Photochemical Synthesis of Noble Metal Nanoparticles: Influence of Metal Salt Concentration on Size and Distribution
Abstract
:1. Introduction
2. Results and Discussion
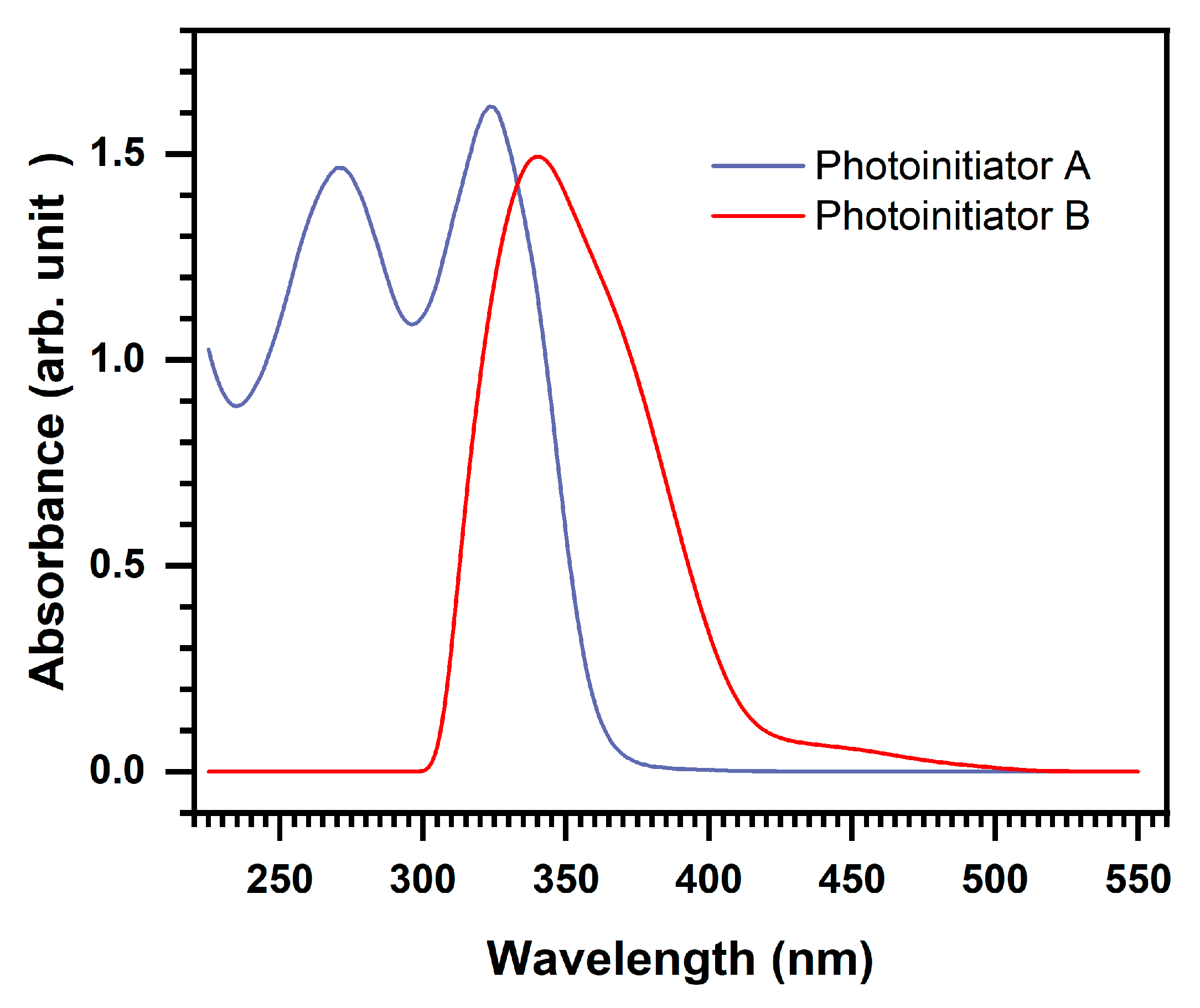
2.1. Light Absorption Properties of the Photoinitiators
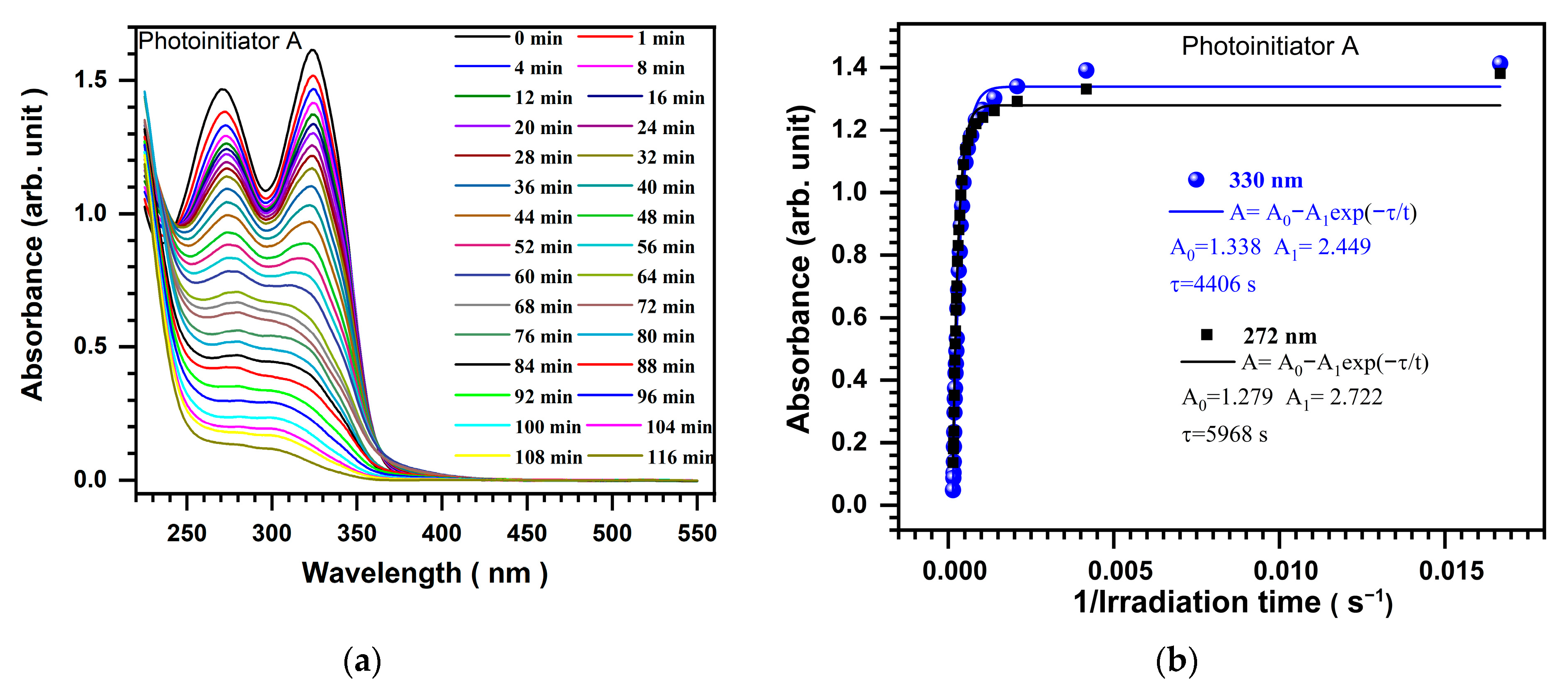
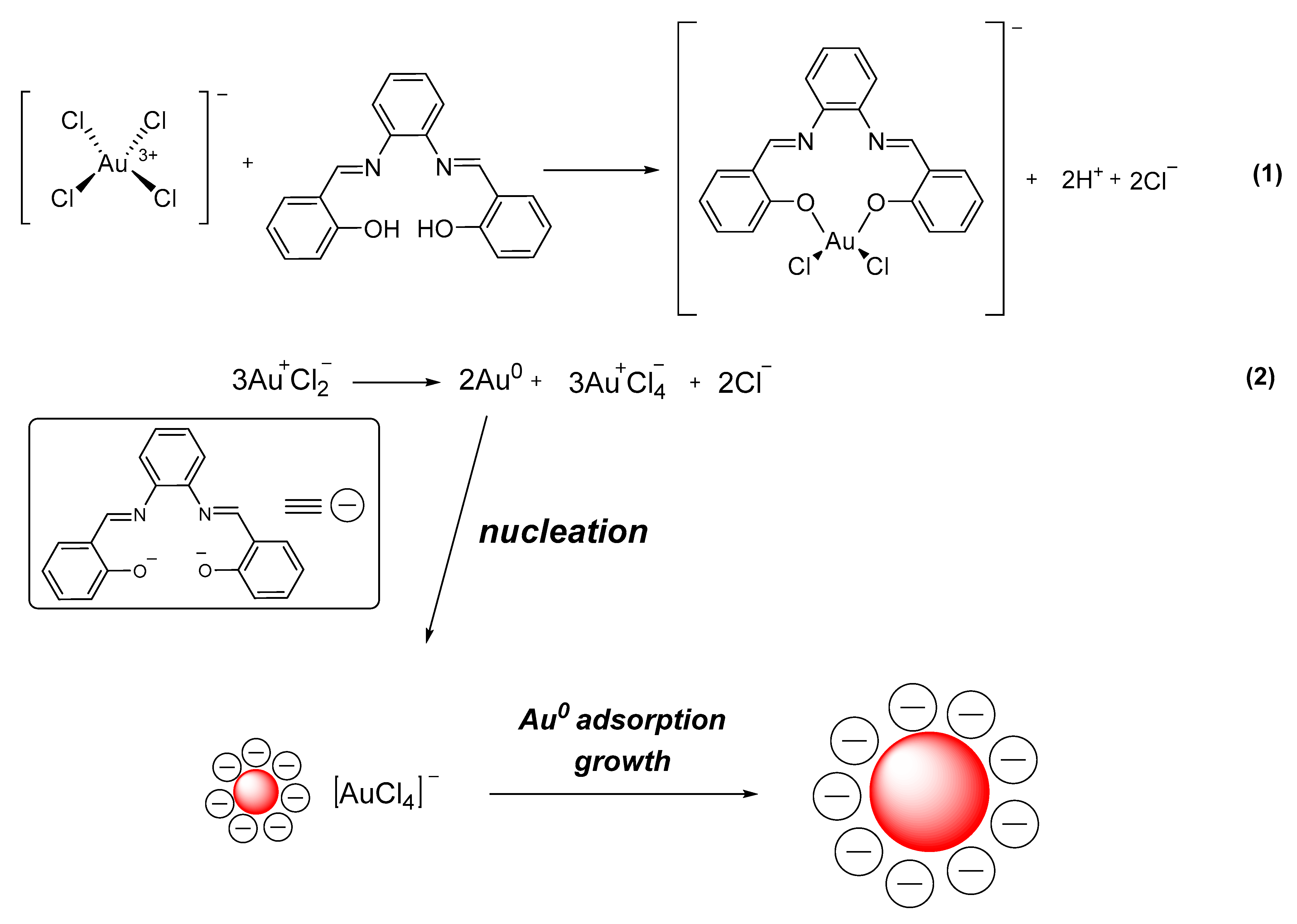
2.2. Photolysis of Photoinitiators
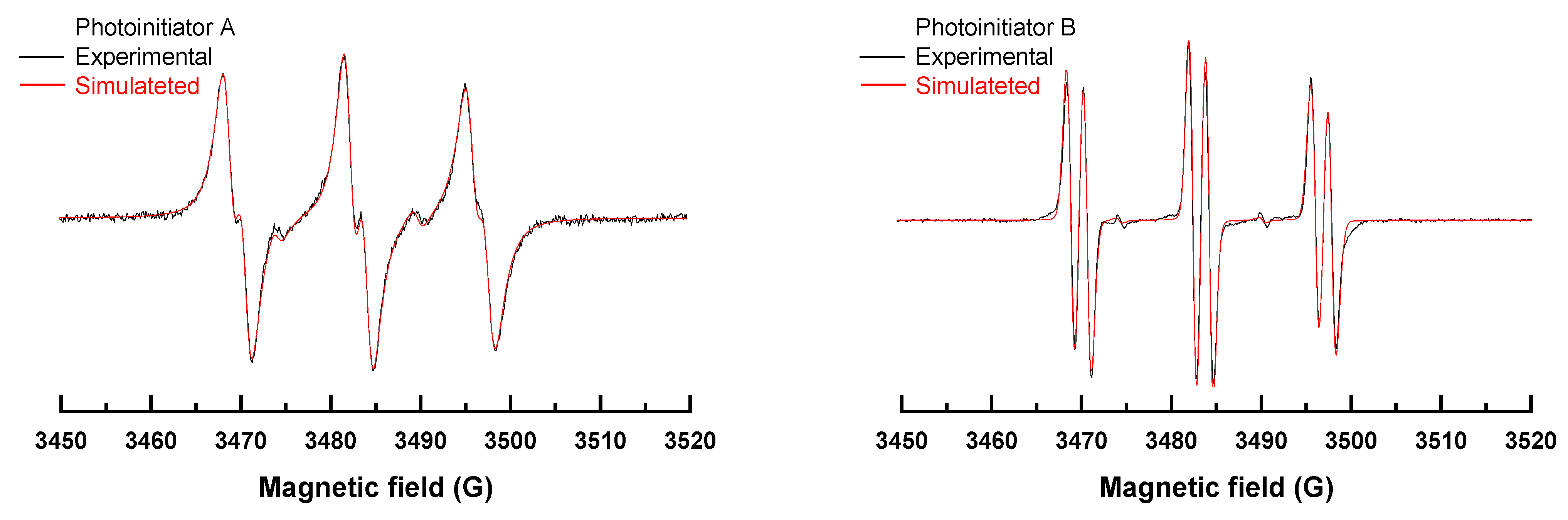
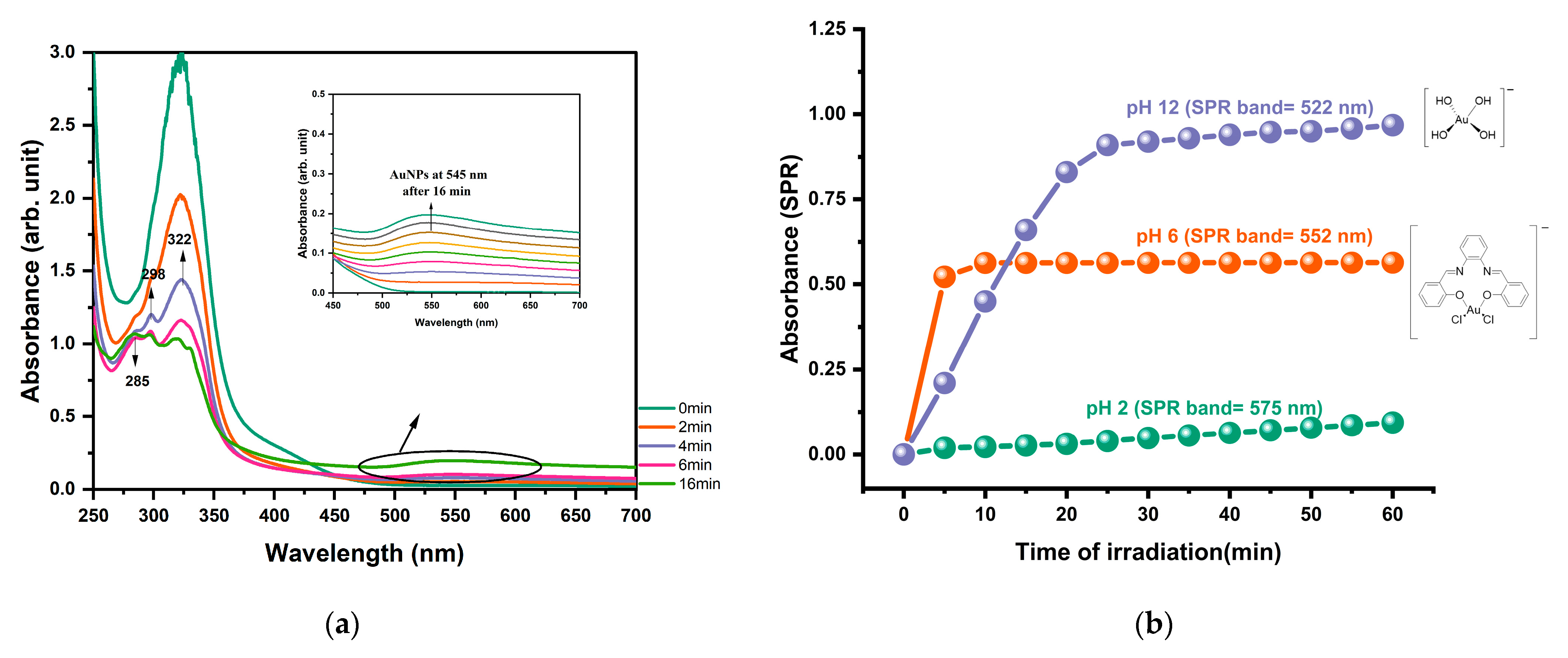
2.3. Photoinitiators Oxidation Process
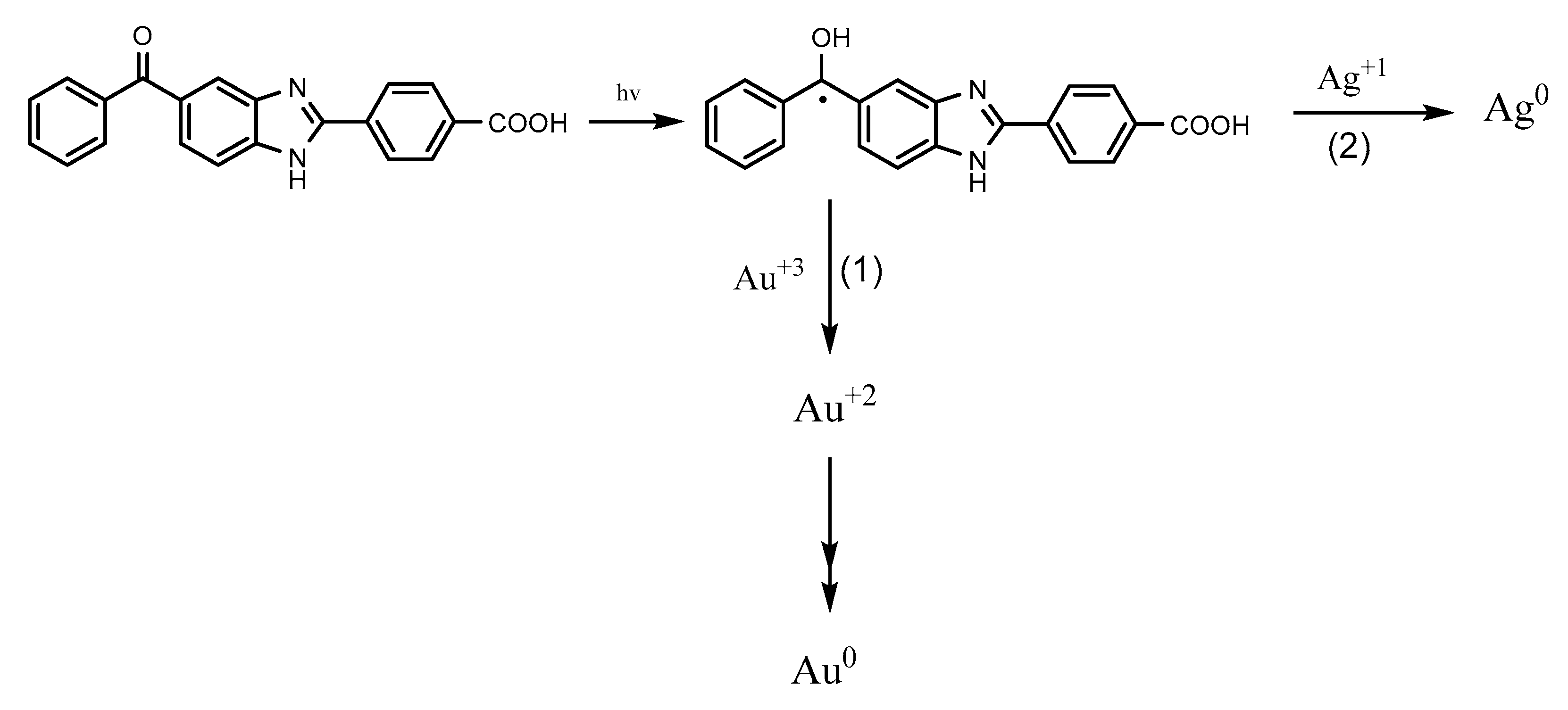
2.4. Photoproduction of Metal NPs
2.4.1. Photoproduction of Au Nanoparticles
2.4.2. Photoproduction of Ag Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. Irradiation Source
3.3. Absorbance Measurements
3.4. ESR Experiments
3.5. Redox Potentials
3.6. Fluorescence Experiments
3.7. Photoproduction of Gold/Silver Nanoparticles by Photoinitiators in Methanol Solution
3.8. Photoproduction of Gold/Silver Nanoparticles by Photoinitiators B at Different pH Values
3.9. Transmission Electron Microscopy (TEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaqad, K.; Saleh, T.A. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 2016, 6, 525–2161. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Kazancioglu, E.O.; Aydin, M.; Arsu, N. Photochemical synthesis of bimetallic gold/silver nanoparticles in polymer matrix with tunable absorption properties: Superior photocatalytic activity for degradation of methylene blue. Mater. Chem. Phys. 2021, 269, 124734. [Google Scholar] [CrossRef]
- Khan, S.A.; Lee, C.-S. Green biological synthesis of nanoparticles and their biomedical applications. Appl. Nanotechnol. Green Synth. 2020, 247–280. [Google Scholar] [CrossRef]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and gold nanoparticles for antimicrobial purposes against multi-drug resistance bacteria. Materials 2022, 15, 1799. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Pitkethly, M.J. Nanomaterials–the driving force. Mater. Today 2004, 7, 20–29. [Google Scholar] [CrossRef]
- Karimadom, B.R.; Kornweitz, H. Mechanism of producing metallic nanoparticles, with an emphasis on silver and gold nanoparticles, using bottom-up methods. Molecules 2021, 26, 2968. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A review of silver nanoparticles: Research trends, global consumption, synthesis, properties, and future challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Amirjani, A.; Firouzi, F.; Haghshenas, D.F. Predicting the size of silver nanoparticles from their optical properties. Plasmonics 2020, 15, 1077–1082. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Babich, E.; Reduto, I.; Lipovskii, A. Diffusive Formation of Au/Ag Alloy Nanoparticles of Governed Composition in Glass. Nanomaterials 2022, 12, 4202. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, J.P.; Martin, J.E.; Parsapour, F.; Wiedenman, B.; Kelley, D.F. Photoluminescence from nanosize gold clusters. J. Chem. Phys. 1998, 108, 9137–9143. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, C.; Yu, M.; Liu, J. Different sized luminescent gold nanoparticles. Nanoscale 2012, 4, 4073–4083. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zeng, Z.; Pang, S. Relationship between crystal structure and luminescent properties of novel red emissive BiVO4: Eu3+ and its photocatalytic performance. J. Nanoparticle Res. 2012, 14, 1076. [Google Scholar] [CrossRef]
- Onubogu, K.; Medina-Ramirez, I.; Bashir, S.; Luo, Z.; Liu, J. Colloidal synthesis and nanocharacterization of engineered noble metal nanoparticles. Int. J. Green Nanotechnol. 2011, 3, 140–151. [Google Scholar] [CrossRef]
- Tuan Anh, M.N.; Nguyen, D.T.D.; Ke Thanh, N.V.; Phuong Phong, N.T.; Nguyen, D.H.; Nguyen-Le, M.-T. Photochemical synthesis of silver nanodecahedrons under blue LED irradiation and their SERS activity. Processes 2020, 8, 292. [Google Scholar] [CrossRef]
- Jara, N.; Milán, N.S.; Rahman, A.; Mouheb, L.; Boffito, D.C.; Jeffryes, C.; Dahoumane, S.A. Photochemical synthesis of gold and silver nanoparticles—A review. Molecules 2021, 26, 4585. [Google Scholar] [CrossRef] [PubMed]
- Çinko, T.; Koyuncu, U.; Ömür, B.C.; Altındal, A.; Arsu, N. In-situ photochemical synthesis of Au nanoparticles in polymer matrix with one-component thioxanthone disulfide for detection of benzene, toluene and xylene vapours. Prog. Org. Coat. 2019, 132, 125–131. [Google Scholar] [CrossRef]
- Kirtay, H.; Omur, B.C.; Altindal, A.; Arsu, N. One step facile in-situ photochemical synthesis of hollow, doughnut-like ZnO nanoparticles and their alcohol vapor sensing properties. Mater. Res. Bull. 2020, 122, 110661. [Google Scholar] [CrossRef]
- Batibay, G.S.; Gunkara, O.T.; Ocal, N.; Arsu, N. In-situ photoinduced formation of self–assembled Ag NPs using POSS-TX as nano-photoinitiator in PEGMEA/PEGDA polymer matrix and creating self-wrinkled pattern. J. Photochem. Photobiol. A Chem. 2018, 359, 73–79. [Google Scholar] [CrossRef]
- Çeper, T.; Arsu, N. Photochemically prepared gold/polymer nanocoatings: Formation of gold mirror. Macromol. Chem. Phys. 2017, 218, 1700030. [Google Scholar] [CrossRef]
- Metin, E.; Batibay, G.S.; Arsu, N. In–situ formation of self-assembled Ag nanoclusters on ct-DNA in the presence of 2-mercaptothioxanthone by using UV–vis light irradiation. J. Photochem. Photobiol. A Chem. 2018, 356, 1–6. [Google Scholar] [CrossRef]
- Tar, H.; Kashar, T.I.; Kouki, N.; Aldawas, R.; Graff, B.; Lalevée, J. Novel copper photoredox catalysts for polymerization: An in situ synthesis of metal nanoparticles. Polymers 2020, 12, 2293. [Google Scholar] [CrossRef]
- Adibelli, M.; Ozcelik, E.; Batibay, G.S.; Arasoglu, T.O.; Arsu, N. A facile and versatile route for preparation AgNp nanocomposite thin films via thiol-acrylate photopolymerization: Determination of antibacterial activity. Prog. Org. Coat. 2020, 143, 105620. [Google Scholar] [CrossRef]
- Esen, D.S.; Arsu, N.; Da Silva, J.P.; Jockusch, S.; Turro, N.J. Benzoin type photoinitiator for free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1865–1871. [Google Scholar] [CrossRef]
- Tar, H.; Sevinc Esen, D.; Aydin, M.; Ley, C.; Arsu, N.; Allonas, X. Panchromatic type II photoinitiator for free radical polymerization based on thioxanthone derivative. Macromolecules 2013, 46, 3266–3272. [Google Scholar] [CrossRef]
- Karaca, N.; Balta, D.K.; Ocal, N.; Arsu, N. Mechanistic studies of thioxanthone–carbazole as a one-component type II photoinitiator. J. Lumin. 2014, 146, 424–429. [Google Scholar] [CrossRef]
- Alnafisah, A.S.; Alqrairy, E.; Tar, H.; Alminderej, M.F.; Aroua, L.M.; Graff, B.; Lalevee, J. Light-Assisted Synthesis of Silver and Gold Nanoparticles by New Benzophenone Derivatives. ACS Omega 2023, 8, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Metin, E.; Arsu, N.; Catak, S.; Aviyente, V. Photophysical, kinetic and thermodynamic study of one-component Type II thioxanthone acetic acid photoinitiators. Eur. Polym. J. 2020, 136, 109909. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The photoinitiators used in resin based dental composite—A review and future perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: Advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-soluble photoinitiators in biomedical applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Noirbent, G.; Mau, A.; Chen, H.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Xiao, P.; Dumur, F. New multifunctional benzophenone-based photoinitiators with high migration stability and their applications in 3D printing. Mater. Chem. Front. 2021, 5, 1982–1994. [Google Scholar] [CrossRef]
- Aldebasi, S.M.; Tar, H.; Alnafisah, A.S.; Salmi-Mani, H.; Kouki, N.; Alminderej, F.M.; Lalevée, J. Surface Modification of PP and PBT Nonwoven Membranes for Enhanced Efficiency in Photocatalytic MB Dye Removal and Antibacterial Activity. Polymers 2023, 15, 3378. [Google Scholar] [CrossRef]
- Alhomaidan, L.M.; Tar, H.; Alnafisah, A.S.; Aroua, L.M.; KouKi, N.; Alminderej, F.M.; Lalevee, J. Copper II Complexes Based on Benzimidazole Ligands as a Novel Photoredox Catalysis for Free Radical Polymerization Embedded Gold and Silver Nanoparticles. Polymers 2023, 15, 1289. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Pardoe, A.; McGilvray, K.L.; Chrétien, M.N.; Scaiano, J.C. Synthesis of copper nanoparticles mediated by photogenerated free radicals: Catalytic role of chloride anions. Photochem. Photobiol. Sci. 2010, 9, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Dizman, H.M.; Kazancioglu, E.O.; Shigemune, T.; Takahara, S.; Arsu, N. High sensitivity colorimetric determination of L-cysteine using gold nanoparticles functionalized graphene oxide prepared by photochemical reduction method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120294. [Google Scholar] [CrossRef] [PubMed]
- Scaiano, J.C.; Stamplecoskie, K.G.; Hallett-Tapley, G.L. Photochemical Norrish type I reaction as a tool for metal nanoparticle synthesis: Importance of proton coupled electron transfer. Chem. Commun. 2012, 48, 4798–4808. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.L.; McGilvray, K.L.; Scaiano, J.C. Photochemical strategies for the synthesis of gold nanoparticles from Au(III) and Au(I) using photoinduced free radical generation. J. Am. Chem. Soc. 2008, 130, 16572–16584. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Shi, S.; Gao, H.; Wu, G.; Nie, J. Cyclic acetal as coinitiator for bimolecular photoinitiating systems. Polymer 2007, 48, 2860–2865. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Hug, G.L.; Andrzejewski, M.; Marciniak, B. Trithianes as coinitiators in benzophenone-induced photopolymerizations. Macromolecules 1999, 32, 2173–2179. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Kometani, N.; Doi, H.; Asami, K.; Yonezawa, Y. Laser flash photolysis study of the photochemical formation of colloidal Ag nanoparticles in the presence of benzophenone. Phys. Chem. Chem. Phys. 2002, 4, 5142–5147. [Google Scholar] [CrossRef]
- Eustis, S.; Krylova, G.; Eremenko, A.; Smirnova, N.; Schill, A.W.; El-Sayed, M. Growth and fragmentation of silver nanoparticles in their synthesis with a fs laser and CW light by photo-sensitization with benzophenone. Photochem. Photobiol. Sci. 2005, 4, 154–159. [Google Scholar] [CrossRef]
- Eustis, S.; Krylova, G.; Smirnova, N.; Eremenko, A.; Tabor, C.; Huang, W.; El-Sayed, M.A. Using silica films and powders modified with benzophenone to photoreduce silver nanoparticles. J. Photochem. Photobiol. A Chem. 2006, 181, 385–393. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Aliaga, C.; Maguire, S.; Wang, D. Magnetic field control of photoinduced silver nanoparticle formation. J. Phys. Chem. B 2006, 110, 12856–12859. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Two-color two-laser fabrication of gold nanoparticles in a PVA film. Chem. Phys. Lett. 2006, 420, 90–94. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Alarcon, E.I.; Hallett-Tapley, G.L.; Murillo-Lopez, J.; Arratia-Perez, R.; Netto-Ferreira, J.C.; Scaiano, J.C. Electron transfer from the benzophenone triplet excited state directs the photochemical synthesis of gold nanoparticles. Photochem. Photobiol. Sci. 2014, 13, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Photochemical formation of Au/Cu bimetallic nanoparticles with different shapes and sizes in a poly (vinyl alcohol) film. Adv. Funct. Mater. 2007, 17, 857–862. [Google Scholar] [CrossRef]
- Zhao, F.; Ren, G.; Zhou, C.; Yang, Y. Study of one-step photosynthesis of Ag nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 65–69. [Google Scholar] [CrossRef]
- Koyuncu, U.; Metin, E.; Ocal, N.; Arsu, N. Synthesis of one-component type II dithioxanthone-disulfide photoinitiator and investigation of photophysical and photochemical properties. Eur. Polym. J. 2021, 153, 110510. [Google Scholar] [CrossRef]
- Mutlu, S.; Metin, E.; Yuksel, S.A.; Bayrak, U.; Nuhoglu, C.; Arsu, N. In-situ photochemical synthesis and dielectric properties of nanocomposite thin films containing Au, Ag and MnO nanoparticles. Eur. Polym. J. 2021, 144, 110238. [Google Scholar] [CrossRef]
- Kazancioglu, E.O.; Aydin, M.; Arsu, N. Photochemical synthesis of nanocomposite thin films containing silver and gold nanoparticles with 2-thioxanthone thioacetic acid-dioxide and their role in photocatalytic degradation of methylene blue. Surf. Interfaces 2021, 22, 100793. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Netto-Ferreira, J.C.; Khmelinskii, I.V.; Garcia, A.R.; Costa, S.M.B. Photochemistry on surfaces: Matrix isolation mechanisms study of interactions of benzophenone adsorbed on microcrystalline cellulose investigated by diffuse reflectance and luminescence techniques. Langmuir 1995, 11, 231–236. [Google Scholar] [CrossRef]
- Jauk, S.; Liska, R. Photoinitiators with functional groups, 8. Macromol. Rapid Commun. 2005, 26, 1687–1692. [Google Scholar] [CrossRef]
- Karasu, F.; Arsu, N.; Jockusch, S.; Turro, N.J. Mechanistic studies of photoinitiated free radical polymerization using a bifunctional thioxanthone acetic acid derivative as photoinitiator. Macromolecules 2009, 42, 7318–7323. [Google Scholar] [CrossRef]
- Newman, J.D.S.; Blanchard, G.J. Formation of gold nanoparticles using amine reducing agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Tanaka, H.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Photoreductive synthesis of monodispersed Au nanoparticles with citric acid as reductant and surface stabilizing reagent. RSC Adv. 2017, 7, 6187–6192. [Google Scholar] [CrossRef]
- Gao, J.; Hu, Y.; Li, S.; Zhang, Y.; Chen, X. Adsorption of benzoic acid, phthalic acid on gold substrates studied by surface-enhanced Raman scattering spectroscopy and density functional theory calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Tanaka, K.; Shirakawa, E.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Light-Triggered Self-Assembly of Gold Nanoparticles Based on Photoisomerization of Spirothiopyran. Angew. Chem. 2013, 125, 8462–8466. [Google Scholar] [CrossRef]
- Boufi, S.; Vilar, M.R.; Ferraria, A.M.; do Rego, A.M.B. In situ photochemical generation of silver and gold nanoparticles on chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 151–158. [Google Scholar] [CrossRef]
- Goh, C.F.; Yu, H.; Yong, S.S.; Mhaisalkar, S.G.; Boey, F.Y.C.; Teo, P.S. Synthesis and cure kinetics of isotropic conductive adhesives comprising sub-micrometer sized nickel particles. Mater. Sci. Eng. B 2005, 117, 153–158. [Google Scholar] [CrossRef]
- Biçer, M.; Şişman, İ. Controlled synthesis of copper nano/microstructures using ascorbic acid in aqueous CTAB solution. Powder Technol. 2010, 198, 279–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Z.-M.; Ding, B.; Lan, X.; Guo, Y. Preparation of copper nanoparticles by chemical reduction method using potassium borohydride. Trans. Nonferrous Met. Soc. China 2010, 20, s240–s244. [Google Scholar] [CrossRef]
- Huang, S.; Minami, K.; Sakaue, H.; Shingubara, S.; Takahagi, T. Optical spectroscopic studies of the dispersibility of gold nanoparticle solutions. J. Appl. Phys. 2002, 92, 7486–7490. [Google Scholar] [CrossRef]
- Ferguson, M.; Giri, N.; Huang, X.; Apperley, D.; James, S.L. One-pot two-step mechanochemical synthesis: Ligand and complex preparation without isolating intermediates. Green Chem. 2014, 16, 1374–1382. [Google Scholar] [CrossRef]
- Aroua, L.M.; Almuhaylan, H.R.; Alminderej, F.M.; Messaoudi, S.; Chigurupati, S.; Al-Mahmoud, S.; Mohammed, H.A. A facile approach synthesis of benzoylaryl benzimidazole as potential α-amylase and α-glucosidase inhibitor with antioxidant activity. Bioorg. Chem. 2021, 114, 105073. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]



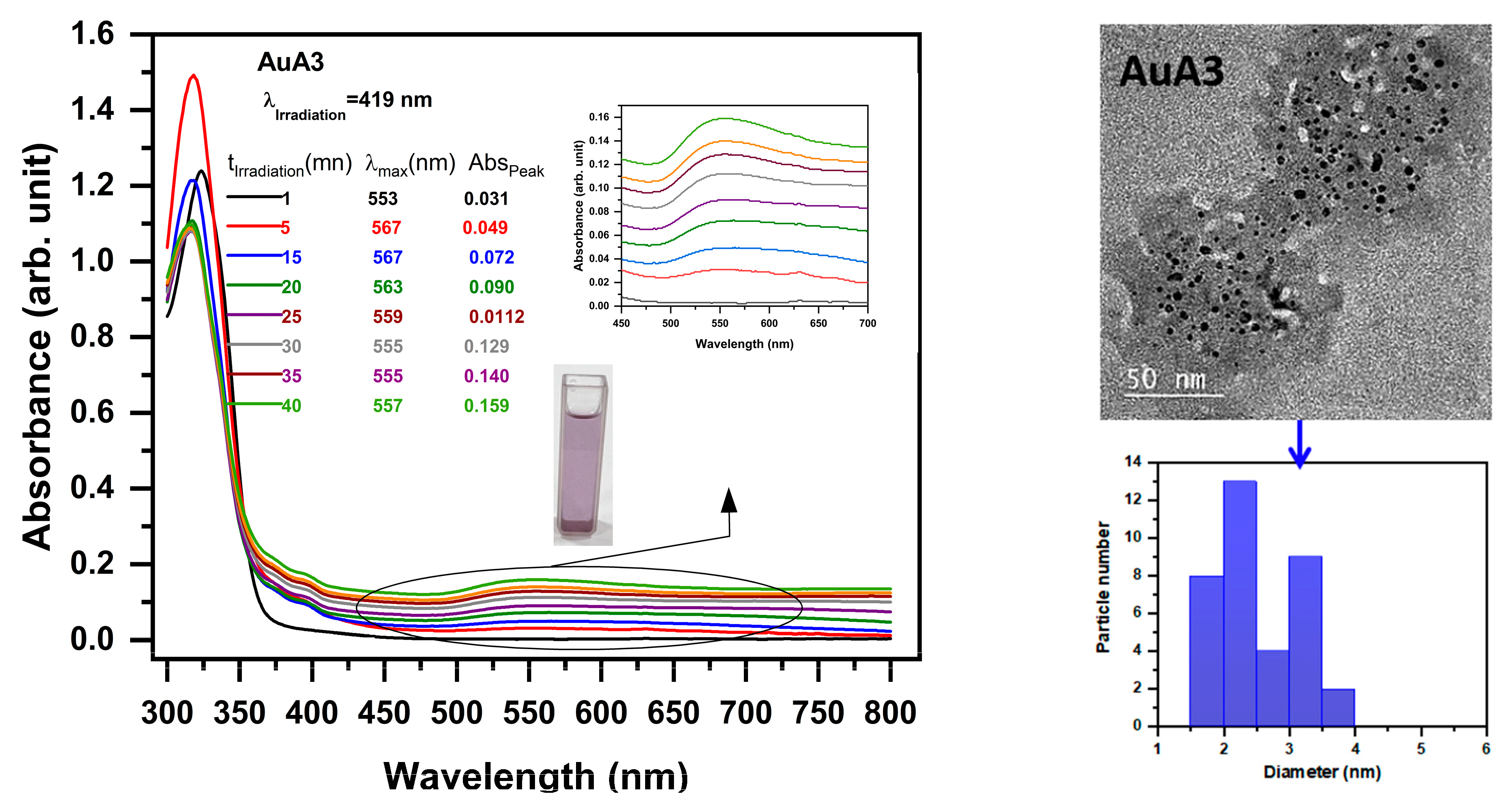
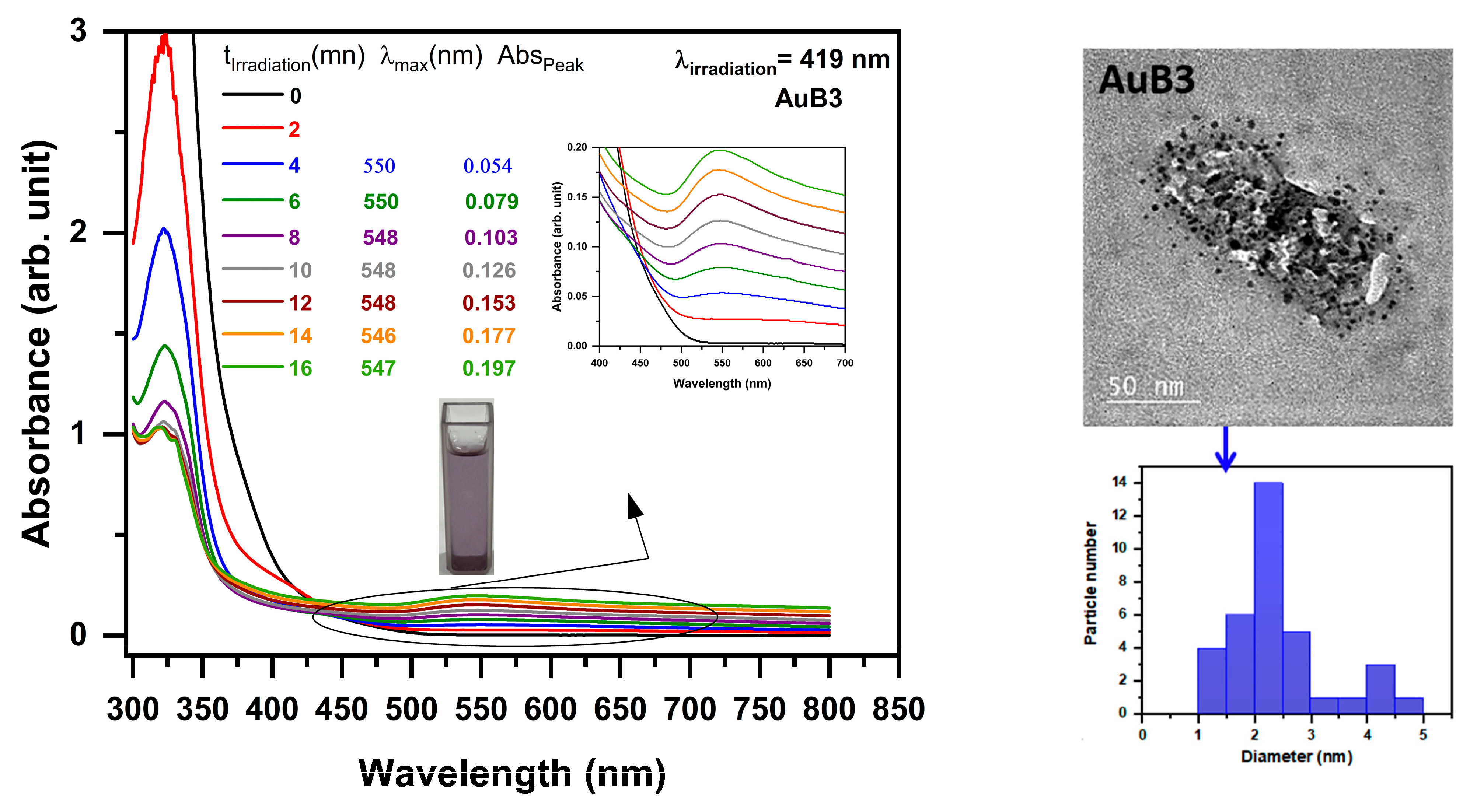
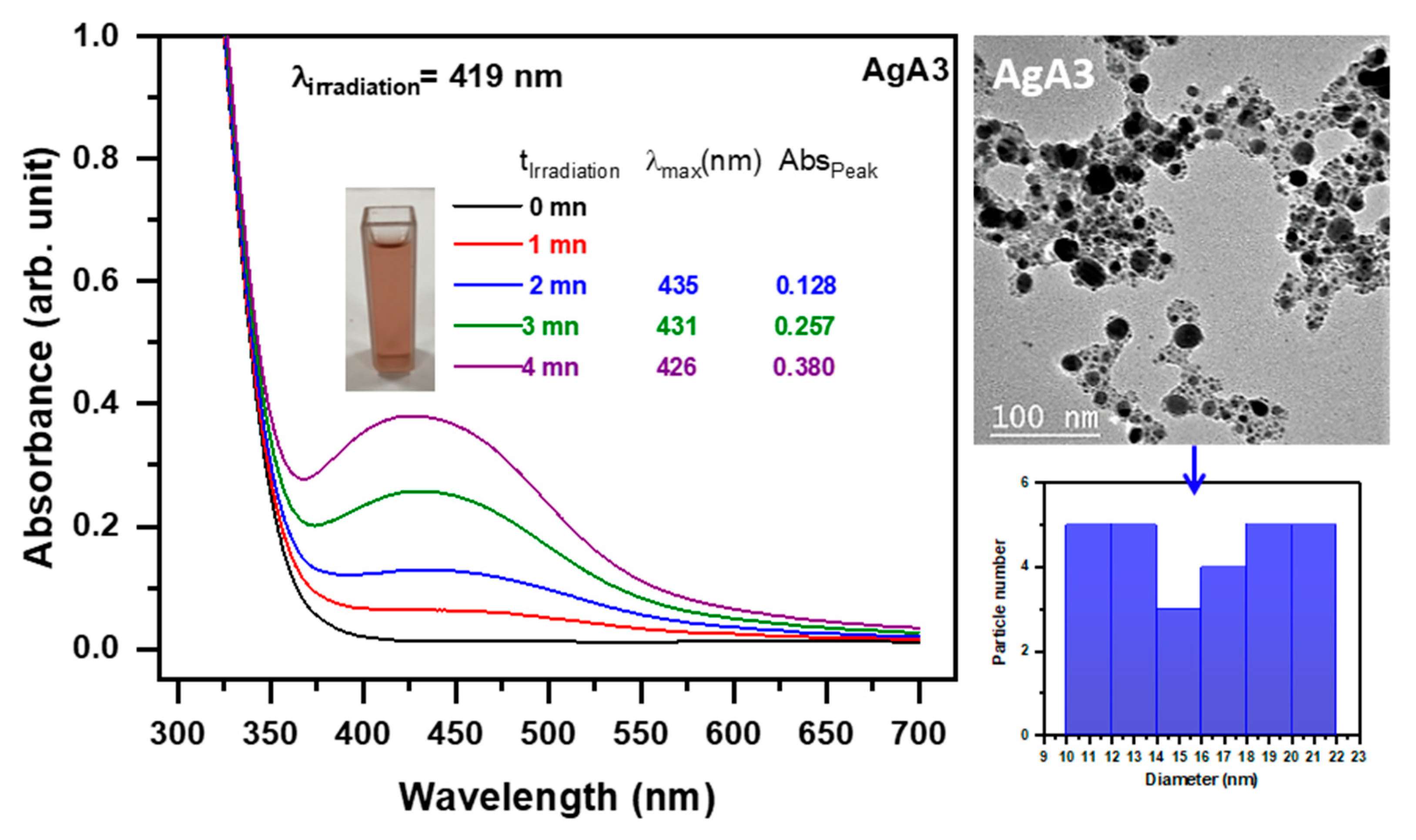

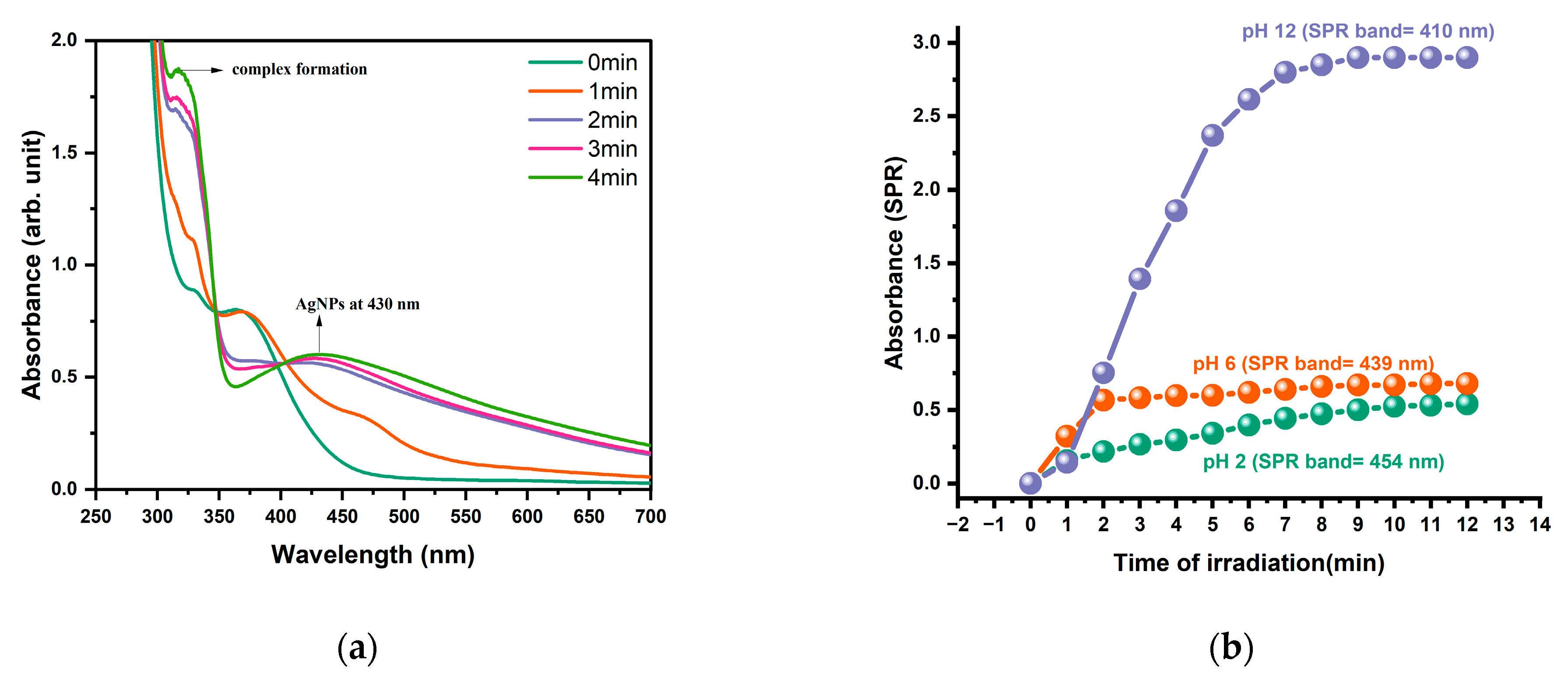
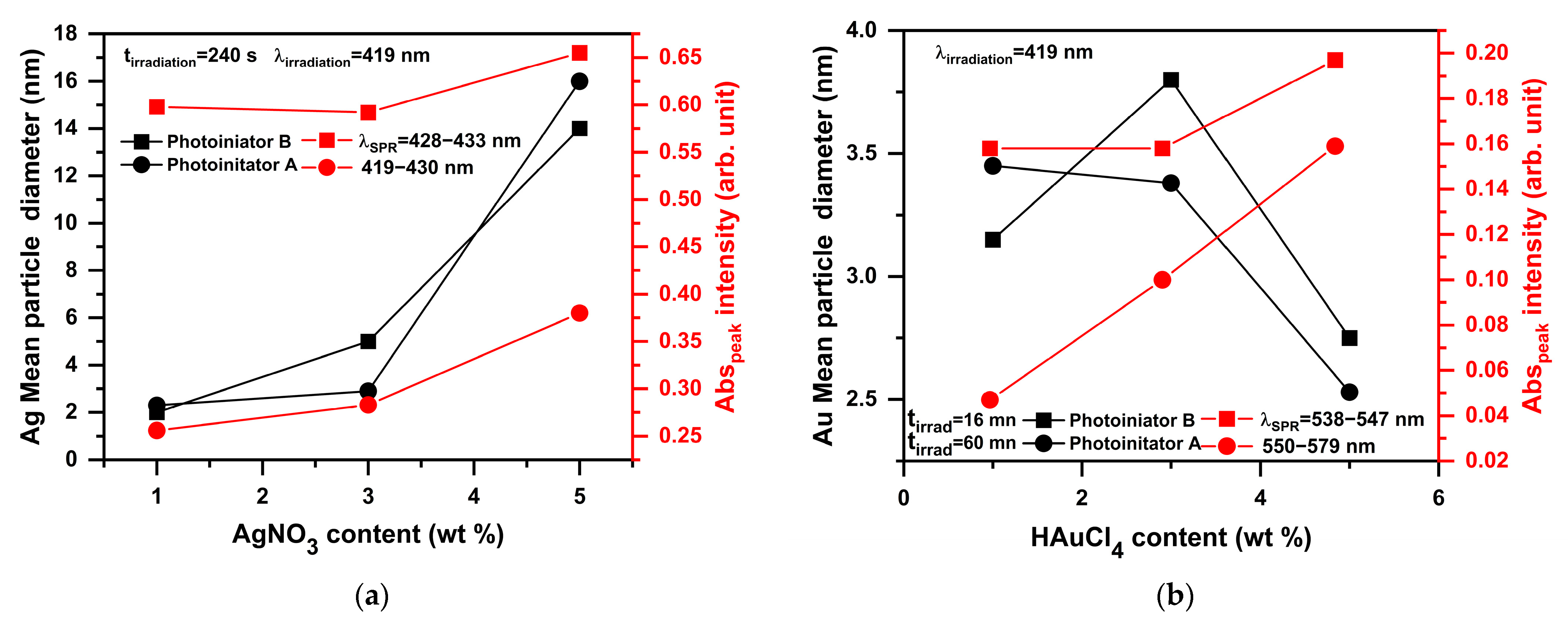

| Photoinitiator | Eox (V) | ΔGAu (ev) | ΔGAg (ev) | |
|---|---|---|---|---|
| A | 0.82 | 3.48 | −3.514 | −3.459 |
| B | 0.91 | 3.02 | −2.964 | −2.909 |
| No. | A0 | At | Conversion (A0 − At/A0) |
|---|---|---|---|
| AuA1 | 1.550 | 1.062 | 31% |
| AuA2 | 1.245 | 0.751 | 39% |
| AuA3 | 3.713 | 1.070 | 71% |
| AuB1 | 3.780 | 0.976 | 74% |
| AuB2 | 3.800 | 1.100 | 71% |
| AuB3 | 3.900 | 1.010 | 74% |
| No. | Photoinitiator | Metal Salt (wt%) | Irradiation Time (min) |
|---|---|---|---|
| AuA1 | A | HAuCl4 (1 wt%) | 40 |
| AuA2 | A | HAuCl4 (3 wt%) | 40 |
| AuA3 | A | HAuCl4 (5 wt%) | 40 |
| AuB1 | B | HAuCl4 (1 wt%) | 16 |
| AuB2 | B | HAuCl4 (3 wt%) | 16 |
| AuB3 | B | HAuCl4 (5 wt%) | 16 |
| AgA1 | A | AgNO3 (1 wt%) | 5 |
| AgA2 | A | AgNO3 (3 wt%) | 5 |
| AgA3 | A | AgNO3 (5 wt%) | 5 |
| AgB1 | B | AgNO3 (1 wt%) | 4 |
| AgB2 | B | AgNO3 (3 wt%) | 4 |
| AgB3 | B | AgNO3 (5 wt%) | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Aldebasi, S.; Tar, H.; S. Alnafisah, A.; Beji, L.; Kouki, N.; Morlet-Savary, F.; Alminderej, F.M.; Aroua, L.M.; Lalevée, J. Photochemical Synthesis of Noble Metal Nanoparticles: Influence of Metal Salt Concentration on Size and Distribution. Int. J. Mol. Sci. 2023, 24, 14018. https://doi.org/10.3390/ijms241814018
M. Aldebasi S, Tar H, S. Alnafisah A, Beji L, Kouki N, Morlet-Savary F, Alminderej FM, Aroua LM, Lalevée J. Photochemical Synthesis of Noble Metal Nanoparticles: Influence of Metal Salt Concentration on Size and Distribution. International Journal of Molecular Sciences. 2023; 24(18):14018. https://doi.org/10.3390/ijms241814018
Chicago/Turabian StyleM. Aldebasi, Shahad, Haja Tar, Abrar S. Alnafisah, Lotfi Beji, Noura Kouki, Fabrice Morlet-Savary, Fahad M. Alminderej, Lotfi M. Aroua, and Jacques Lalevée. 2023. "Photochemical Synthesis of Noble Metal Nanoparticles: Influence of Metal Salt Concentration on Size and Distribution" International Journal of Molecular Sciences 24, no. 18: 14018. https://doi.org/10.3390/ijms241814018
APA StyleM. Aldebasi, S., Tar, H., S. Alnafisah, A., Beji, L., Kouki, N., Morlet-Savary, F., Alminderej, F. M., Aroua, L. M., & Lalevée, J. (2023). Photochemical Synthesis of Noble Metal Nanoparticles: Influence of Metal Salt Concentration on Size and Distribution. International Journal of Molecular Sciences, 24(18), 14018. https://doi.org/10.3390/ijms241814018







