Pregnancy by Oocyte Donation: Reviewing Fetal–Maternal Risks and Complications
Abstract
1. Introduction
2. Egg Donation in Europe and Worldwide
3. Major Risky Events for Fetus in IVF-Assisted Pregnancy
3.1. IVF and Recurrent Miscarriages
3.2. IVF and Fetal–Maternal Risks
4. Recurrent Complications in IVF Pregnancies by Oocyte Donation
4.1. Alterations due to Manipulation of Gametes including Epigenetic Derangements
4.2. Abnormal Embryo Implantation
4.3. Latent Endometritis
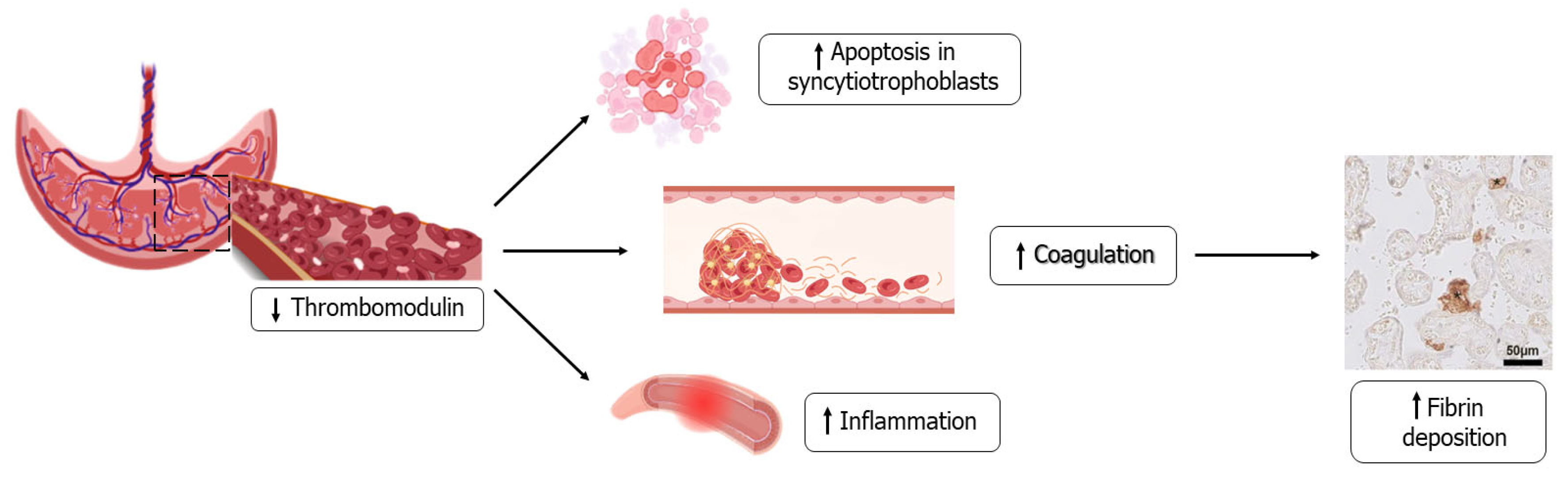
4.4. Role of Abnormal Placentation
5. Immune System Interaction and Deregulation
6. Natural Protection for Heterologous Implantation
6.1. Endocrine Function of the Corpus Luteum in Implantation, Placentation and the Risk of PE
6.2. The Angiogenic Factors
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Infertility. 2020. Available online: www.who.int/news-room/fact-sheets/detail/infertility (accessed on 8 July 2022).
- Silvestris, E.; D’Oronzo, S.; Cafforio, P.; D’Amato, G.; Loverro, G. Perspective in infertility: The ovarian stem cells. J. Ovarian Res. 2015, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C. Towards the global coverage of a unified registry of IVF outcomes. Reprod. Biomed. Online 2019, 38, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, M. The Consequences of In Vitro Fertilization Treatment. J. Reprod. Health Contracept 2022, 7, 152. [Google Scholar] [CrossRef]
- Spandorfer, S.D.; Bendikson, K.; Dragisic, K.; Schattman, G.; Davis, O.K.; Rosenwaks, Z. Outcome of in vitro fertilization in women 45 years and older who use autologous oocytes. Fertil. Steril. 2007, 87, 74–76. [Google Scholar] [CrossRef]
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; Goossens, V. European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE), ART in Europe, 2018: Results generated from European registries by ESHRE. Hum. Reprod. Open 2022, 3, hoac022. [Google Scholar] [CrossRef]
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2014: Results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. 2018, 33, 1586–1601. [Google Scholar] [CrossRef]
- Calhaz-Jorge, C.; De Geyter, C.h.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. Survey on ART and IUI: Legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. Open 2020, 1, hoz044. [Google Scholar] [CrossRef]
- Jain, M.; Singh, M. Assisted Reproductive Technology (ART) Techniques. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pellicer, A.; Rubio, C.; Vidal, F.; Mínguez, Y.; Giménez, C.; Egozcue, J.; Remohí, J.; Simón, C. In vitro fertilization plus preimplantation genetic diagnosis in patients with recurrent miscarriage: An analysis of chromosome abnormalities in human preimplantation embryos. Fertil. Steril. 1999, 71, 1033–1039. [Google Scholar] [CrossRef]
- Simón, C.; Rubio, C.; Vidal, F.; Gimenez, C.; Moreno, C.; Parrilla, J.J.; Pellicer, A. Increased chromosome abnormalities in human preimplantation embryos after in-vitro fertilization in patients with recurrent miscarriage. Reprod. Fertil. Dev. 1998, 10, 87–92. [Google Scholar] [CrossRef]
- Sacks, G.; Yang, Y.; Gowen, E.; Smith, S.; Fay, L.; Chapman, M. Detailed analysis of peripheral blood natural killer cells in women with repeated IVF failure. Am. J. Reprod. Immunol. 2012, 67, 434–442. [Google Scholar] [CrossRef]
- Sugiura-Ogasawara, M.; Nozawa, K.; Nakanishi, T.; Hattori, Y.; Ozaki, Y. Complement as a predictor of further miscarriage in couples with recurrent miscarriages. Hum. Reprod. 2006, 21, 2711–2714. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Chamley, L.; Hale, L.; Kloss, M.; Speirs, A.; Baker, H.W. Antibodies to beta2 glycoprotein I are associated with in vitro fertilization implantation failure as well as recurrent miscarriage: Results of a prevalence study. Fertil. Steril. 1998, 70, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Lykke, J.A.; Bare, L.A.; Olsen, J.; Lagier, R.; Arellano, A.R.; Tong, C.; Paidas, M.J.; Langhoff-Roos, J. Thrombophilias and adverse pregnancy outcomes: Results from the Danish National Birth Cohort. J. Thromb. Haemost. 2012, 10, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Laufer, N. Repeated implantation failure: Clinical approach. Fertil. Steril. 2012, 97, 1039–1043. [Google Scholar] [CrossRef]
- Vitagliano, A.; Paffoni, A.; Viganò, P. Does maternal age affect assisted reproduction technology success rates after euploid embryo transfer? A systematic review and meta-analysis. Fertil. Steril. 2023, 210, 251–265. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef]
- Vitagliano, A.; Saccardi, C.; Litta, P.S.; Noventa, M. Chronic endometritis: Really so relevant in repeated IVF failure? Am. J. Reprod. Immunol. 2017, 78, 6. [Google Scholar] [CrossRef]
- Bilibio, J.P.; Gama, T.B.; Nascimento, I.C.M.; Meireles, A.J.C.; de Aguiar, A.S.C.; do Nascimento, F.C.; Lorenzzoni, P.L. Causes of recurrent miscarriage after spontaneous pregnancy and after in vitro fertilization. Am. J. Reprod. Immunol. 2020, 83, e13226. [Google Scholar] [CrossRef]
- Ou, J.; Wang, W.; Feng, T.; Liao, L.; Meng, Q.; Zou, Q.; Ding, J.; Zheng, A.; Duan, C.; Li, P. Identification of small segmental translocations in patients with repeated implantation failure and recurrent miscarriage using next generation sequencing after in vitro fertilization/intracytoplasmic sperm injection. Mol. Cytogenet. 2015, 8, 105. [Google Scholar] [CrossRef]
- Friedenthal, J.; Maxwell, S.M.; Munné, S.; Kramer, Y.; McCulloh, D.H.; McCaffrey, C.; Grifo, J.A. Next generation sequencing for preimplantation genetic screening improves pregnancy outcomes compared with array comparative genomic hybridization in single thawed euploid embryo transfer cycles. Fertil. Steril. 2018, 109, 627–632. [Google Scholar] [CrossRef]
- Sewell, W.A.; Jolles, S. Immunomodulatory action of intravenous immunoglobulin. Immunology 2002, 107, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.F.; LaCoursiere, Y.; Scott, J.R. Immunotherapy for recurrent miscarriage. Cochrane Database Syst. Rev. 2006, CD000112. [Google Scholar] [CrossRef]
- Ata, B.; Tan, S.L.; Shehata, F.; Holzer, H.; Buckett, W. A systematic review of intravenous immunoglobulin for treatment of unexplained recurrent miscarriage. Fertil. Steril. 2011, 95, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Virro, M.R.; Winger, E.E.; Reed, J.L. Intravenous immunoglobulin for repeated IVF failure and unexplained infertility. Am. J. Reprod. Immunol. 2012, 68, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, C.M.; Keay, S.D.; Macklon, N.S. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst. Rev. 2012, 6, CD005996. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, K.M.; Kolte, A.M.; Larsen, E.C.; Christiansen, O.B. Immunomodulatory treatment with intravenous immunoglobulin and prednisone in patients with recurrent miscarriage and implantation failure after in vitro fertilization/intracytoplasmic sperm injection. Fertil. Steril. 2014, 102, 1650–1655. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Liu, Y.; Zhang, R.; Wu, Y.; Huang, Y.; Liu, F.; Li, M.; Sun, S.; Xing, L.; et al. Maternal and Live-birth Outcomes of Pregnancies following Assisted Reproductive Technology: A Retrospective Cohort Study. Sci. Rep. 2016, 6, 35141. [Google Scholar] [CrossRef]
- Oberg, A.S.; VanderWeele, T.J.; Almqvist, C.; Hernandez-Diaz, S. Pregnancy complications following fertility treatment-disentangling the role of multiple gestation. Int. J. Epidemiol. 2018, 47, 1333–1342. [Google Scholar] [CrossRef]
- Johnson, K.M.; Hacker, M.R.; Thornton, K.; Young, B.C.; Modest, A.M. Association between in vitro fertilization and ischemic placental disease by gestational age. Fertil. Steril. 2020, 114, 579–586. [Google Scholar] [CrossRef]
- Roberts, J.M. Pathophysiology of ischemic placental disease. Semin. Perinatol. 2014, 38, 139–145. [Google Scholar] [CrossRef]
- Keukens, A.; van Wely, M.; van der Meulen, C.; Mochtar, M.H. Pre-eclampsia in pregnancies resulting from oocyte donation, natural conception or IVF: A systematic review and meta-analysis. Hum. Reprod. 2022, 37, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, C.; Salamalekis, G.; Kintis, K.; Andrianopoulou, I.; Michalopoulou, H.; Skalis, G.; Archontakis, S.; Argyri, O.; Tsioufis, C.; Makris, T.K.; et al. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: Overview and meta-analysis. J. Clin. Hypertens. 2017, 19, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Almasi-Hashiani, A.; Omani-Samani, R.; Mohammadi, M.; Amini, P.; Navid, B.; Alizadeh, A.; Khedmati Morasae, E.; Maroufizadeh, S. Assisted reproductive technology and the risk of preeclampsia: An updated systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, X.; Sheng, X.; Wang, H.; Gao, S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: A meta-analysis of cohort studies. Fertil. Steril. 2016, 105, 73–85.e6. [Google Scholar] [CrossRef] [PubMed]
- Okun, N.; Sierra, S. Pregnancy outcomes after assisted human reproduction. J. Obstet. Gynaecol. Can. 2014, 36, 64–83. [Google Scholar] [CrossRef]
- Roque, M.; Valle, M.; Sampaio, M.; Geber, S. Obstetric outcomes after fresh versus frozen-thawed embryo transfers: A systematic review and meta-analysis. JBRA Assist. Reprod. 2018, 22, 253–260. [Google Scholar] [CrossRef]
- Chih, H.J.; Elias, F.T.S.; Gaudet, L.; Velez, M.P. Assisted reproductive technology and hypertensive disorders of pregnancy: Systematic review and meta-analyses. BMC Pregnancy Childbirth 2021, 21, 449. [Google Scholar] [CrossRef]
- Bulletins-Obstetrics, C. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol. 2013, 122 Pt 1, 406–416. [Google Scholar]
- Ashrafi, M.; Gosili, R.; Hosseini, R.; Arabipoor, A.; Ahmadi, J.; Chehrazi, M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 176, 149–152. [Google Scholar] [CrossRef]
- Pandey, S.; Shetty, A.; Hamilton, M.; Bhattacharya, S.; Maheshwari, A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 485–503. [Google Scholar] [CrossRef]
- Marino, J.L.; Moore, V.M.; Willson, K.J.; Rumbold, A.; Whitrow, M.J.; Giles, L.C.; Davies, M.J. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS ONE 2014, 9, e80398. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.K.; Ratcliffe, S.J.; Coutifaris, C.; Molinaro, T.; Barnhart, K.T. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet. Gynecol. 2011, 118, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Seggers, J.; Pontesilli, M.; Ravelli, A.C.J.; Painter, R.C.; Hadders-Algra, M.; Heineman, M.J.; Repping, S.; Mol, B.W.J.; Roseboom, T.J.; Ensing, S. Effects of in vitro fertilization and maternal characteristics on perinatal outcomes: A population-based study using siblings. Fertil. Steril. 2016, 105, 590–598.e2. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Bower, C.; Milne, E.; de Klerk, N.; Kurinczuk, J.J. Assisted reproductive technologies and the risk of birth defects--a systematic review. Hum. Reprod. 2005, 20, 328–338. [Google Scholar] [CrossRef]
- Davies, M.J.; Moore, V.M.; Willson, K.J.; Van Essen, P.; Priest, K.; Scott, H.; Haan, E.A.; Chan, A. Reproductive technologies and the risk of birth defects. New Engl. J. Med. 2012, 366, 1803–1813. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Q.; Wang, Y.; Wang, B.; Lyu, Q.; Kuang, Y. Comparative study on risk for birth defects among infants after in vitro fertilization and intracytoplasmic sperm injection. Syst. Biol. Reprod. Med. 2019, 65, 54–60. [Google Scholar] [CrossRef]
- Tararbit, K.; Lelong, N.; Thieulin, A.C.; Houyel, L.; Bonnet, D.; Goffinet, F.; Khoshnood, B. The risk for four specific congenital heart defects associated with assisted reproductive techniques: A population-based evaluation. Hum. Reprod. 2013, 28, 367–374. [Google Scholar] [CrossRef]
- Talebi, T.; Mohsen-Pour, N.; Hesami, M.; Maleki, M.; Kalayinia, S. The association between in vitro fertilization and intracytoplasmic sperm injection treatment and the risk of congenital heart defects. J. Matern. Fetal Neonatal Med. 2022, 35, 7471–7485. [Google Scholar] [CrossRef]
- Giorgione, V.; Parazzini, F.; Fesslova, V.; Cipriani, S.; Candiani, M.; Inversetti, A.; Sigismondi, C.; Tiberio, F.; Cavoretto, P. Congenital heart defects in IVF/ICSI pregnancy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 33–42. [Google Scholar] [CrossRef]
- Vermeiden, J.P.; Bernardus, R.E. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil. Steril. 2013, 99, 642–651. [Google Scholar] [CrossRef]
- Strömberg, B.; Dahlquist, G.; Ericson, A.; Finnström, O.; Köster, M.; Stjernqvist, K. Neurological sequelae in children born after in-vitro fertilisation: A population-based study. Lancet 2002, 359, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Pontesilli, M.; Painter, R.C.; Grooten, I.J.; van der Post, J.A.; Mol, B.W.; Vrijkotte, T.G.; Repping, S.; Roseboom, T.J. Subfertility and assisted reproduction techniques are associated with poorer cardiometabolic profiles in childhood. Reprod. Biomed. Online 2015, 30, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ghosh, J.; Mainigi, M.; Turan, N.; Weinerman, R.; Truongcao, M.; Coutifaris, C.; Sapienza, C. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clin. Epigenetics 2015, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; D’Amato, G.; Caroppo, E.; Ménézo, Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: Mechanisms, clinical results, insights on gene-environment interactions and the role of diet. J. Assist. Reprod. Genet. 2016, 33, 1633–1648. [Google Scholar] [CrossRef] [PubMed]
- Donkena, K.V.; Young, C.Y.; Tindall, D.J. Oxidative stress and DNA methylation in prostate cancer. Obstet. Gynecol. Int. 2010, 2010, 302051. [Google Scholar] [CrossRef]
- Maltseva, D.V.; Baykov, A.A.; Jeltsch, A.; Gromova, E.S. Impact of 7,8-dihydro-8-oxoguanine on methylation of the CpG site by Dnmt3a. Biochemistry 2009, 48, 1361–1368. [Google Scholar] [CrossRef]
- Menezo, Y.; Khatchadourian, C.; Gharib, A.; Hamidi, J.; Greenland, T.; Sarda, N. Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sci. 1989, 44, 1601–1609. [Google Scholar] [CrossRef]
- Huffman, S.R.; Pak, Y.; Rivera, R.M. Superovulation induces alterations in the epigenome of zygotes, and results in differences in gene expression at the blastocyst stage in mice. Mol. Reprod. Dev. 2015, 83, 207–217. [Google Scholar] [CrossRef]
- Evenson, D.P.; Djira, G.; Kasperson, K.; Christianson, J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil. Steril. 2020, 114, 311–320. [Google Scholar] [CrossRef]
- Van der Hoorn, M.L.; Lashley, E.E.; Bianchi, D.W.; Claas, F.H.; Schonkeren, C.M.; Scherjon, S.A. Clinical and immunologic aspects of egg donation pregnancies: A systematic review. Hum. Reprod. Update 2010, 16, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, V.P.; Tumanova, L.E.; Sudoma, I.A.; Bannikov, V.I. Th1 and Th2 in human IVF pregnancy with allogenic fetus. Am. J. Reprod. Immunol. 2008, 59, 352–358. [Google Scholar] [CrossRef]
- Von Woon, E.; Greer, O.; Shah, N.; Nikolau, D.; Johnson, M.; Male, V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 548–582. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, N.; Bocking, A.; Machin, G.; Rizek, R.; Watson, C.; Keating, S. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr. Dev. Pathol. 2009, 12, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Boog, G. Chronic villitis of unknown etiology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.C.; Predanic, M.; Cho, J.E.; Baergen, R.N. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J. Perinat. Med. 2005, 33, 186. [Google Scholar] [CrossRef]
- Huppertz, B.; Kadyrov, M.; Kingdom, J.C. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 2006, 195, 29–39. [Google Scholar] [CrossRef]
- Allaire, A.D.; Ballenger, K.A.; Wells, S.R.; McMahon, M.J.; Lessey, B.A. Placental apoptosis in preeclampsia. Obstet. Gynecol. 2000, 96, 271–276. [Google Scholar] [CrossRef]
- Ishihara, N.; Matsuo, H.; Murakoshi, H.; Laoag-Fernandez, J.B.; Samoto, T.; Maruo, T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002, 186, 158–166. [Google Scholar] [CrossRef]
- Minakami, H.; Takahashi, T.; Izumi, A.; Tamada, T. Increased levels of plasma thrombomodulin in preeclampsia. Gynecol. Obstet. Investig. 1993, 36, 208–210. [Google Scholar] [CrossRef]
- Conway, E.M. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012, 34, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Bloemenkamp, K.W.; Bruijn, J.A.; Baelde, H.J. Loss of Thrombomodulin in Placental Dysfunction in Preeclampsia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 728–735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bos, M.; Baelde, H.J.; Bruijn, J.A.; Bloemenkamp, K.V.M.; van der Hoorn, M.P.; Turner, R.J. Loss of placental thrombomodulin in oocyte donation pregnancies. Fertil. Steril. 2017, 107, 119–129.e5. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nakabayashi, Y.; Nakashima, A.; Shima, T.; Yoshino, O. A new era in reproductive medicine: Consequences of third-party oocyte donation for maternal and fetal health. Semin Immunopathol. 2016, 38, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Papúchová, H.; Meissner, T.B.; Li, Q.; Strominger, J.L.; Tilburgs, T. The Dual Role of HLA-C in Tolerance and Immunity at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2730. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Barrio, A.; Garrido, N.; Aparicio, P.; Pellicer, A.; Moffett, A.; García-Velasco, J.A. Parental human leukocyte antigen-C allotypes are predictive of live birth rate and risk of poor placentation in assisted reproductive treatment. Fertil. Steril. 2020, 114, 809–817. [Google Scholar] [CrossRef]
- Tilburgs, T.; Scherjon, S.A.; van der Mast, B.J.; Haasnoot, G.W.; Versteeg-V D Voort-Maarschalk, M.; Roelen, D.L.; van Rood, J.J.; Claas, F.H. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 2009, 82, 148–157. [Google Scholar] [CrossRef]
- Shima, T.; Sasaki, Y.; Itoh, M.; Nakashima, A.; Ishii, N.; Sugamura, K.; Saito, S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010, 85, 121–129. [Google Scholar] [CrossRef]
- van Bentem, K.; Bos, M.; van der Keur, C.; Brand-Schaaf, S.H.; Haasnoot, G.W.; Roelen, D.L.; Eikmans, M.; Heidt, S.; Claas, F.H.J.; Lashley, E.E.L.O.; et al. The development of preeclampsia in oocyte donation pregnancies is related to the number of fetal-maternal HLA class II mismatches. J. Reprod. Immunol. 2020, 137, 103074. [Google Scholar] [CrossRef]
- Alecsandru, D.; Garrido, N.; Vicario, J.L.; Barrio, A.; Aparicio, P.; Requena, A.; García-Velasco, J.A. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum. Reprod. 2014, 29, 2637–2643. [Google Scholar] [CrossRef]
- Lashley, L.E.; Buurma, A.; Swings, G.M.; Eikmans, M.; Anholts, J.D.; Bakker, J.A.; Claas, F.H. Preeclampsia in autologous and oocyte donation pregnancy: Is there a different pathophysiology? J. Reprod. Immunol. 2015, 109, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schonkeren, D.; Swings, G.; Roberts, D.; Claas, F.; de Heer, E.; Scherjon, S. Pregnancy close to the edge: An immunosuppressive infiltrate in the chorionic plate of placentas from uncomplicated egg cell donation. PLoS ONE 2012, 7, e32347. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, M.; Sfakianoudis, K.; Maziotis, E.; Grigoriadis, S.; Giannelou, P.; Rapani, A.; Tsioulou, P.; Pantou, A.; Kalampokas, T.; Vlahos, N.; et al. The Impact of Autoantibodies on IVF Treatment and Outcome: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 892. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P. Maternal vasodilation in pregnancy: The emerging role of relaxin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R267–R275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 2013, 34, 939–980. [Google Scholar] [CrossRef]
- Young, S.L. Oestrogen and progesterone action on endometrium: A translational approach to understanding endometrial receptivity. Reprod. Biomed. Online 2013, 27, 497–505. [Google Scholar] [CrossRef]
- Hervé, M.A.; Meduri, G.; Petit, F.G.; Domet, T.S.; Lazennec, G.; Mourah, S.; Perrot-Applanat, M. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J. Endocrinol. 2006, 188, 91–99. [Google Scholar] [CrossRef][Green Version]
- Pereira, M.M.; Mainigi, M.; Strauss, J.F. Secretory products of the corpus luteum and preeclampsia. Hum. Reprod. Update 2021, 27, 651–672. [Google Scholar] [CrossRef]
- Berkane, N.; Liere, P.; Oudinet, J.P.; Hertig, A.; Lefèvre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef]
- Henríquez, S.; Kohen, P.; Xu, X.; Veenstra, T.D.; Muñoz, A.; Palomino, W.A.; Strauss, J.F., 3rd; Devoto, L. Estrogen metabolites in human corpus luteum physiology: Differential effects on angiogenic activity. Fertil. Steril. 2016, 106, 230–237.e1. [Google Scholar] [CrossRef]
- Jobe, S.O.; Tyler, C.T.; Magness, R.R. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013, 61, 480–487. [Google Scholar] [CrossRef]
- Poch, A.; Villanelo, F.; Henriquez, S.; Kohen, P.; Muñoz, A.; Strauss, J.F., 3rd; Devoto, L. Molecular modelling predicts that 2-methoxyestradiol disrupts HIF function by binding to the PAS-B domain. Steroids 2019, 144, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Wong, A.P.; Kanasaki, K.; Xu, Y.; Shenoy, V.K.; McElrath, T.F.; Whitesides, G.M.; Kalluri, R. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am. J. Pathol. 2010, 176, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Pertegal, M.; Fenoy, F.J.; Bonacasa, B.; Mendiola, J.; Delgado, J.L.; Hernández, M.; Salom, M.G.; Bosch, V.; Hernández, I. 2-methoxyestradiol plasma levels are associated with clinical severity indices and biomarkers of preeclampsia. Reprod. Sci. 2015, 22, 198–206. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestris, E.; Petracca, E.A.; Mongelli, M.; Arezzo, F.; Loizzi, V.; Gaetani, M.; Nicolì, P.; Damiani, G.R.; Cormio, G. Pregnancy by Oocyte Donation: Reviewing Fetal–Maternal Risks and Complications. Int. J. Mol. Sci. 2023, 24, 13945. https://doi.org/10.3390/ijms241813945
Silvestris E, Petracca EA, Mongelli M, Arezzo F, Loizzi V, Gaetani M, Nicolì P, Damiani GR, Cormio G. Pregnancy by Oocyte Donation: Reviewing Fetal–Maternal Risks and Complications. International Journal of Molecular Sciences. 2023; 24(18):13945. https://doi.org/10.3390/ijms241813945
Chicago/Turabian StyleSilvestris, Erica, Easter Anna Petracca, Michele Mongelli, Francesca Arezzo, Vera Loizzi, Maria Gaetani, Pierpaolo Nicolì, Gianluca Raffaello Damiani, and Gennaro Cormio. 2023. "Pregnancy by Oocyte Donation: Reviewing Fetal–Maternal Risks and Complications" International Journal of Molecular Sciences 24, no. 18: 13945. https://doi.org/10.3390/ijms241813945
APA StyleSilvestris, E., Petracca, E. A., Mongelli, M., Arezzo, F., Loizzi, V., Gaetani, M., Nicolì, P., Damiani, G. R., & Cormio, G. (2023). Pregnancy by Oocyte Donation: Reviewing Fetal–Maternal Risks and Complications. International Journal of Molecular Sciences, 24(18), 13945. https://doi.org/10.3390/ijms241813945










