies for Coronary Artery Disease: The Challenges Ahead. Cardiovasc. Res. 2018, 114, 1241–1257. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Zemaitis, M.R.; Boll, J.M.; Dreyer, M.A. Peripheral Arterial Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Basatemur, G.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Grootaert, M.O.J.; Bennett, M.R. Vascular Smooth Muscle Cells in Atherosclerosis: Time for a Re-Assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef]
- Yurdagul, A.; Doran, A.C.; Cai, B.; Fredman, G.; Tabas, I.A. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front. Cardiovasc. Med. 2018, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Moons, A.H.M.; Levi, M.; Peters, R.J.G. Tissue Factor and Coronary Artery Disease. Cardiovasc. Res. 2002, 53, 313–325. [Google Scholar] [CrossRef]
- Bacigaluppi, M.; Semerano, A.; Gullotta, G.S.; Strambo, D. Insights from Thrombi Retrieved in Stroke Due to Large Vessel Occlusion. J. Cereb. Blood Flow. Metab. 2019, 39, 1433–1451. [Google Scholar] [CrossRef]
- Patil, S.; Darcourt, J.; Messina, P.; Bozsak, F.; Cognard, C.; Doyle, K. Characterising Acute Ischaemic Stroke Thrombi: Insights from Histology, Imaging and Emerging Impedance-Based Technologies. Stroke Vasc. Neurol. 2022, 7, 001038. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170,000 Participants in 26 Randomised Trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Peng, Z.; Gu, H.; Wang, M.; Wang, G.; Zhang, D. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 764038. [Google Scholar] [CrossRef]
- Hummelgaard, S.; Vilstrup, J.P.; Gustafsen, C.; Glerup, S.; Weyer, K. Targeting PCSK9 to Tackle Cardiovascular Disease. Pharmacol. Ther. 2023, 249, 108480. [Google Scholar] [CrossRef]
- Langsted, A.; Nordestgaard, B.G.; Benn, M.; Tybjærg-Hansen, A.; Kamstrup, P.R. PCSK9 R46L Loss-of-Function Mutation Reduces Lipoprotein(a), LDL Cholesterol, and Risk of Aortic Valve Stenosis. J. Clin. Endocrinol. Metab. 2016, 101, 3281–3287. [Google Scholar] [CrossRef]
- Fasano, T.; Cefalù, A.B.; Di Leo, E.; Noto, D.; Pollaccia, D.; Bocchi, L.; Valenti, V.; Bonardi, R.; Guardamagna, O.; Averna, M.; et al. A Novel Loss of Function Mutation of PCSK9 Gene in White Subjects with Low-Plasma Low-Density Lipoprotein Cholesterol. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 677–681. [Google Scholar] [CrossRef]
- Kent, S.T.; Rosenson, R.S.; Avery, C.L.; Chen, Y.-D.I.; Correa, A.; Cummings, S.R.; Cupples, L.A.; Cushman, M.; Evans, D.S.; Gudnason, V.; et al. PCSK9 Loss-of-Function Variants, Low-Density Lipoprotein Cholesterol, and Risk of Coronary Heart Disease and Stroke. Circ. Cardiovasc. Genet. 2017, 10, e001632. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.; Hobbs, H.H. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Cohen, J.; Pertsemlidis, A.; Kotowski, I.K.; Graham, R.; Garcia, C.K.; Hobbs, H.H. Low LDL Cholesterol in Individuals of African Descent Resulting from Frequent Nonsense Mutations in PCSK9. Nat. Genet. 2005, 37, 161–165. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Xhaard, C.; Lamiral, Z.; Borges-Canha, M.; Neves, J.S.; Dandine-Roulland, C.; LeFloch, E.; Deleuze, J.; Bacq-Daian, D.; Bozec, E.; et al. PCSK9 Protein and Rs562556 Polymorphism Are Associated with Arterial Plaques in Healthy Middle-Aged Population: The STANISLAS Cohort. J. Am. Heart Assoc. 2020, 9, e014758. [Google Scholar] [CrossRef]
- Deedwania, P.; Murphy, S.A.; Scheen, A.; Badariene, J.; Pineda, A.L.; Honarpour, N.; Keech, A.C.; Sever, P.S.; Pedersen, T.R.; Sabatine, M.S.; et al. Efficacy and Safety of PCSK9 Inhibition with Evolocumab in Reducing Cardiovascular Events in Patients with Metabolic Syndrome Receiving Statin Therapy: Secondary Analysis From the FOURIER Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 139–147. [Google Scholar] [CrossRef]
- Raal, F.J.; Honarpour, N.; Blom, D.J.; Hovingh, G.K.; Xu, F.; Scott, R.; Wasserman, S.M.; Stein, E.A. Inhibition of PCSK9 with Evolocumab in Homozygous Familial Hypercholesterolaemia (TESLA Part B): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2015, 385, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.T.; Turner, T.; Visseren, F.L.J.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Wright, R.S.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-Term Efficacy and Safety of Inclisiran in Patients with High Cardiovascular Risk and Elevated LDL Cholesterol (ORION-3): Results from the 4-Year Open-Label Extension of the ORION-1 Trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid Restriction Enzymes: Zinc Finger Fusions to Fok I Cleavage Domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef]
- Bibikova, M.; Beumer, K.; Trautman, J.K.; Carroll, D. Enhancing Gene Targeting with Designed Zinc Finger Nucleases. Science 2003, 300, 764. [Google Scholar] [CrossRef]
- Santiago, Y.; Chan, E.; Liu, P.-Q.; Orlando, S.; Zhang, L.; Urnov, F.D.; Holmes, M.C.; Guschin, D.; Waite, A.; Miller, J.C.; et al. Targeted Gene Knockout in Mammalian Cells by Using Engineered Zinc-Finger Nucleases. Proc. Natl. Acad. Sci. USA 2008, 105, 5809–5814. [Google Scholar] [CrossRef]
- Ou, L.; DeKelver, R.C.; Rohde, M.; Tom, S.; Radeke, R.; Martin, S.J.S.; Santiago, Y.; Sproul, S.; Przybilla, M.J.; Koniar, B.L.; et al. ZFN-Mediated In Vivo Genome Editing Corrects Murine Hurler Syndrome. Mol. Ther. 2019, 27, 178–187. [Google Scholar] [CrossRef]
- Yan, H.; Niimi, M.; Matsuhisa, F.; Zhou, H.; Kitajima, S.; Chen, Y.; Wang, C.; Yang, X.; Yao, J.; Yang, D.; et al. Apolipoprotein CIII Deficiency Protects Against Atherosclerosis in Knockout Rabbits. ATVB 2020, 40, 2095–2107. [Google Scholar] [CrossRef] [PubMed]
- Sithu, S.D.; Malovichko, M.V.; Riggs, K.A.; Wickramasinghe, N.S.; Winner, M.G.; Agarwal, A.; Hamed-Berair, R.E.; Kalani, A.; Riggs, D.W.; Bhatnagar, A.; et al. Atherogenesis and Metabolic Dysregulation in LDL Receptor–Knockout Rats. JCI Insight 2017, 2, 86442. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Quan, C.; Hu, C.; Xie, B.; Du, Y.; Chen, L.; Yang, W.; Yang, L.; Chen, Q.; Shen, B.; et al. A Lipidomics Study Reveals Hepatic Lipid Signatures Associating with Deficiency of the LDL Receptor in a Rat Model. Biol. Open 2016, 5, 979–986. [Google Scholar] [CrossRef]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA Double-Strand Breaks with TAL Effector Nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef]
- Boch, J.; Scholze, H.; Schornack, S.; Landgraf, A.; Hahn, S.; Kay, S.; Lahaye, T.; Nickstadt, A.; Bonas, U. Breaking the Code of DNA Binding Specificity of TAL-Type III Effectors. Science 2009, 326, 1509–1512. [Google Scholar] [CrossRef]
- Miller, J.C.; Tan, S.; Qiao, G.; Barlow, K.A.; Wang, J.; Xia, D.F.; Meng, X.; Paschon, D.E.; Leung, E.; Hinkley, S.J.; et al. A TALE Nuclease Architecture for Efficient Genome Editing. Nat. Biotechnol. 2011, 29, 143–148. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A Widely Applicable Technology for Targeted Genome Editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, H. Transcription Activator-like Effector Nucleases (TALENs): A Highly Efficient and Versatile Tool for Genome Editing. Biotechnol. Bioeng. 2013, 110, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Boch, J. TALE and TALEN Genome Editing Technologies. Gene Genome Ed. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Benjamin, R.; Berges, B.K.; Solis-Leal, A.; Igbinedion, O.; Strong, C.L.; Schiller, M.R. TALEN Gene Editing Takes Aim on HIV. Hum. Genet. 2016, 135, 1059–1070. [Google Scholar] [CrossRef]
- Jain, S.; Shukla, S.; Yang, C.; Zhang, M.; Fatma, Z.; Lingamaneni, M.; Abesteh, S.; Lane, S.T.; Xiong, X.; Wang, Y.; et al. TALEN Outperforms Cas9 in Editing Heterochromatin Target Sites. Nat. Commun. 2021, 12, 606. [Google Scholar] [CrossRef]
- Karakikes, I.; Termglinchan, V.; Cepeda, D.A.; Lee, J.; Diecke, S.; Hendel, A.; Itzhaki, I.; Ameen, M.; Shrestha, R.; Wu, H.; et al. A Comprehensive TALEN-Based Knockout Library for Generating Human-Induced Pluripotent Stem Cell–Based Models for Cardiovascular Diseases. Circ. Res. 2017, 120, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Moiani, A. Non-Viral DNA Delivery Associated to TALEN® Gene Editing Leads to Highly Efficient Correction of Sickle Cell Mutation in Long-Term Repopulating Hematopoietic Stem Cells. Available online: https://cellectis.com/uploads/files/2022_ESGCT_Moiani._October_14._FINAL3.pdf (accessed on 2 August 2023).
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide Sequence of the Iap Gene, Responsible for Alkaline Phosphatase Isozyme Conversion in Escherichia Coli, and Identification of the Gene Product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Terns, M.P.; Terns, R.M. CRISPR-Based Adaptive Immune Systems. Curr. Opin. Microbiol. 2011, 14, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, D.; Davison, M.; Barrangou, R. CRISPR-Cas Systems in Bacteria and Archaea: Versatile Small RNAs for Adaptive Defense and Regulation. Annu. Rev. Genet. 2011, 45, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Horvath, P.; Barrangou, R. CRISPR/Cas, the Immune System of Bacteria and Archaea. Science 2010, 327, 167–170. [Google Scholar] [CrossRef]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA Maturation by Trans-Encoded Small RNA and Host Factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Collias, D.; Beisel, C.L. CRISPR Technologies and the Search for the PAM-Free Nuclease. Nat. Commun. 2021, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, Classification and Evolution of CRISPR-Cas Systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and Evolution of Class 2 CRISPR–Cas Systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-Homologous DNA End Joining and Alternative Pathways to Double-Strand Break Repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yang, S.; Hwang, G.-H.; Yu, J.; Bae, S. Analysis of NHEJ-Based DNA Repair after CRISPR-Mediated DNA Cleavage. Int. J. Mol. Sci. 2021, 22, 6397. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Staahl, B.T.; Alla, R.K.; Doudna, J.A. Enhanced Homology-Directed Human Genome Engineering by Controlled Timing of CRISPR/Cas9 Delivery. eLife 2014, 3, e04766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Li, X.-L.; Li, G.-H.; Chen, W.; Arakaki, C.; Botimer, G.D.; Baylink, D.; Zhang, L.; Wen, W.; Fu, Y.-W.; et al. Efficient Precise Knockin with a Double Cut HDR Donor after CRISPR/Cas9-Mediated Double-Stranded DNA Cleavage. Genome Biol. 2017, 18, 35. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable Editing of a Target Base in Genomic DNA without Double-Stranded DNA Cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Rees, H.A.; Liu, D.R. Base Editing: Precision Chemistry on the Genome and Transcriptome of Living Cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef]
- Porto, E.M.; Komor, A.C.; Slaymaker, I.M.; Yeo, G.W. Base Editing: Advances and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2020, 19, 839–859. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Linder, S.J.; Cascio, V.M.; Fu, Y.; Ho, Q.H.; Joung, J.K. CRISPR RNA–Guided Activation of Endogenous Human Genes. Nat. Methods 2013, 10, 977–979. [Google Scholar] [CrossRef]
- Forstnerič, V.; Oven, I.; Ogorevc, J.; Lainšček, D.; Praznik, A.; Lebar, T.; Jerala, R.; Horvat, S. CRISPRa-Mediated FOXP3 Gene Upregulation in Mammalian Cells. Cell Biosci. 2019, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Steinhart, Z.; Layeghi, M.; Freimer, J.W.; Bueno, R.; Nguyen, V.Q.; Blaeschke, F.; Ye, C.J.; Marson, A. CRISPR Activation and Interference Screens Decode Stimulation Responses in Primary Human T Cells. Science 2022, 375, eabj4008. [Google Scholar] [CrossRef]
- Jensen, T.I.; Mikkelsen, N.S.; Gao, Z.; Foßelteder, J.; Pabst, G.; Axelgaard, E.; Laustsen, A.; König, S.; Reinisch, A.; Bak, R.O. Targeted Regulation of Transcription in Primary Cells Using CRISPRa and CRISPRi. Genome Res. 2021, 31, 2120–2130. [Google Scholar] [CrossRef]
- Thakore, P.I.; Kwon, J.B.; Nelson, C.E.; Rouse, D.C.; Gemberling, M.P.; Oliver, M.L.; Gersbach, C.A. RNA-Guided Transcriptional Silencing in Vivo with S. Aureus CRISPR-Cas9 Repressors. Nat. Commun. 2018, 9, 1674. [Google Scholar] [CrossRef]
- Qiu, M.; Glass, Z.; Chen, J.; Haas, M.; Jin, X.; Zhao, X.; Rui, X.; Ye, Z.; Li, Y.; Zhang, F.; et al. Lipid Nanoparticle-Mediated Codelivery of Cas9 MRNA and Single-Guide RNA Achieves Liver-Specific in Vivo Genome Editing of Angptl3. Proc. Natl. Acad. Sci. USA 2021, 118, e2020401118. [Google Scholar] [CrossRef]
- Jarrett, K.E.; Lee, C.M.; Yeh, Y.-H.; Hsu, R.H.; Gupta, R.; Zhang, M.; Rodriguez, P.J.; Lee, C.S.; Gillard, B.K.; Bissig, K.-D.; et al. Somatic Genome Editing with CRISPR/Cas9 Generates and Corrects a Metabolic Disease. Sci. Rep. 2017, 7, 44624. [Google Scholar] [CrossRef]
- Zha, Y.; Lu, Y.; Zhang, T.; Yan, K.; Zhuang, W.; Liang, J.; Cheng, Y.; Wang, Y. CRISPR/Cas9-Mediated Knockout of APOC3 Stabilizes Plasma Lipids and Inhibits Atherosclerosis in Rabbits. Lipids Health Dis. 2021, 20, 180. [Google Scholar] [CrossRef]
- Guo, M.; Xu, Y.; Dong, Z.; Zhou, Z.; Cong, N.; Gao, M.; Huang, W.; Wang, Y.; Liu, G.; Xian, X. Inactivation of ApoC3 by CRISPR/Cas9 Protects Against Atherosclerosis in Hamsters. Circ. Res. 2020, 127, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Ren, X.; Wang, Y.; Li, Z.; Zhao, L.; Zhang, M.; Li, C.; Zhang, Z.; Chen, L.; Li, X.; et al. Apolipoprotein E Deficiency Accelerates Atherosclerosis Development in Miniature Pigs. Dis. Models Mech. 2018, 11, dmm036632. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hua, Z.; Xiao, H.; Cheng, Y.; Xu, K.; Gao, Q.; Xia, Y.; Liu, Y.; Zhang, X.; Zheng, X.; et al. CRISPR/Cas9-Mediated ApoE -/- and LDLR -/- Double Gene Knockout in Pigs Elevates Serum LDL-C and TC Levels. Oncotarget 2017, 8, 37751–37760. [Google Scholar] [CrossRef] [PubMed]
- Fedoseienko, A.; Wijers, M.; Wolters, J.C.; Dekker, D.; Smit, M.; Huijkman, N.; Kloosterhuis, N.; Klug, H.; Schepers, A.; Willems van Dijk, K.; et al. The COMMD Family Regulates Plasma LDL Levels and Attenuates Atherosclerosis Through Stabilizing the CCC Complex in Endosomal LDLR Trafficking. Circ. Res. 2018, 122, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shi, H.; Zhao, M.; Zhang, X.; Huang, W.; Wang, Y.; Zheng, L.; Xian, X.; Liu, G. Loss of LCAT Activity in the Golden Syrian Hamster Elicits Pro-Atherogenic Dyslipidemia and Enhanced Atherosclerosis. Metabolism 2018, 83, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; He, L.; Pu, W.; Yu, W.; Li, Y.; Wu, Y.-T.; Xu, C.; Wei, Y.; Ding, Q.; et al. In Vivo AAV-CRISPR/Cas9–Mediated Gene Editing Ameliorates Atherosclerosis in Familial Hypercholesterolemia. Circulation 2020, 141, 67–79. [Google Scholar] [CrossRef]
- Lu, R.; Yuan, T.; Wang, Y.; Zhang, T.; Yuan, Y.; Wu, D.; Zhou, M.; He, Z.; Lu, Y.; Chen, Y.; et al. Spontaneous Severe Hypercholesterolemia and Atherosclerosis Lesions in Rabbits with Deficiency of Low-Density Lipoprotein Receptor (LDLR) on Exon 7. EBioMedicine 2018, 36, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhong, Y.; Wang, Y.; Zhang, T.; Lu, R.; Zhou, M.; Lu, Y.; Yan, K.; Chen, Y.; Hu, Z.; et al. Generation of Hyperlipidemic Rabbit Models Using Multiple SgRNAs Targeted CRISPR/Cas9 Gene Editing System. Lipids Health Dis. 2019, 18, 69. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.; Cho, B.; Byun, J.; Kim, Y.S.; Ahn, Y.; Hur, J.; Oh, Y. In Vivo Therapeutic Genome Editing via CRISPR/Cas9 Magnetoplexes for Myocardial Infarction. Biomaterials 2021, 281, 121327. [Google Scholar] [CrossRef]
- Wang, X.; Raghavan, A.; Chen, T.; Qiao, L.; Zhang, Y.; Ding, Q.; Musunuru, K. CRISPR-Cas9 Targeting of PCSK9 in Human Hepatocytes In Vivo—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 783–786. [Google Scholar] [CrossRef]
- Carreras, A.; Pane, L.S.; Nitsch, R.; Madeyski-Bengtson, K.; Porritt, M.; Akcakaya, P.; Taheri-Ghahfarokhi, A.; Ericson, E.; Bjursell, M.; Perez-Alcazar, M.; et al. In Vivo Genome and Base Editing of a Human PCSK9 Knock-in Hypercholesterolemic Mouse Model. BMC Biol. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, J.; Liu, Y.; Jin, X.; Zhong, X.; Mo, L.; Wang, Q.; Deng, H.; Yang, Y. In Vivo PCSK9 Gene Editing Using an All-in-One Self-Cleavage AAV-CRISPR System. Mol. Ther.-Methods Clin. Dev. 2021, 20, 652–659. [Google Scholar] [CrossRef]
- Ding, Q.; Strong, A.; Patel, K.M.; Ng, S.-L.; Gosis, B.S.; Regan, S.N.; Cowan, C.A.; Rader, D.J.; Musunuru, K. Permanent Alteration of PCSK9 With In Vivo CRISPR-Cas9 Genome Editing. Circ. Res. 2014, 115, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Xie, Y.; Wang, P.; Deng, S.; Qin, A.; Zhang, J.; Yu, X.; Zheng, W.; Jiang, X. Triple-Targeting Delivery of CRISPR/Cas9 To Reduce the Risk of Cardiovascular Diseases. Angew. Chem. Int. Ed. 2019, 58, 12404–12408. [Google Scholar] [CrossRef] [PubMed]
- Ibraheim, R.; Song, C.-Q.; Mir, A.; Amrani, N.; Xue, W.; Sontheimer, E.J. All-in-One Adeno-Associated Virus Delivery and Genome Editing by Neisseria Meningitidis Cas9 in Vivo. Genome Biol. 2018, 19, 137. [Google Scholar] [CrossRef]
- Jarrett, K.E.; Lee, C.M.; De Giorgi, M.; Hurley, A.; Gillard, B.K.; Doerfler, A.M.; Li, A.; Pownall, H.J.; Henry, J.; Pownall, H.J.; et al. Somatic Editing of Ldlr With Adeno-Associated Viral-CRISPR Is an Efficient Tool for Atherosclerosis Research. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1997–2006. [Google Scholar] [CrossRef]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In Vivo Genome Editing Using Staphylococcus Aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In Vivo CRISPR Base Editing of PCSK9 Durably Lowers Cholesterol in Primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered Virus-like Particles for Efficient in Vivo Delivery of Therapeutic Proteins. Cell 2022, 185, 250–265.e16. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, C.; Zhou, Y.; Li, H.; Xiao, W.; Herzog, R.W.; Xu, J.; Zhang, J.; Chen, Y.E.; Han, R. Liver-Specific in Vivo Base Editing of Angptl3 via AAV Delivery Efficiently Lowers Blood Lipid Levels in Mice. Cell Biosci. 2023, 13, 109. [Google Scholar] [CrossRef]
- Lebek, S.; Chemello, F.; Caravia, X.M.; Tan, W.; Li, H.; Chen, K.; Xu, L.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Ablation of CaMKIIδ Oxidation by CRISPR-Cas9 Base Editing as a Therapy for Cardiac Disease. Science 2023, 379, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhi, S.; Liu, W.; Wen, J.; Hu, S.; Cao, T.; Sun, H.; Li, Y.; Huang, L.; Liu, Y.; et al. Development of Highly Efficient Dual-AAV Split Adenosine Base Editor for In Vivo Gene Therapy. Small Methods 2020, 4, 2000309. [Google Scholar] [CrossRef]
- Lee, R.G.; Mazzola, A.M.; Braun, M.C.; Platt, C.; Vafai, S.B.; Kathiresan, S.; Rohde, E.; Bellinger, A.M.; Khera, A.V. Efficacy and Safety of an Investigational Single-Course CRISPR Base-Editing Therapy Targeting PCSK9 in Nonhuman Primate and Mouse Models. Circulation 2023, 147, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Nguyen, G.N.; Zhang, C.; Zeng, C.; Yan, J.; Du, S.; Hou, X.; Li, W.; Jiang, J.; et al. Functionalized Lipid-like Nanoparticles for in Vivo MRNA Delivery and Base Editing. Sci. Adv. 2020, 6, eabc2315. [Google Scholar] [CrossRef]
- Rothgangl, T.; Dennis, M.K.; Lin, P.J.C.; Oka, R.; Witzigmann, D.; Villiger, L.; Qi, W.; Hruzova, M.; Kissling, L.; Lenggenhager, D.; et al. In Vivo Adenine Base Editing of PCSK9 in Macaques Reduces LDL Cholesterol Levels. Nat. Biotechnol. 2021, 39, 949–957. [Google Scholar] [CrossRef]
- Chadwick, A.C.; Evitt, N.H.; Lv, W.; Musunuru, K. Reduced Blood Lipid Levels with In Vivo CRISPR-Cas9 Base Editing of ANGPTL3. Circulation 2018, 137, 975–977. [Google Scholar] [CrossRef]
- Chadwick, A.C.; Wang, X.; Musunuru, K. In Vivo Base Editing of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) as a Therapeutic Alternative to Genome Editing. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1741–1747. [Google Scholar] [CrossRef]
- Wang, L.; Smith, J.; Breton, C.; Clark, P.; Zhang, J.; Ying, L.; Che, Y.; Lape, J.; Bell, P.; Calcedo, R.; et al. Meganuclease Targeting of PCSK9 in Macaque Liver Leads to Stable Reduction in Serum Cholesterol. Nat. Biotechnol. 2018, 36, 717–725. [Google Scholar] [CrossRef]
- Wang, L.; Breton, C.; Warzecha, C.C.; Bell, P.; Yan, H.; He, Z.; White, J.; Zhu, Y.; Li, M.; Buza, E.L.; et al. Long-Term Stable Reduction of Low-Density Lipoprotein in Nonhuman Primates Following In Vivo Genome Editing of PCSK9. Mol. Ther. 2021, 29, 2019–2029. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Su, L.; He, K.; Wang, Q.; Zhang, Y.; Yang, D.; Yang, Y.; Ma, S. Apolipoprotein E-Deficient Rats Develop Atherosclerotic Plaques in Partially Ligated Carotid Arteries. Atherosclerosis 2015, 243, 589–592. [Google Scholar] [CrossRef]
- Koike, T.; Koike, Y.; Yang, D.; Guo, Y.; Rom, O.; Song, J.; Xu, J.; Chen, Y.; Wang, Y.; Zhu, T.; et al. Human Apolipoprotein A-II Reduces Atherosclerosis in Knock-in Rabbits. Atherosclerosis 2021, 316, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.; Mendel, M.; Kim, K.; McGovern, K.; Boyko, A.; Zhang, L.; Miller, J.C.; DeKelver, R.C.; Paschon, D.E.; Mui, B.L.; et al. Non-Viral Delivery of Zinc Finger Nuclease MRNA Enables Highly Efficient In Vivo Genome Editing of Multiple Therapeutic Gene Targets. Mol. Ther. 2019, 27, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.C.; Malik, R.; Valdés-Márquez, E.; Worrall, B.B.; Collins, R. METASTROKE Collaboration of the ISGC Differential Effects of PCSK9 Variants on Risk of Coronary Disease and Ischaemic Stroke. Eur. Heart J. 2018, 39, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Small, A.M.; Huffman, J.E.; Klarin, D.; Lynch, J.A.; Assimes, T.; DuVall, S.; Sun, Y.V.; Shere, L.; Natarajan, P.; Gaziano, M.; et al. PCSK9 Loss of Function Is Protective against Extra-Coronary Atherosclerotic Cardiovascular Disease in a Large Multi-Ethnic Cohort. PLoS ONE 2020, 15, e0239752. [Google Scholar] [CrossRef] [PubMed]
- Amrani, N.; Gao, X.D.; Liu, P.; Edraki, A.; Mir, A.; Ibraheim, R.; Gupta, A.; Sasaki, K.E.; Wu, T.; Donohoue, P.D.; et al. NmeCas9 Is an Intrinsically High-Fidelity Genome-Editing Platform. Genome Biol. 2018, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Breton, C.; Furmanak, T.; Avitto, A.N.; Smith, M.K.; Latshaw, C.; Yan, H.; Greig, J.A.; Wilson, J.M. Increasing the Specificity of AAV-Based Gene Editing through Self-Targeting and Short-Promoter Strategies. Mol. Ther. 2021, 29, 1047–1056. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Lam, D.K.; Rees, H.A.; Solá-Esteves, N.M.; Barrera, L.A.; Born, D.A.; Edwards, A.; Gehrke, J.M.; Lee, S.-J.; Liquori, A.J.; et al. Directed Evolution of Adenine Base Editors with Increased Activity and Therapeutic Application. Nat. Biotechnol. 2020, 38, 892–900. [Google Scholar] [CrossRef]
- Shimizugawa, T.; Ono, M.; Shimamura, M.; Yoshida, K.; Ando, Y.; Koishi, R.; Ueda, K.; Inaba, T.; Minekura, H.; Kohama, T.; et al. ANGPTL3 Decreases Very Low Density Lipoprotein Triglyceride Clearance by Inhibition of Lipoprotein Lipase*. J. Biol. Chem. 2002, 277, 33742–33748. [Google Scholar] [CrossRef]
- Stitziel, N.O.; Khera, A.V.; Wang, X.; Bierhals, A.J.; Vourakis, A.C.; Sperry, A.E.; Natarajan, P.; Klarin, D.; Emdin, C.A.; Zekavat, S.M.; et al. ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 2054–2063. [Google Scholar] [CrossRef]
- Khera, A.; Lee, R.; Rohde, E.; Jayaram, H.; Kathiresan, S.; Bellinger, A. An in Vivo CRISPR Base Editing Therapy to Inactivate the ANGPTL3 Gene: Nomination of a Development Candidate for VERVE-201. Eur. Heart J. 2022, 43, ehac544.3087. [Google Scholar] [CrossRef]
- Lee, R.; Denizio, J.; Dutta, C.; Hsu, H.-T.; Garrity, R.; Pacheco, A.; Rohde, E.; Jayaram, H.; Kathiresan, S.; Bellinger, A.; et al. Preclinical Data Supporting Potential Efficacy of Verve-201—An Investigational Crispr Base Editing Medicine Targeting Angptl3—In Primary Human Cells, Mice, and Non-Human Primates. J. Am. Coll. Cardiol. 2023, 81, 1115. [Google Scholar] [CrossRef]
- Giammanco, A.; Spina, R.; Cefalù, A.B.; Averna, M. APOC-III: A Gatekeeper in Controlling Triglyceride Metabolism. Curr. Atheroscler. Rep. 2023, 25, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Corder, C.; Mueller, G.; Centurion, H.; Hallum, G.; Fesmire, J.; McConathy, W.D.; Alaupovic, P. Triglyceride Enriched Lipoprotein Particles Correlate with the Severity of Coronary Artery Disease. Atherosclerosis 1996, 122, 105–115. [Google Scholar] [CrossRef] [PubMed]
- The TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-Function Mutations in APOC3, Triglycerides, and Coronary Disease. N. Engl. J. Med. 2014, 371, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Karwatowska-Prokopczuk, E.; Amour, E.S.; Ballantyne, C.M.; Shapiro, M.D.; Moriarty, P.M.; Baum, S.J.; Hurh, E.; Bartlett, V.J.; Kingsbury, J.; et al. Apolipoprotein C-III Reduction in Subjects with Moderate Hypertriglyceridaemia and at High Cardiovascular Risk. Eur. Heart J. 2022, 43, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, J.; Zhang, L.; Miao, G.; Lai, P.; Zhang, W.; Liu, L.; Hou, X.; Wang, Y.; Huang, W.; et al. Targeting ApoC3 Paradoxically Aggravates Atherosclerosis in Hamsters With Severe Refractory Hypercholesterolemia. Front. Cardiovasc. Med. 2022, 9, 840358. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and Function in Lipid Metabolism, Neurobiology, and Alzheimer’s Diseases. Neurobiol. Dis. 2014, 72 Pt A, 3–12. [Google Scholar] [CrossRef]
- Bouchareychas, L.; Raffai, R.L. Apolipoprotein E and Atherosclerosis: From Lipoprotein Metabolism to MicroRNA Control of Inflammation. J. Cardiovasc. Dev. Dis. 2018, 5, 30. [Google Scholar] [CrossRef]
- Jeon, H.; Blacklow, S.C. Structure and Physiologic Function of the Low-Density Lipoprotein Receptor. Annu. Rev. Biochem. 2005, 74, 535–562. [Google Scholar] [CrossRef]
- Sun, X.M.; Patel, D.D.; Webb, J.C.; Knight, B.L.; Fan, L.M.; Cai, H.J.; Soutar, A.K. Familial Hypercholesterolemia in China. Identification of Mutations in the LDL-Receptor Gene That Result in a Receptor-Negative Phenotype. Arterioscler. Thromb. A J. Vasc. Biol. 1994, 14, 85–94. [Google Scholar] [CrossRef]
- Singla, A.; Fedoseienko, A.; Giridharan, S.S.P.; Overlee, B.L.; Lopez, A.; Jia, D.; Song, J.; Huff-Hardy, K.; Weisman, L.; Burstein, E.; et al. Endosomal PI(3)P Regulation by the COMMD/CCDC22/CCDC93 (CCC) Complex Controls Membrane Protein Recycling. Nat. Commun. 2019, 10, 4271. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, P.; Billadeau, D.D.; Favier, R.; Rong, S.; Dekker, D.; Fedoseienko, A.; Fieten, H.; Wijers, M.; Levels, J.H.; Huijkman, N.; et al. CCC- and WASH-Mediated Endosomal Sorting of LDLR Is Required for Normal Clearance of Circulating LDL. Nat. Commun. 2016, 7, 10961. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early Expression of Angiogenesis Factors in Acute Myocardial Ischemia and Infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-G.; Hausenloy, D.J. Hypoxia-Inducible Factor as a Therapeutic Target for Cardioprotection. Pharmacol. Ther. 2012, 136, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Wang, X.; Park, K.H.; Lee, K.E.; Kim, J.; Chen, P.; Zhou, X.; Tan, T.; Yang, C.; Zou, X.; et al. UCHL1 Protects against Ischemic Heart Injury via Activating HIF-1α Signal Pathway. Redox Biol. 2022, 52, 102295. [Google Scholar] [CrossRef] [PubMed]
- Hovsepian, J.; Albanèse, V.; Becuwe, M.; Ivashov, V.; Teis, D.; Léon, S. The Yeast Arrestin-Related Protein Bul1 Is a Novel Actor of Glucose-Induced Endocytosis. MBoC 2018, 29, 1012–1020. [Google Scholar] [CrossRef]
- Nakayama, Y.; Mukai, N.; Kreitzer, G.; Patwari, P.; Yoshioka, J. Interaction of ARRDC4 with GLUT1 Mediates Metabolic Stress in the Ischemic Heart. Circ. Res. 2022, 131, 510–527. [Google Scholar] [CrossRef]
- Karamanavi, E.; McVey, D.G.; van der Laan, S.W.; Stanczyk, P.J.; Morris, G.E.; Wang, Y.; Yang, W.; Chan, K.; Poston, R.N.; Luo, J.; et al. The FES Gene at the 15q26 Coronary-Artery-Disease Locus Inhibits Atherosclerosis. Circ. Res. 2022, 131, 1004–1017. [Google Scholar] [CrossRef]
- Luo, C.; Wang, F.; Ren, X.; Ke, T.; Xu, C.; Tang, B.; Qin, S.; Yao, Y.; Chen, Q.; Wang, Q.K. Identification of a Molecular Signaling Gene-Gene Regulatory Network between GWAS Susceptibility Genes ADTRP and MIA3/TANGO1 for Coronary Artery Disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1640–1653. [Google Scholar] [CrossRef]
- Aherrahrou, R.; Guo, L.; Nagraj, V.P.; Aguhob, A.; Hinkle, J.; Chen, L.; Yuhl Soh, J.; Lue, D.; Alencar, G.F.; Boltjes, A.; et al. Genetic Regulation of Atherosclerosis-Relevant Phenotypes in Human Vascular Smooth Muscle Cells. Circ. Res. 2020, 127, 1552–1565. [Google Scholar] [CrossRef]
- Duran, J.; Nickel, L.; Estrada, M.; Backs, J.; van den Hoogenhof, M.M.G. CaMKIIδ Splice Variants in the Healthy and Diseased Heart. Front. Cell Dev. Biol. 2021, 9, 644630. [Google Scholar] [CrossRef]
- Paz-García, M.; Povo-Retana, A.; Jaén, R.I.; Prieto, P.; Peraza, D.A.; Zaragoza, C.; Hernandez-Jimenez, M.; Pineiro, D.; Regadera, J.; García-Bermejo, M.L.; et al. Beneficial Effect of TLR4 Blockade by a Specific Aptamer Antagonist after Acute Myocardial Infarction. Biomed. Pharmacother. 2023, 158, 114214. [Google Scholar] [CrossRef] [PubMed]
- Schary, Y.; Rotem, I.; Caller, T.; Lewis, N.; Shaihov-Teper, O.; Brzezinski, R.Y.; Lendengolts, D.; Raanani, E.; Sternik, L.; Naftali-Shani, N.; et al. CRISPR-Cas9 Editing of TLR4 to Improve the Outcome of Cardiac Cell Therapy. Sci. Rep. 2023, 13, 4481. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Farbas, C.F., III. ZFN, TALEN and CRISPR/Cas-based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Nain, V. TALENs—An Indespensable Tool in the Era of CRISPR: A Mini Review. J. Genet. Eng. Biotechnol. 2021, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.M.; Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Investig. 2014, 124, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.; Yu, T. CRISPR/Cas9 Therapeutics: Progress and Prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef]
- Zhu, L.J.; Holmes, B.R.; Aronin, N.; Brodsky, M.H. CRISPRseek: A Bioconductor Package to Identify Target-Specific Guide RNAs for CRISPR-Cas9 Genome-Editing Systems. PLoS ONE 2014, 9, e108424. [Google Scholar] [CrossRef]
- Cradick, T.J.; Qiu, P.; Lee, C.M.; Fine, E.J.; Bao, G. COSMID: A Web-Based Tool for Identifying and Validating CRISPR/Cas Off-Target Sites. Mol. Ther.-Nucleic Acids 2014, 3, e214. [Google Scholar] [CrossRef]
- Rasul, M.F.; Hussen, B.M.; Salihi, A.; Ismael, B.S.; Jalal, P.J.; Zanichelli, A.; Jamali, E.; Banlahmad, A.; Ghafouri-Fard, S.; Basiri, A.; et al. Strategies to Overcome the Main Challenges of the Use of CRISPR/Cas9 as a Replacement for Cancer Therapy. Mol. Cancer 2022, 21, 64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).