Novel Long Non-Coding RNA (lncRNA) Transcript AL137782.1 Promotes the Migration of Normal Lung Epithelial Cells through Positively Regulating LMO7
Abstract
:1. Introduction
2. Results
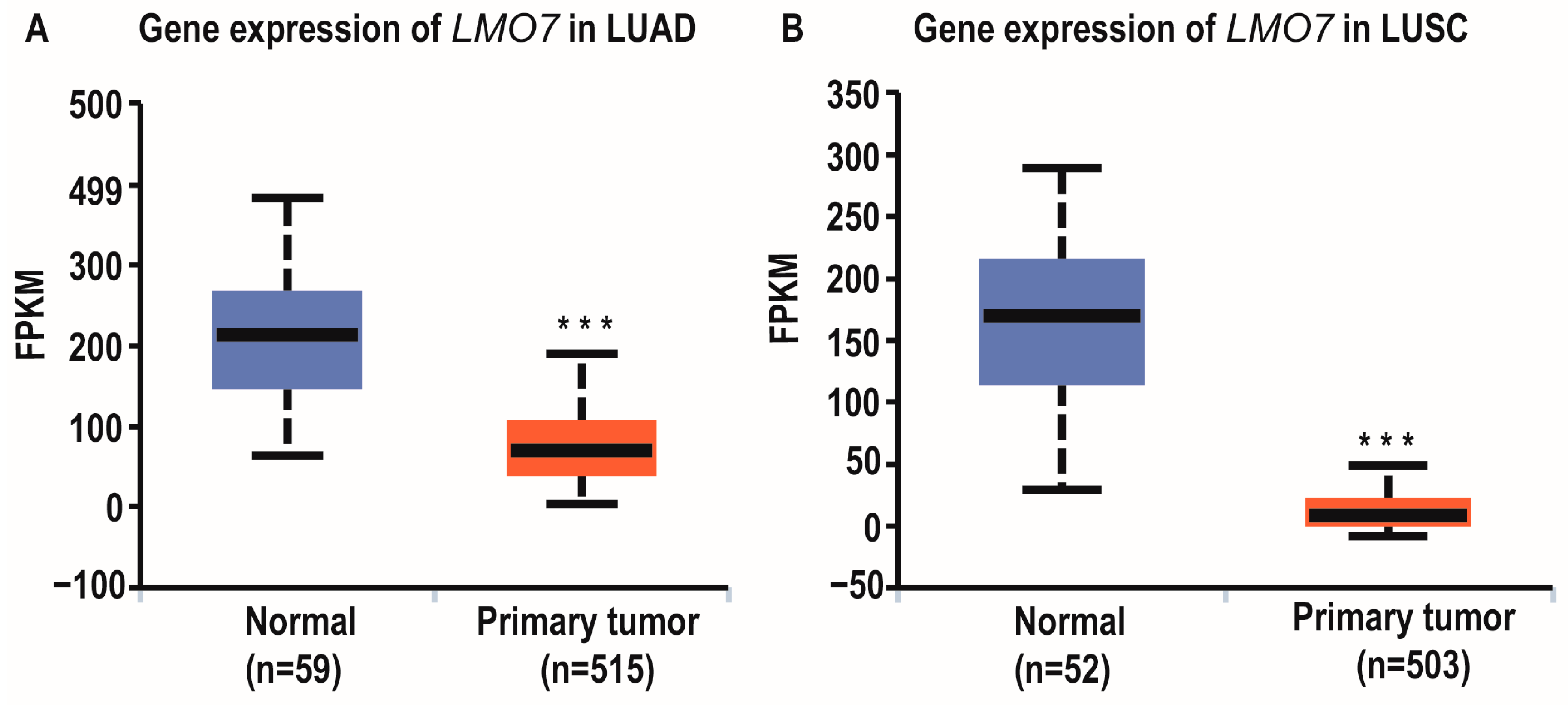
2.1. LncRNA AL137782.1 Highly Expressed in Para-Cancerous Samples Than Lung Adenocarcinoma
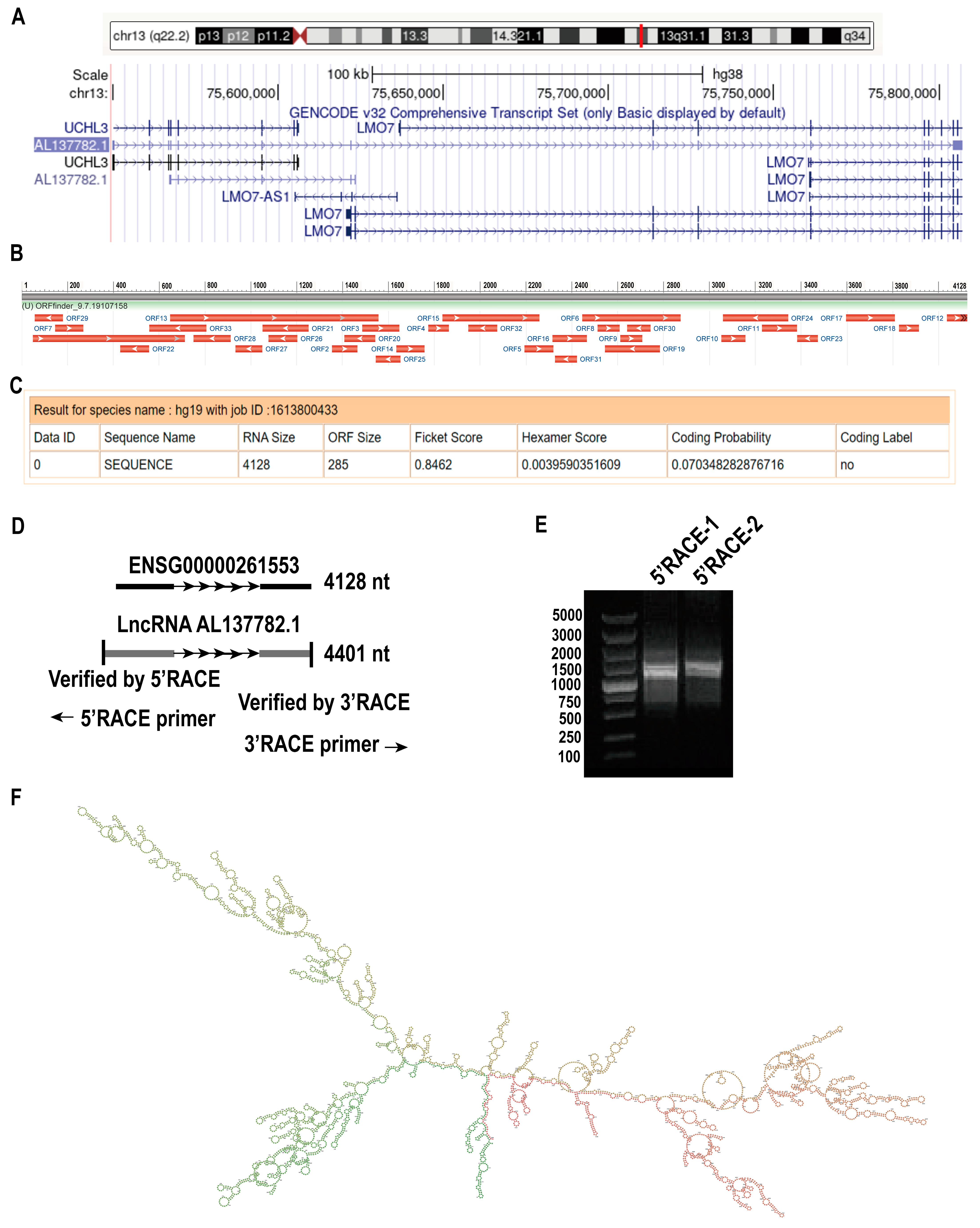
2.2. The Profile of lncRNA AL137782.1 in Human Bronchial Epithelial Cells and Type II Alveolar Epithelial Cell
2.3. AL137782.1 Is Located Both in Nucleus and Cytoplasm of Lung Epithelial Cells
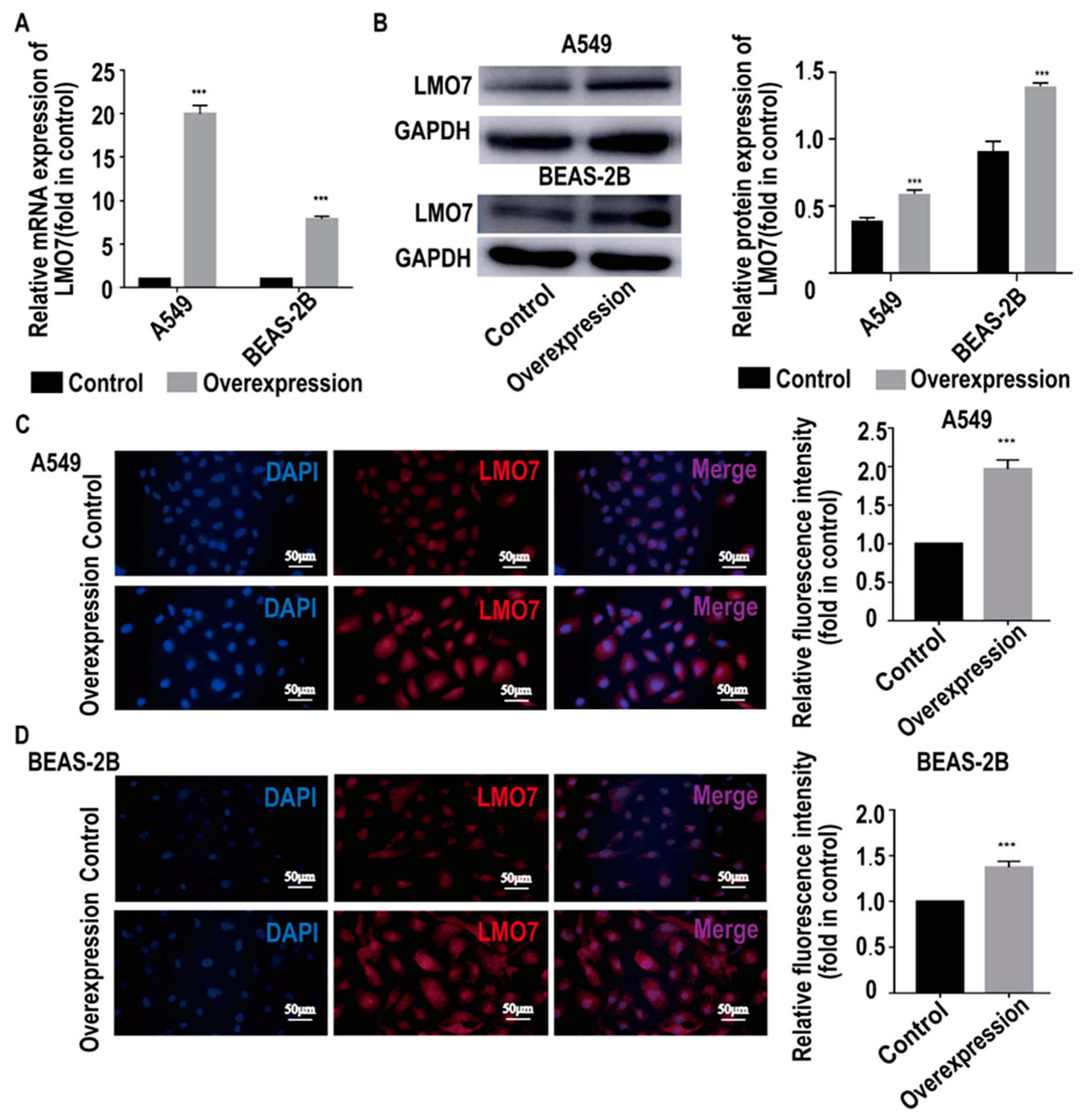
2.4. AL137782.1 Is Co-Expressed with the mRNA Expression of LMO7 in Lung Epithelial Cells
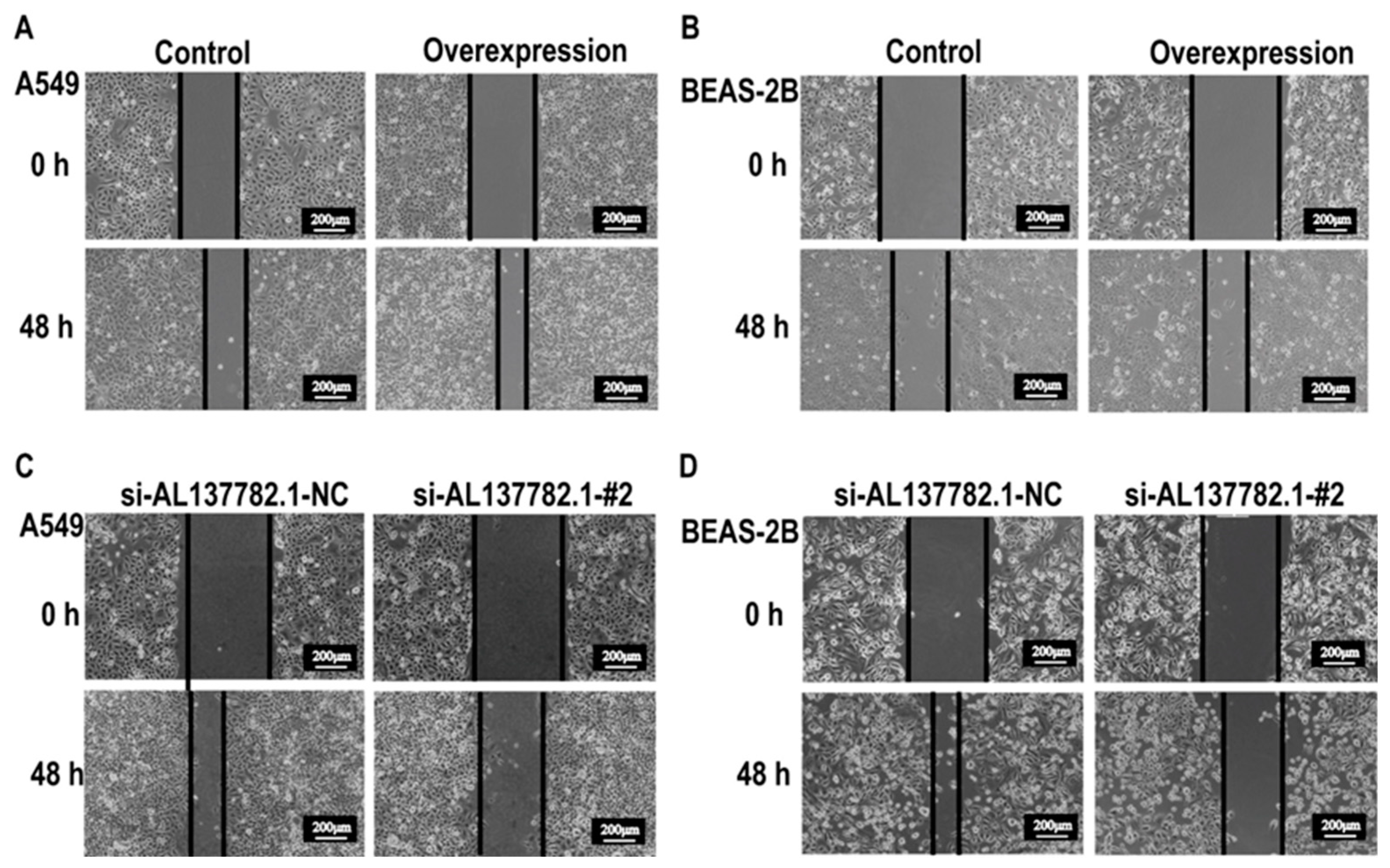
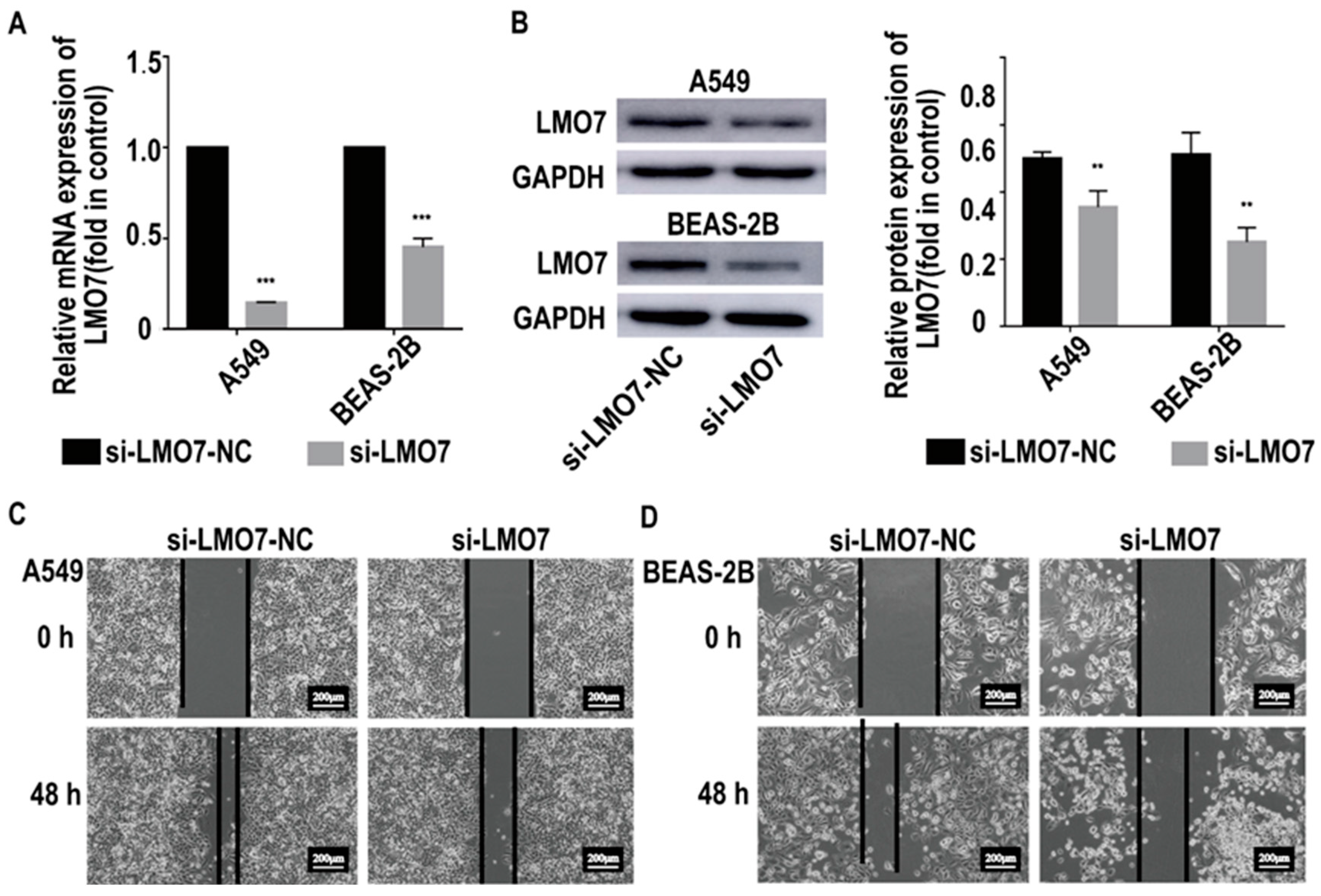
2.5. AL137782.1 Regulates the Migration of Lung Epithelial Cells though LMO7
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Overexpression of AL137782.1 by Recombinant Adenovirus
4.3. Rapid-Amplification of cDNA Ends (RACE)
4.4. Fluorescence In Situ Hybridization (FISH)
4.5. RNA Extraction and Real-Time PCR
4.6. Subcellular Fractionation
4.7. Transcriptome Sequence
4.8. Western Blot
4.9. RNA Knockdown by Small Interfering RNA
4.10. Wound Healing Assay
4.11. Databases and Bioinformatics Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, N.; Toba, H.; Miyoshi, K.; Sakamoto, S.; Matsumoto, D.; Takashima, M.; Aoyama, M.; Inoue, S.; Morimoto, M.; Nishino, T.; et al. Bronchioalveolar stem cells derived from mouse-induced pluripotent stem cells promote airway epithelium regeneration. Stem Cell Res. Ther. 2020, 11, 430. [Google Scholar] [CrossRef]
- Bhargava, M.; Dey, S.; Becker, T.; Steinbach, M.; Wu, B.; Lee, S.M.; Higgins, L.; Kumar, V.; Bitterman, P.B.; Ingbar, D.H.; et al. Protein expression profile of rat type two alveolar epithelial cells during hyperoxic stress and recovery. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L604–L614. [Google Scholar] [CrossRef]
- Becker, P.M.; Tran, T.S.; Delannoy, M.J.; He, C.; Shannon, J.M.; McGrath-Morrow, S. Semaphorin 3A Contributes to Distal Pulmonary Epithelial Cell Differentiation and Lung Morphogenesis. PLoS ONE 2011, 6, e27449. [Google Scholar] [CrossRef]
- Jansing, N.L.; McClendon, J.; Henson, P.M.; Tuder, R.M.; Hyde, D.M.; Zemans, R.L. Unbiased Quantitation of Alveolar Type II to Alveolar Type I Cell Transdifferentiation during Repair after Lung Injury in Mice. Am. J. Respir. Cell Mol. Biol. 2017, 57, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and Functions of Long Noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Guttman, M.; Russell, P.; Ingolia, N.T.; Weissman, J.S.; Lander, E.S. Ribosome Profiling Provides Evidence that Large Noncoding RNAs Do Not Encode Proteins. Cell 2013, 154, 240–251. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, J.; Luan, Y. LncRNA-Encoded Short Peptides Identification Using Feature Subset Recombination and Ensemble Learning. Interdiscip. Sci. Comput. Life Sci. 2022, 14, 101–112. [Google Scholar] [CrossRef]
- Xing, J.; Liu, H.; Jiang, W.; Wang, L. LncRNA-Encoded Peptide: Functions and Predicting Methods. Front. Oncol. 2020, 10, 622294. [Google Scholar] [CrossRef]
- Hou, M.; Wu, N.; Yao, L. LncRNA CBR3-AS1 potentiates Wnt/β-catenin signaling to regulate lung adenocarcinoma cells proliferation, migration and invasion. Cancer Cell Int. 2021, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, X.; Wang, Y.; Zhang, J. Up-Regulation of FSTL3, Regulated by lncRNA DSCAM-AS1/miR-122-5p Axis, Promotes Proliferation and Migration of Non-Small Cell Lung Cancer Cells. OncoTargets Ther. 2020, 13, 2725–2738. [Google Scholar] [CrossRef]
- Gokey, J.J.; Snowball, J.; Sridharan, A.; Speth, J.P.; Black, K.E.; Hariri, L.P.; Perl, A.-K.T.; Xu, Y.; Whitsett, J.A. MEG3 is increased in idiopathic pulmonary fibrosis and regulates epithelial cell differentiation. JCI Insight 2018, 3, e122490. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Cai, R.; Wang, G.; Shu, X.; Pang, W. Long noncoding RNA: Multiple players in gene expression. BMB Rep. 2018, 51, 280–289. [Google Scholar] [CrossRef]
- Horabin, J.I. Long noncoding RNAs as metazoan developmental regulators. Chromosom. Res. 2013, 21, 673–684. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Chen, F.; Li, X.; Ma, J.; Ma, X.; Zhao, Y.; Li, M. Effect of Mycoplasma ovipneumoniae on the activity and inflammatory response of human alveolar type II epithelial cells. Chin. J. Prev. Vet. Med. 2020, 42, 1051–1057. [Google Scholar]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Li, Y.; Wu, Y.; Ma, H.; Jiang, X.; Fu, L.; Zhang, G.; Wang, H.; Liu, X.; et al. Constructing a novel signature and predicting the immune landscape of colon cancer using N6-methylandenosine-related lncRNAs. Front. Genet. 2023, 14, 906346. [Google Scholar] [CrossRef]
- Lagarde, J.; Uszczynska-Ratajczak, B.; Santoyo-Lopez, J.; Gonzalez, J.M.; Tapanari, E.; Mudge, J.M.; Steward, C.A.; Wilming, L.; Tanzer, A.; Howald, C.; et al. Extension of human lncRNA transcripts by RACE coupled with long-read high-throughput sequencing (RACE-Seq). Nat. Commun. 2016, 7, 12339. [Google Scholar] [CrossRef]
- Nishikawa, M.; Yuri, S.; Kimura, H.; Yanagawa, N.; Hamon, M.; Hauser, P.; Zhao, L.; Jo, O.D.; Yanagawa, N. Comprehensive analysis of chromatin signature and transcriptome uncovers functional lncRNAs expressed in nephron progenitor cells. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2019, 1862, 58–70. [Google Scholar] [CrossRef]
- Pastori, C.; Velmeshev, D.; Peschansky, V.J. Deep-RACE: Comprehensive Search for Novel ncRNAs Associated to a Specific Locus. Methods Mol. Biol. 2017, 1543, 129–143. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Ji, L.; Kuang, L.; Yu, Z.; Li, H.; Zhang, J.; Zhao, J. LncRNA-MALAT1/miRNA-204-5p/Smad4 Axis Regulates Epithelial–Mesenchymal Transition, Proliferation and Migration of Lens Epithelial Cells. Curr. Eye Res. 2020, 46, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; You, J.; Wei, W.; Gu, J.; Xu, C.; Gu, X. Downregulated lncRNA RCPCD promotes differentiation of embryonic stem cells into cardiac pacemaker-like cells by suppressing HCN4 promoter methylation. Cell Death Dis. 2021, 12, 667. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef]
- Capitanchik, C.; Dixon, C.R.; Swanson, S.K.; Florens, L.; Kerr, A.R.W.; Schirmer, E.C. Analysis of RNA-Seq datasets reveals enrichment of tissue-specific splice variants for nuclear envelope proteins. Nucleus 2018, 9, 410–430. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, J.; Mei, S.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J. Cell. Mol. Med. 2017, 21, 1228–1236. [Google Scholar] [CrossRef]
- Lao, D.H.; Esparza, M.C.; Bremner, S.N.; Banerjee, I.; Zhang, J.; Veevers, J.; Bradford, W.H.; Gu, Y.; Dalton, N.D.; Knowlton, K.U.; et al. Lmo7 is dispensable for skeletal muscle and cardiac function. Am. J. Physiol. Physiol. 2015, 309, C470–C479. [Google Scholar] [CrossRef]
- Hu, Q.; Guo, C.; Li, Y.; Aronow, B.J.; Zhang, J. LMO7 Mediates Cell-Specific Activation of the Rho-Myocardin-Related Transcription Factor-Serum Response Factor Pathway and Plays an Important Role in Breast Cancer Cell Migration. Mol. Cell. Biol. 2011, 31, 3223–3240. [Google Scholar] [CrossRef]
- Karlsson, T.; Kvarnbrink, S.; Holmlund, C.; Botling, J.; Micke, P.; Henriksson, R.; Johansson, M.; Hedman, H. LMO7 and LIMCH1 interact with LRIG proteins in lung cancer, with prognostic implications for early-stage disease. Lung Cancer 2018, 125, 174–184. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y. LRIG1 acts as a critical regulator of melanoma cell invasion, migration, and vasculogenic mimicry upon hypoxia by regulating EGFR/ERK-triggered epithelial–mesenchymal transition. Biosci. Rep. 2019, 39, BSR20181165. [Google Scholar] [CrossRef]
- Yu, S.; Yang, M.; Lim, K.-M.; Cho, Y.; Kim, H.; Lee, K.; Jeong, S.-H.; Coffey, R.J.; Goldenring, J.R.; Nam, K.T. Expression of LRIG1, a Negative Regulator of EGFR, Is Dynamically Altered during Different Stages of Gastric Carcinogenesis. Am. J. Pathol. 2018, 188, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Hori, K.; Tanaka-Okamoto, M.; Higashiyama, M.; Itoh, Y.; Inoue, M.; Morinaka, S.; Miyoshi, J. Decreased expression of LMO7 and its clinicopathological significance in human lung adenocarcinoma. Exp. Ther. Med. 2011, 2, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Ooshio, T.; Irie, K.; Morimoto, K.; Fukuhara, A.; Imai, T.; Takai, Y. Involvement of LMO7 in the Association of Two Cell-Cell Adhesion Molecules, Nectin and E-cadherin, through Afadin and α-Actinin in Epithelial Cells. J. Biol. Chem. 2004, 279, 31365–31373. [Google Scholar] [CrossRef]
- Krcmery, J.; Camarata, T.; Kulisz, A.; Simon, H.-G. Nucleocytoplasmic functions of the PDZ-LIM protein family: New insights into organ development. BioEssays 2010, 32, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.H.; Disney, M.D.; Childs, J.L.; Schroeder, S.J.; Zuker, M.; Turner, D.H. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc. Natl. Acad. Sci. USA 2004, 101, 7287–7292. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, W.; Duan, C.; Li, M.; Gao, L. Novel Long Non-Coding RNA (lncRNA) Transcript AL137782.1 Promotes the Migration of Normal Lung Epithelial Cells through Positively Regulating LMO7. Int. J. Mol. Sci. 2023, 24, 13904. https://doi.org/10.3390/ijms241813904
Zhang Y, Wang W, Duan C, Li M, Gao L. Novel Long Non-Coding RNA (lncRNA) Transcript AL137782.1 Promotes the Migration of Normal Lung Epithelial Cells through Positively Regulating LMO7. International Journal of Molecular Sciences. 2023; 24(18):13904. https://doi.org/10.3390/ijms241813904
Chicago/Turabian StyleZhang, Ying, Weili Wang, Chunchun Duan, Min Li, and Liyang Gao. 2023. "Novel Long Non-Coding RNA (lncRNA) Transcript AL137782.1 Promotes the Migration of Normal Lung Epithelial Cells through Positively Regulating LMO7" International Journal of Molecular Sciences 24, no. 18: 13904. https://doi.org/10.3390/ijms241813904
APA StyleZhang, Y., Wang, W., Duan, C., Li, M., & Gao, L. (2023). Novel Long Non-Coding RNA (lncRNA) Transcript AL137782.1 Promotes the Migration of Normal Lung Epithelial Cells through Positively Regulating LMO7. International Journal of Molecular Sciences, 24(18), 13904. https://doi.org/10.3390/ijms241813904





