A Case Study from the Past: “The RGC-5 vs. the 661W Cell Line: Similarities, Differences and Contradictions—Are They Really the Same?”
Abstract
1. Introduction
- The origin and nature of the RGC-5 cell line: A fundamental research gap exists concerning the uncertain origin of the RGC-5 cell line. Lingering concerns regarding possible contamination with or genetic similarity to 661W cone photoreceptor cells have cast doubt on the lineage of RGC-5 cells. As such, there is a pressing need to investigate the true source and nature of the RGC-5 cell line to ensure its reliability as a retinal ganglion cell model.
- Clarifying differentiation status: Another key research gap involves determining the true differentiation status of the RGC-5 and 661W cell lines. A thorough examination is essential to ascertain whether these cell lines genuinely represent retinal ganglion cells or exhibit properties more akin to photoreceptor cells. Resolving this uncertainty is vital for accurate interpretation of experimental outcomes and targeted therapeutic studies.
- Evaluating research findings reliability: Previous studies utilizing RGC-5 cells have reported conflicting results, with some indicating ganglion cell-specific markers, while others suggested photoreceptor-like behavior. This discrepancy calls into question the reliability and validity of research findings based on RGC-5 cells. Addressing this limitation is crucial to ensure the credibility of ocular pathology studies and pave the way for robust scientific advancements.
- Lack of standardized differentiation protocols: The absence of standardized differentiation protocols for the RGC-5 and 661W cell lines has hindered accurate comparisons of their properties. Addressing this limitation by employing rigorous and consistent differentiation procedures will enhance the reliability and interpretability of experimental results.
- Concerns about contamination and cell properties: Persistent concerns regarding potential contamination and loss of cell properties have cast doubts on the trustworthiness of research conducted using RGC-5 cells. These concerns necessitate systematic investigations to ascertain the integrity and authenticity of the cell lines under study.
2. Results
2.1. Karyotyping
- 661W: 60~64,XY,−2,+der(3;3),−4,+5,+5,−6,−7,+10,−11,+12,+17,+17,+mar,+mar,+mar,+mar
- RGC-5: 55~59,X,−4,−5,−6,+10,+11,−12,−13,−13,−14,+15,−16,+17,−18,+mar,+mar,+mar,+mar,+mar
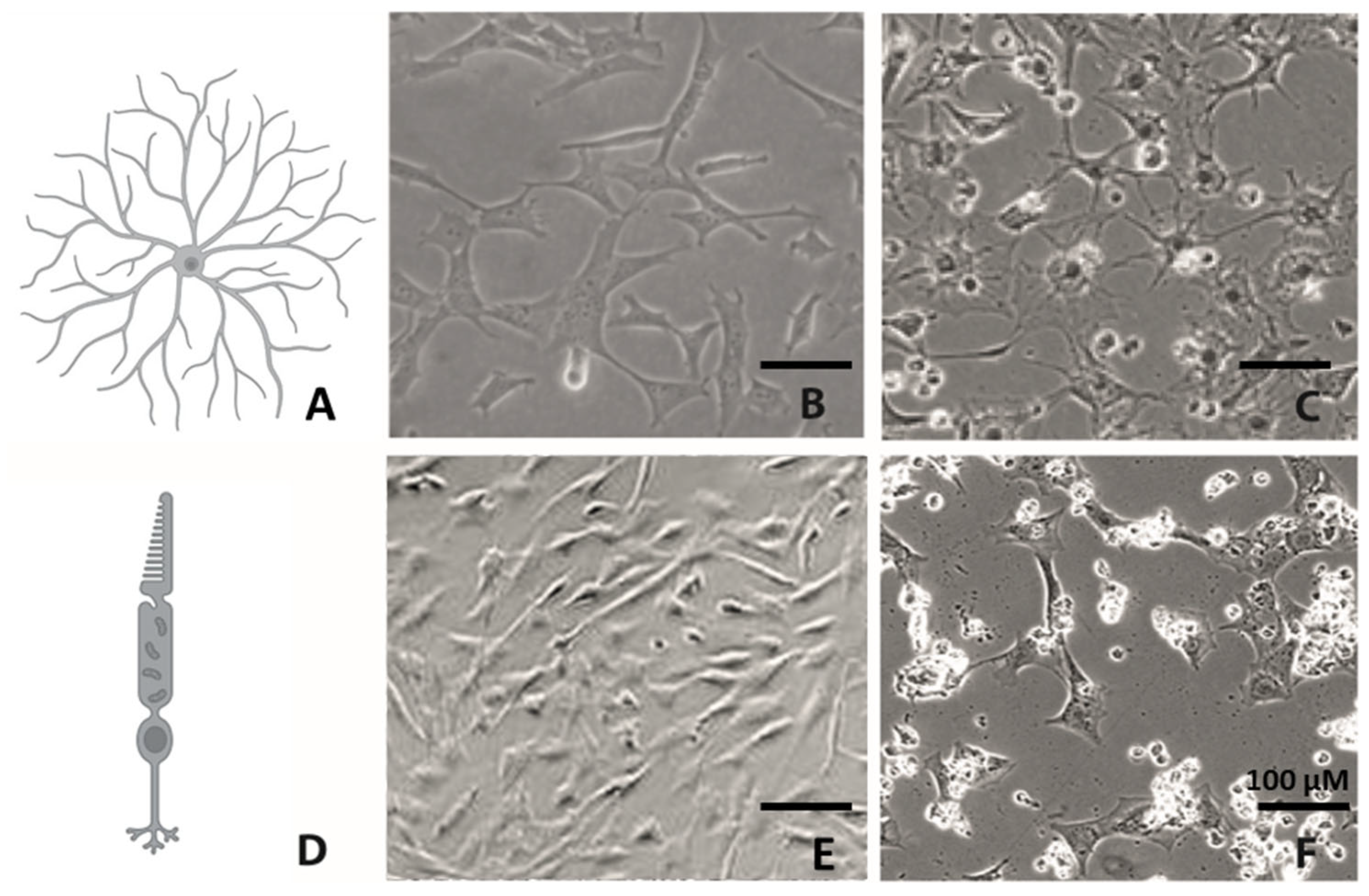
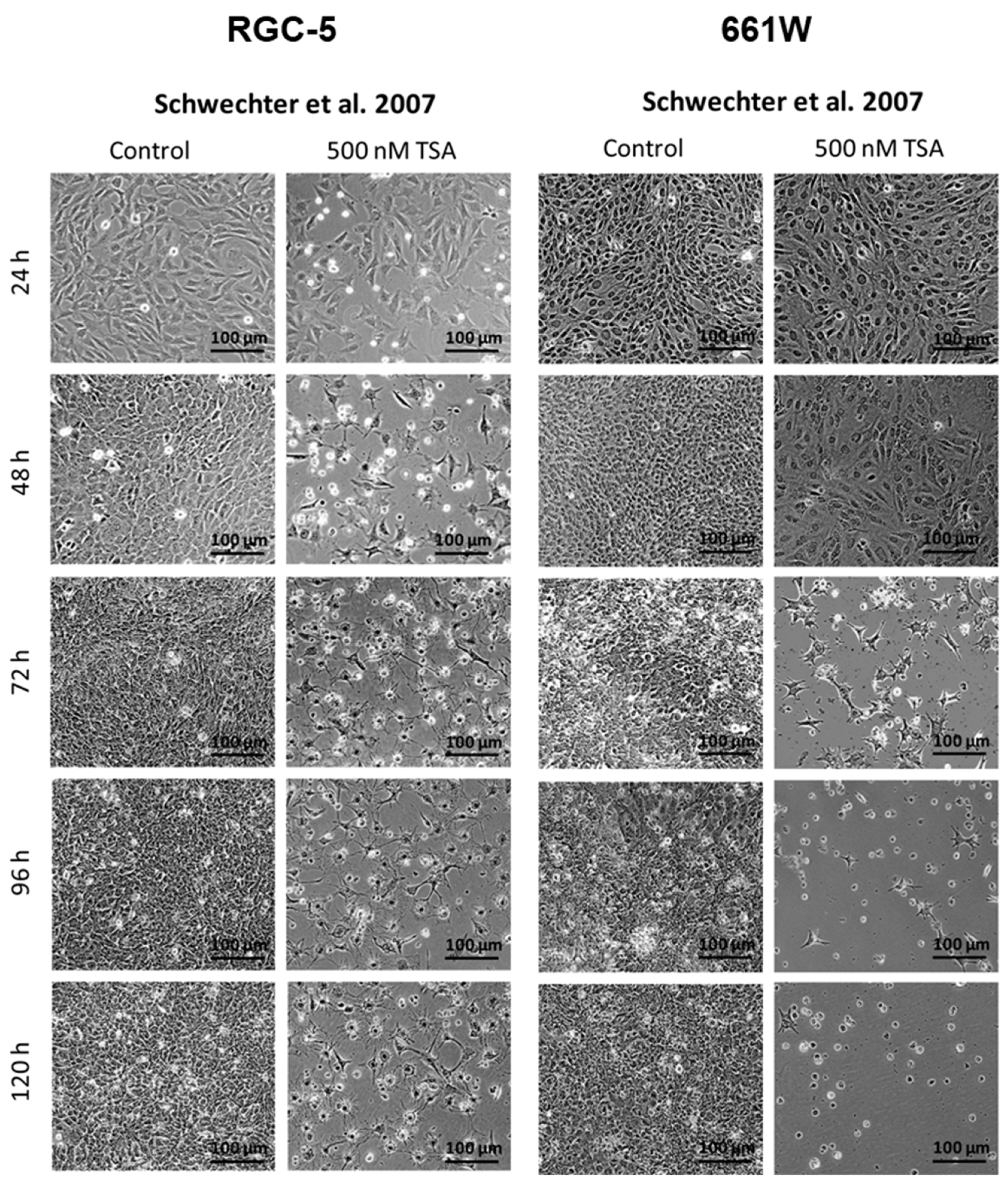
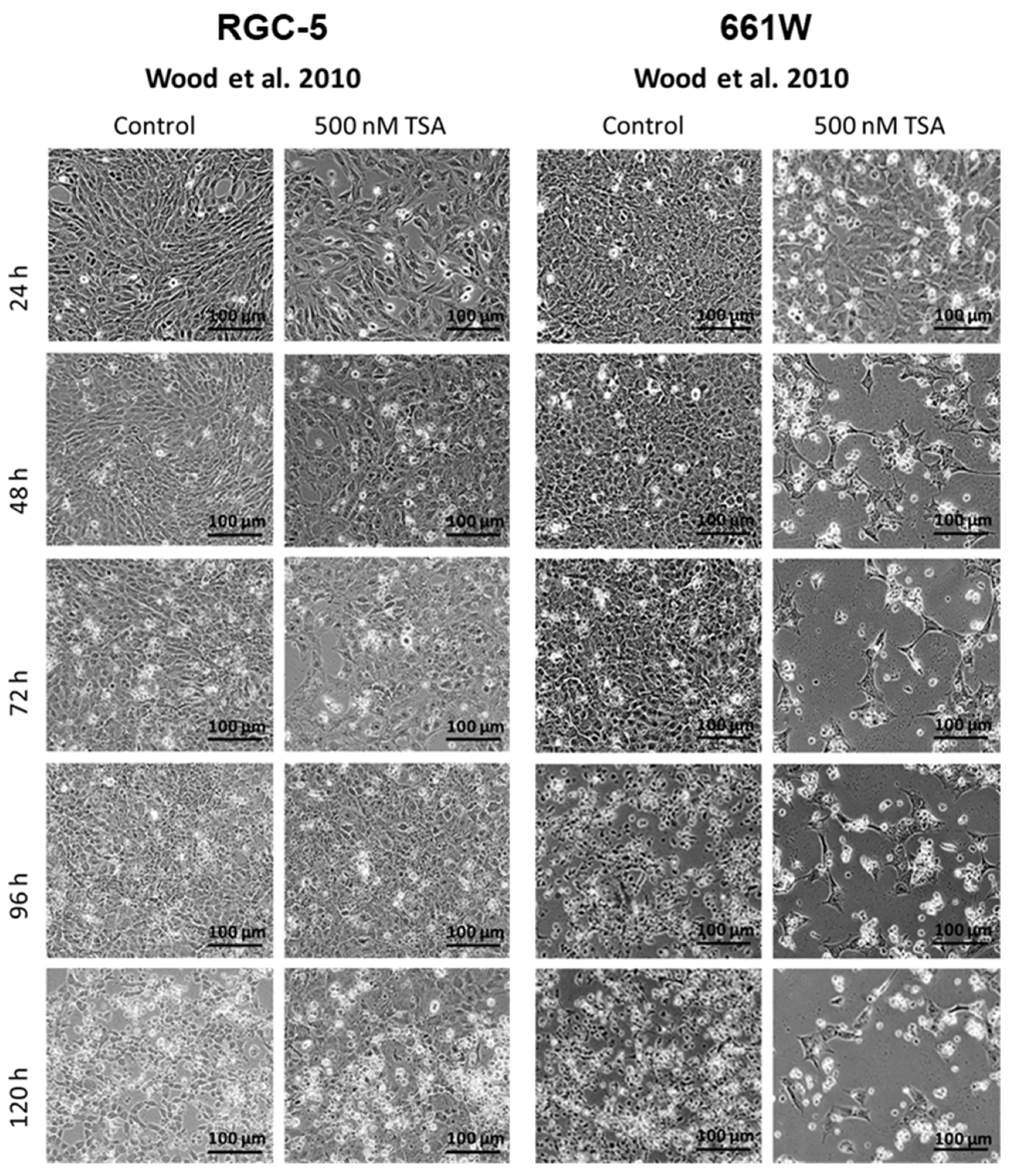
2.2. Morphology Changes in RGC-5 Cells and 661W Cells Due to TSA Treatment
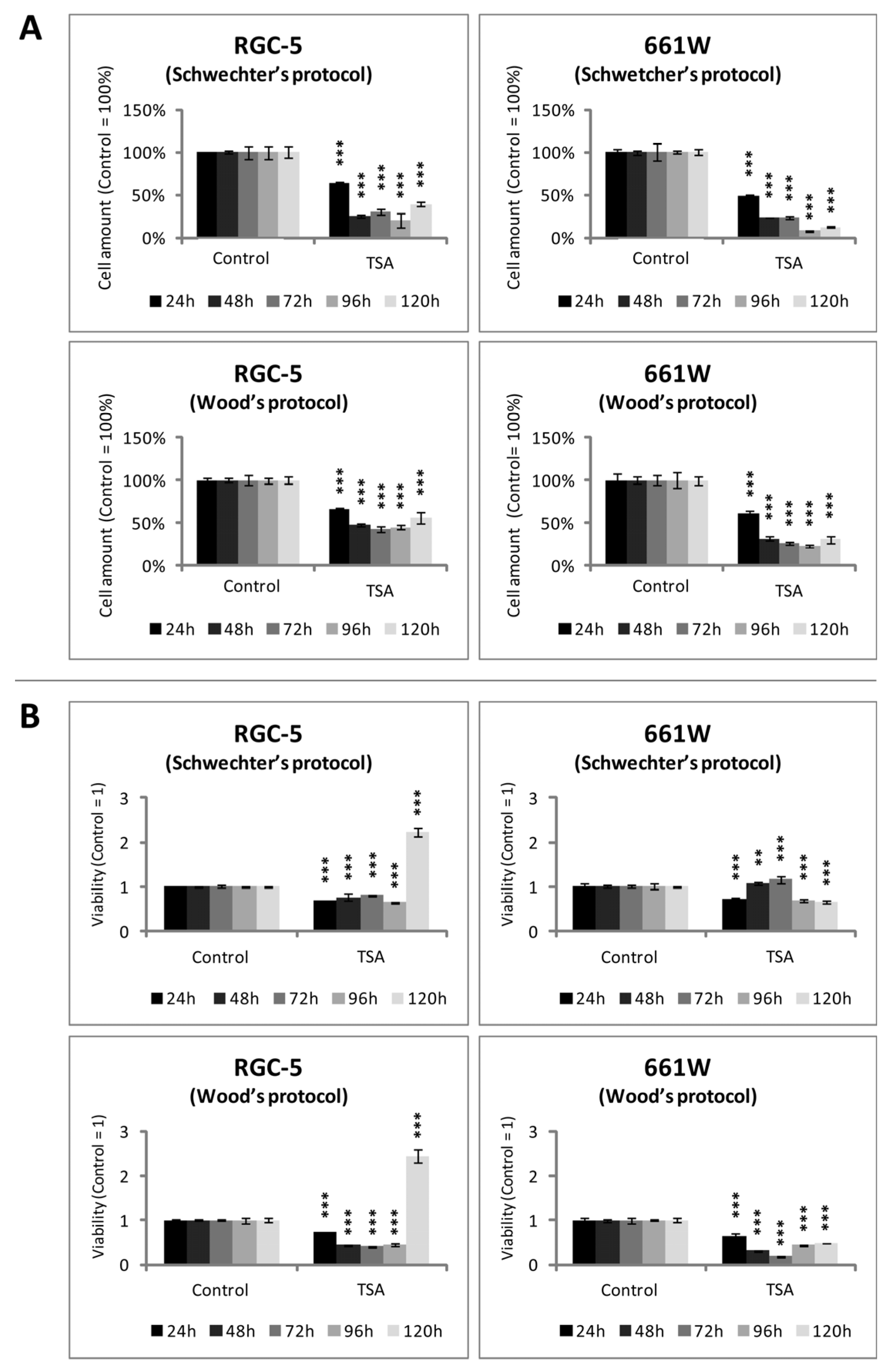
2.3. Cell Amount and Viability of RGC-5 Cells and 661W Cells during TSA Incubation
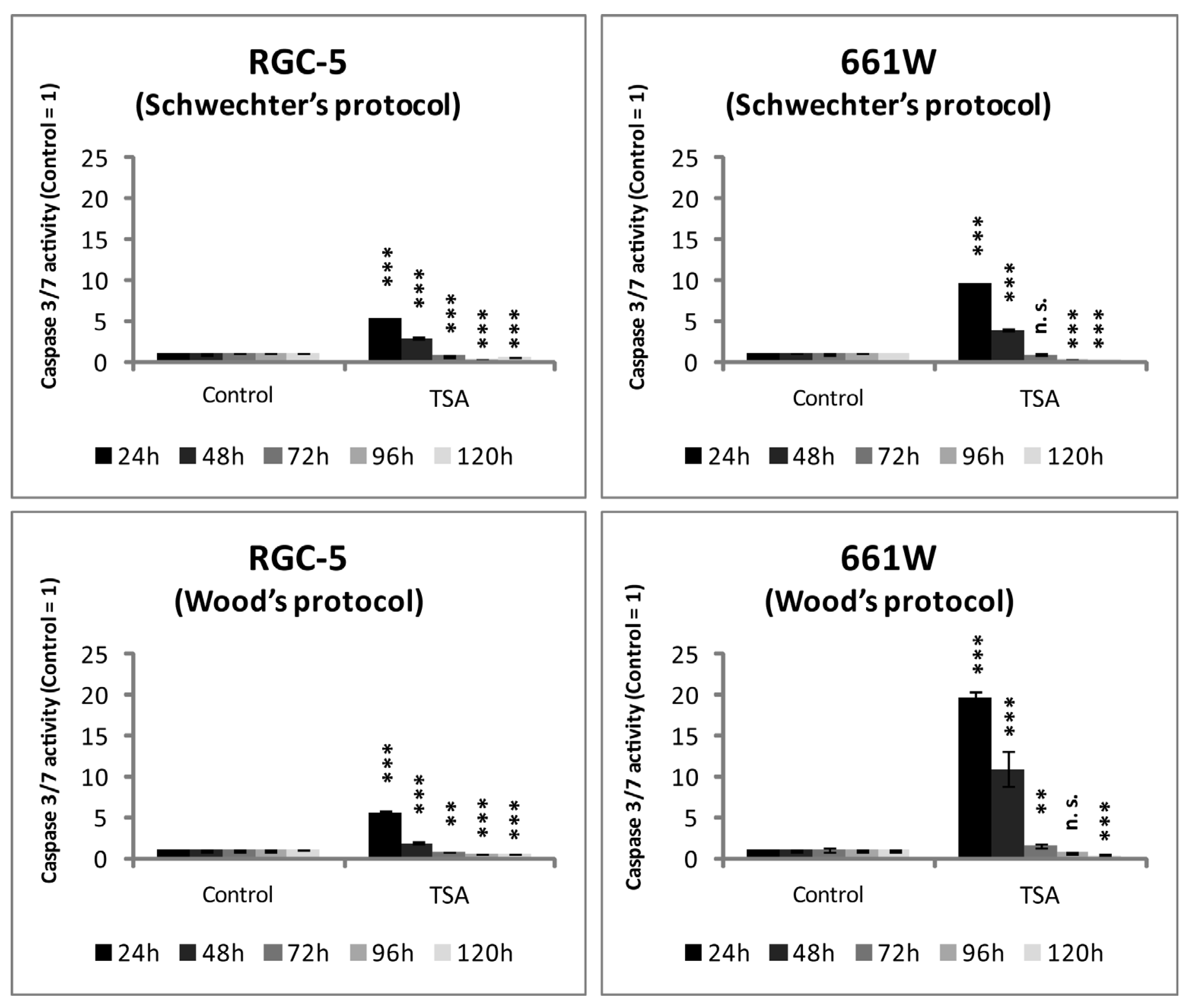
2.4. The Effect of TSA on Apoptosis in RGC-5 Cells and 661W Cells
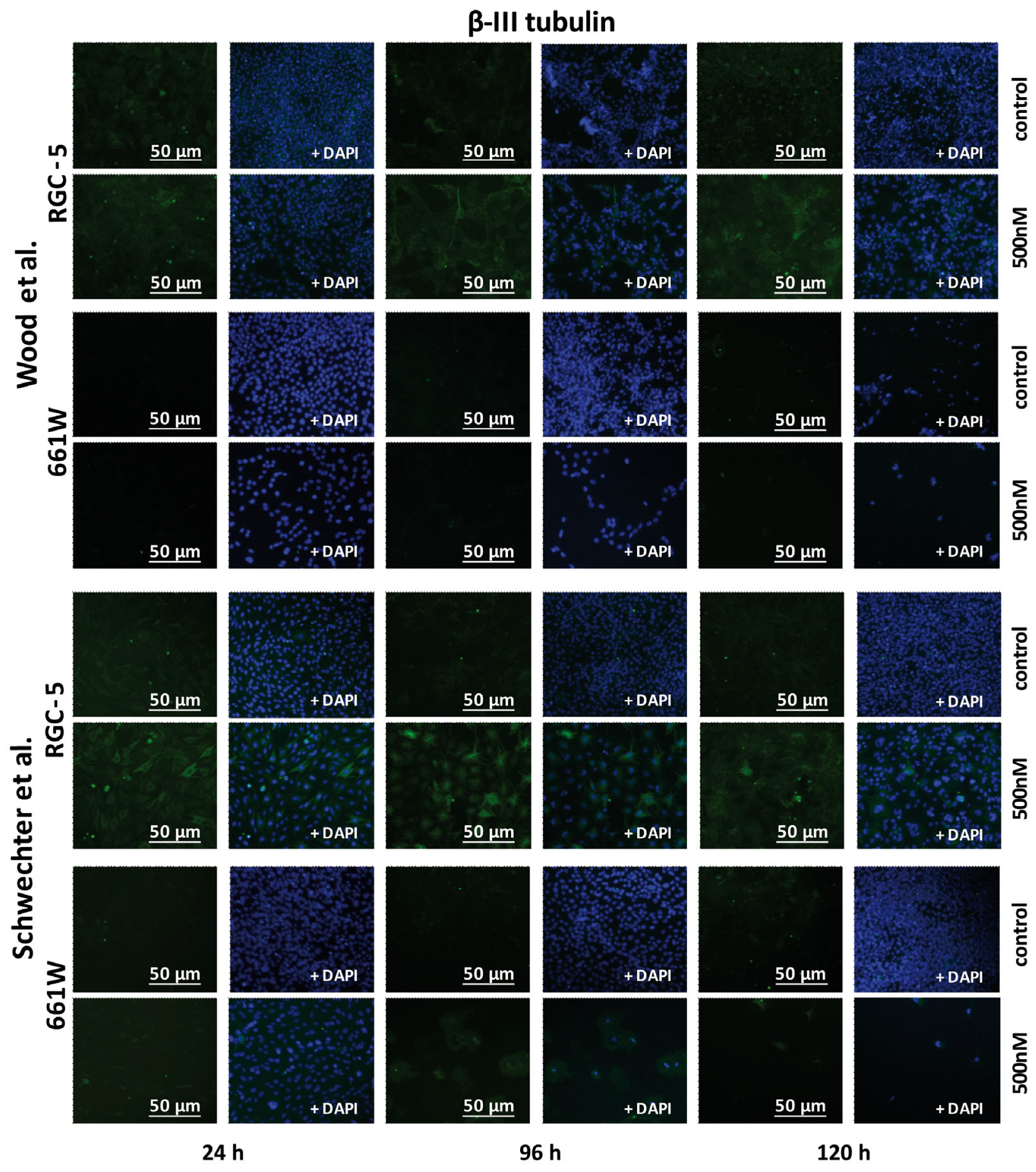
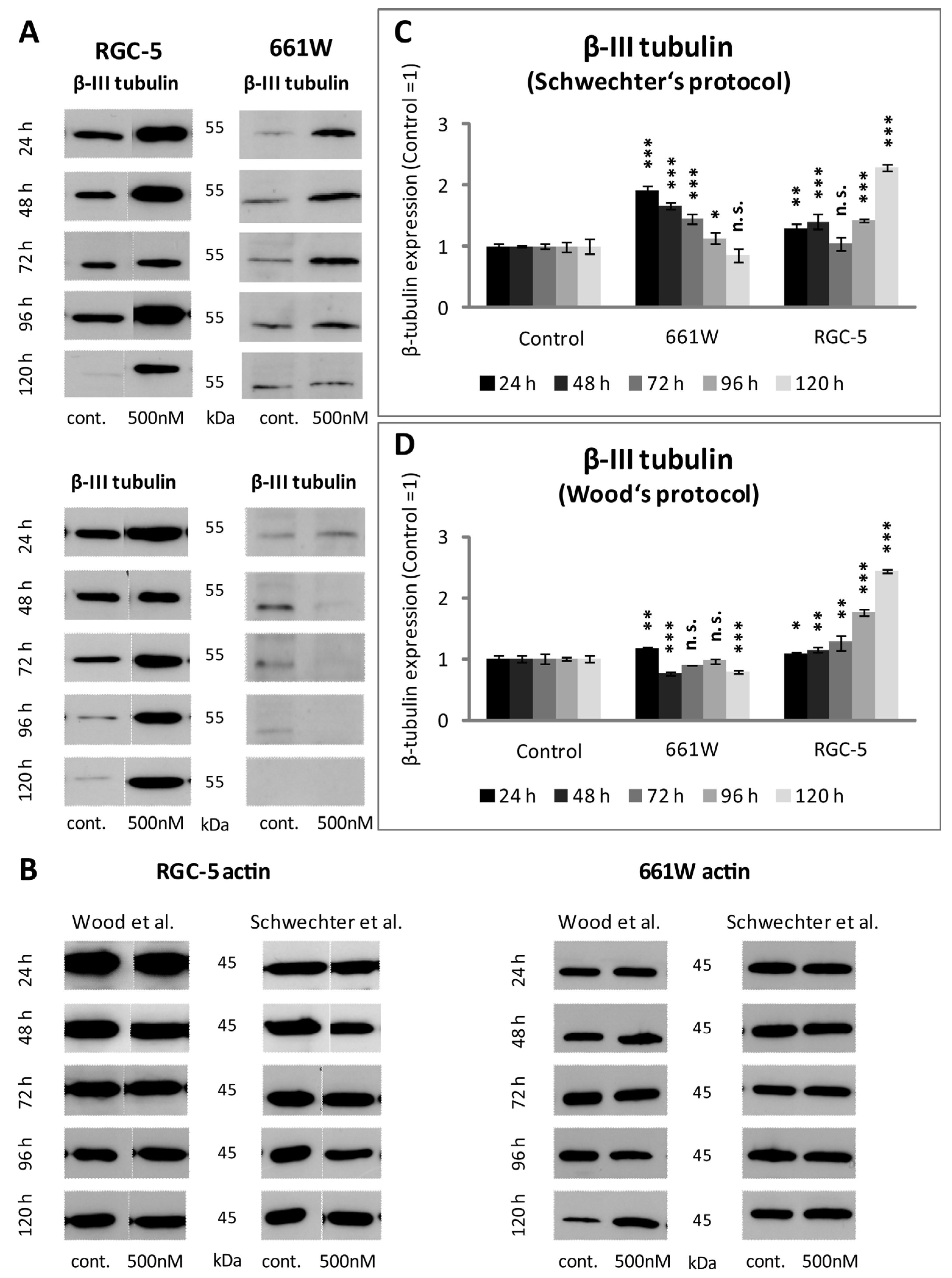
2.5. β-III Tubulin Expression in RGC-5 Cells and 661W Cells Caused by TSA Treatment
2.6. Relative Expression of Thy-1, PGP9.5, CRX- and GFAP-mRNA in RGC-5 and 661W Cells after TSA Incubation
3. Discussion
3.1. Characteristics of the RGC-5 Cells in the Present Study
3.2. The Reaction of 661W Cells to TSA Incubation
3.3. The Effects of TSA
3.4. The Problem of Cell Line Contamination
3.5. Contributions to Knowledge
4. Materials and Methods
4.1. Cell Culture
4.2. Chromosome Analysis
4.3. SDS-PAGE and Western Blot
4.4. Immunofluorescence Staining
4.5. qRT-PCR
4.6. MTS Viability Assay
4.7. Crystal Violet Staining
4.8. Caspase 3/7 Activity Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Directive to Prioritize Efforts to Reduce Animal Testing; United States Environmental Protection Agency: Washington, DC, USA, 2019.
- Schnichels, S.; Paquet-Durand, F.; Loscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a dish: Cell cultures, retinal explants and animal models for common diseases of the retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.R.; Agarwal, P.; Prasanna, G.; Vopat, K.; Lambert, W.; Sheedlo, H.J.; Pang, I.H.; Shade, D.; Wordinger, R.J.; Yorio, T.; et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res. Mol. Brain Res. 2001, 86, 112. [Google Scholar] [CrossRef]
- Agarwal, N.; Agarwal, R.; Kumar, D.M.; Ondricek, A.; Clark, A.F.; Wordinger, R.J.; Pang, I.H. Comparison of expression profile of neurotrophins and their receptors in primary and transformed rat retinal ganglion cells. Mol. Vis. 2007, 13, 1311–1318. [Google Scholar] [PubMed]
- Nieto, P.S.; Acosta-Rodriguez, V.A.; Valdez, D.J.; Guido, M.E. Differential responses of the mammalian retinal ganglion cell line RGC-5 to physiological stimuli and trophic factors. Neurochem. Int. 2010, 57, 216–226. [Google Scholar] [CrossRef]
- Aoun, P.; Simpkins, J.W.; Agarwal, N. Role of PPAR-gamma ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2999–3004. [Google Scholar] [CrossRef]
- Fan, W.; Agarwal, N.; Cooper, N.G. The role of CaMKII in BDNF-mediated neuroprotection of retinal ganglion cells (RGC-5). Brain Res. 2006, 1067, 48–57. [Google Scholar] [CrossRef]
- Ganapathy, P.S.; Dun, Y.; Ha, Y.; Duplantier, J.; Allen, J.B.; Farooq, A.; Bozard, B.R.; Smith, S.B. Sensitivity of staurosporine-induced differentiated RGC-5 cells to homocysteine. Curr. Eye Res. 2010, 35, 80–90. [Google Scholar] [CrossRef]
- Harper, M.M.; Adamson, L.; Blits, B.; Bunge, M.B.; Grozdanic, S.D.; Sakaguchi, D.S. Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp. Eye Res. 2009, 89, 538–548. [Google Scholar] [CrossRef]
- Maher, P.; Hanneken, A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4796–4803. [Google Scholar] [CrossRef]
- Maher, P.; Hanneken, A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Investig. Ophthalmol. Vis. Sci. 2005, 46, 749–757. [Google Scholar] [CrossRef]
- Van Bergen, N.J.; Wood, J.P.; Chidlow, G.; Trounce, I.A.; Casson, R.J.; Ju, W.K.; Weinreb, R.N.; Crowston, J.G. Recharacterization of the RGC-5 retinal ganglion cell line. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4267–4272. [Google Scholar] [CrossRef]
- Wood, J.P.; Chidlow, G.; Tran, T.; Crowston, J.G.; Casson, R.J. A comparison of differentiation protocols for RGC-5 cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Schultheiss, M.; Hofmann, J.; Szurman, P.; Bartz-Schmidt, K.U.; Spitzer, M.S. Trichostatin A induces cell death at the concentration recommended to differentiate the RGC-5 cell line. Neurochem. Int. 2012, 60, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Schwechter, B.R.; Millet, L.E.; Levin, L.A. Histone deacetylase inhibition-mediated differentiation of RGC-5 cells and interaction with survival. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2845–2857. [Google Scholar] [CrossRef]
- Frassetto, L.J.; Schlieve, C.R.; Lieven, C.J.; Utter, A.A.; Jones, M.V.; Agarwal, N.; Levin, L.A. Kinase-dependent differentiation of a retinal ganglion cell precursor. Investig. Ophthalmol. Vis. Sci. 2006, 47, 427–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harvey, R.; Chintala, S.K. Inhibition of plasminogen activators attenuates the death of differentiated retinal ganglion cells and stabilizes their neurite network in vitro. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1884–1891. [Google Scholar] [CrossRef]
- Lieven, C.J.; Millet, L.E.; Hoegger, M.J.; Levin, L.A. Induction of axon and dendrite formation during early RGC-5 cell differentiation. Exp. Eye Res. 2007, 85, 678–683. [Google Scholar] [CrossRef]
- Schultheiss, M.; Schnichels, S.; Miteva, K.; Warstat, K.; Szurman, P.; Spitzer, M.S.; Van Linthout, S. Staurosporine-induced differentiation of the RGC-5 cell line leads to apoptosis and cell death at the lowest differentiating concentration. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1221–1229. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.R.; Clark, A.F.; Daudt, D.; Vishwanatha, J.K.; Yorio, T. A Forensic Path to RGC-5 Cell Line Identification: Lessons Learned. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5712–5719. [Google Scholar] [CrossRef]
- Kanan, Y.; Hoffhines, A.; Rauhauser, A.; Murray, A.; Al-Ubaidi, M.R. Protein tyrosine-O-sulfation in the retina. Exp. Eye Res. 2009, 89, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kanan, Y.; Moiseyev, G.; Agarwal, N.; Ma, J.X.; Al-Ubaidi, M.R. Light induces programmed cell death by activating multiple independent proteases in a cone photoreceptor cell line. Investig. Ophthalmol. Vis. Sci. 2007, 48, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Hagemann, U.; Januschowski, K.; Hofmann, J.; Bartz-Schmidt, K.U.; Szurman, P.; Spitzer, M.S.; Aisenbrey, S. Comparative toxicity and proliferation testing of aflibercept, bevacizumab and ranibizumab on different ocular cells. Br. J. Ophthalmol. 2013, 97, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Tsuruma, K.; Yamauchi, M.; Inokuchi, Y.; Sugitani, S.; Shimazawa, M.; Hara, H. Role of oxidative stress in retinal photoreceptor cell death in N-methyl-N-nitrosourea-treated mice. J. Pharmacol. Sci. 2012, 118, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H. Morphology and Circuitry of Ganglion Cells. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Greeff, R. Mikroskopische Anatomie des Sehnerven und der Netzhaut. In Handbuch der gesamten Augenheilkunde: I; Engelmann: Leipzig, Germany, 1900. [Google Scholar]
- Mu, L.; Dong, Z.; Zhang, Y. Mechanisms of Qing-Gan Li-Shui Formulation in Ameliorating Primary Open Angle Glaucoma: An Analysis Based on Network Pharmacology. Evid. Based Complement. Alternat. Med. 2022, 2022, 8336131. [Google Scholar] [CrossRef]
- Duan, C.Y.; Fan, W.L.; Chen, F. Roles of Optineurin and Extracellular Vesicles in Glaucomatous Retinal Cell Loss. Curr. Med. Sci. 2023, 43, 367–375. [Google Scholar] [CrossRef]
- Xi, X.; Ma, J.; Chen, Q.; Wang, X.; Xia, Y.; Wen, X.; Yuan, J.; Li, Y. Acteoside attenuates hydrogen peroxide-induced injury of retinal ganglion cells via the CASC2/miR-155/mTOR axis. Ann. Transl. Med. 2022, 10, 5. [Google Scholar] [CrossRef]
- Gaub, P.; Tedeschi, A.; Puttagunta, R.; Nguyen, T.; Schmandke, A.; Di Giovanni, S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010, 17, 1392–1408. [Google Scholar] [CrossRef]
- Kretsovali, A.; Hadjimichael, C.; Charmpilas, N. Histone deacetylase inhibitors in cell pluripotency, differentiation, and reprogramming. Stem Cells Int. 2012, 2012, 184154. [Google Scholar] [CrossRef]
- Olney, J.W. Glutaate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J. Neuropathol. Exp. Neurol. 1969, 28, 455–474. [Google Scholar] [CrossRef]
- Vorwerk, C.K.; Lipton, S.A.; Zurakowski, D.; Hyman, B.T.; Sabel, B.A.; Dreyer, E.B. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1618–1624. [Google Scholar]
- Sippl, C.; Bosserhoff, A.K.; Fischer, D.; Tamm, E.R. Depletion of optineurin in RGC-5 cells derived from retinal neurons causes apoptosis and reduces the secretion of neurotrophins. Exp. Eye Res. 2011, 93, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.K.; Liu, Q.; Kim, K.Y.; Crowston, J.G.; Lindsey, J.D.; Agarwal, N.; Ellisman, M.H.; Perkins, G.A.; Weinreb, R.N. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.P.; Lascaratos, G.; Bron, A.J.; Osborne, N.N. The influence of visible light exposure on cultured RGC-5 cells. Mol. Vis. 2007, 14, 334–344. [Google Scholar]
- Beale, R.; Osborne, N.N. Localization of the Thy-1 antigen to the surfaces of rat retinal ganglion cells. Neurochem. Int. 1982, 4, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Zhou, L.; Macke, J.P.; Yoshioka, T.; Hendry, S.H.; Eddy, R.L.; Shows, T.B.; Nathans, J. The Brn-3 family of POU-domain factors: Primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J. Neurosci. 1995, 15, 4762–4785. [Google Scholar] [CrossRef]
- Nieto, P.S.; Valdez, D.J.; Acosta-Rodriguez, V.A.; Guido, M.E. Expression of novel opsins and intrinsic light responses in the mammalian retinal ganglion cell line RGC-5. Presence of OPN5 in the rat retina. PLoS ONE 2011, 6, e26417. [Google Scholar] [CrossRef]
- Al-Ubaidi, M.R. RGC-5: Are they really 661W? The saga continues. Exp. Eye Res. 2014, 119, 115. [Google Scholar] [CrossRef]
- Thompson, A.F.; Crowe, M.E.; Lieven, C.J.; Levin, L.A. Induction of Neuronal Morphology in the 661W Cone Photoreceptor Cell Line with Staurosporine. PLoS ONE 2015, 10, e0145270. [Google Scholar] [CrossRef]
- Thompson, A.F.; Levin, L.A. Neuronal differentiation by analogs of staurosporine. Neurochem. Int. 2010, 56, 554–560. [Google Scholar] [CrossRef]
- Furukawa, T.; Morrow, E.M.; Cepko, C.L. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 1997, 91, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.L.; Nie, Z.; Sun, H.; Lennon, G.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Zack, D.J. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 1997, 19, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.F.; Morin, F.; Shi, Q.; Klein, D.C.; Moller, M. Ontogenetic expression of the Otx2 and Crx homeobox genes in the retina of the rat. Exp. Eye Res. 2007, 85, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, M.C.; Arnold, M.F.; Collet, B.; Munro, E.S. The effect of sub-culturing on the basal level of type I interferon (IFN) gene expression in the Salmon Head Kidney (SHK-1) cell line. Fish Shellfish Immunol. 2009, 27, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Muff, R.; Rath, P.; Ram Kumar, R.M.; Husmann, K.; Born, W.; Baudis, M.; Fuchs, B. Genomic instability of osteosarcoma cell lines in culture: Impact on the prediction of metastasis relevant genes. PLoS ONE 2015, 10, e0125611. [Google Scholar] [CrossRef]
- AbuHammad, S.; Zihlif, M. Gene expression alterations in doxorubicin resistant MCF7 breast cancer cell line. Genomics 2013, 101, 213–220. [Google Scholar] [CrossRef]
- Chen, B.; Cepko, C.L. Requirement of histone deacetylase activity for the expression of critical photoreceptor genes. BMC Dev. Biol. 2007, 7, 78. [Google Scholar] [CrossRef]
- Wallace, D.M.; Cotter, T.G. Histone deacetylase activity in conjunction with E2F-1 and p53 regulates Apaf-1 expression in 661W cells and the retina. J. Neurosci. Res. 2009, 87, 887–905. [Google Scholar] [CrossRef]
- Glozak, M.A.; Sengupta, N.; Zhang, X.; Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. [Google Scholar] [CrossRef]
- Quivy, V.; Van Lint, C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem. Pharmacol. 2004, 68, 1221–1229. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, J.; Olofsson, B.A.; Mwidau, A.; Dedeoglu, A.; Escudero, M.; Flemington, E.; Azizkhan-Clifford, J.; Ferrante, R.J.; Ratan, R.R. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 4281–4286. [Google Scholar] [CrossRef]
- Hrabeta, J.; Stiborova, M.; Adam, V.; Kizek, R.; Eckschlager, T. Histone deacetylase inhibitors in cancer therapy. A review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2014, 158, 161–169. [Google Scholar] [CrossRef]
- Xu, W.S.; Parmigiani, R.B.; Marks, P.A. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene 2007, 26, 5541–5552. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Ruefli, A.; Beamish, H.; Warrener, R.; Saunders, N.; Johnstone, R.; Gabrielli, B. Histone deacetylase inhibitors specifically kill nonproliferating tumour cells. Oncogene 2004, 23, 6693–6701. [Google Scholar] [CrossRef]
- Insinga, A.; Monestiroli, S.; Ronzoni, S.; Gelmetti, V.; Marchesi, F.; Viale, A.; Altucci, L.; Nervi, C.; Minucci, S.; Pelicci, P.G. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat. Med. 2005, 11, 71–76. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Sowa, Y.; Xu, W.S.; Shao, Y.; Dokmanovic, M.; Perez, G.; Ngo, L.; Holmgren, A.; Jiang, X.; Marks, P.A. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2005, 102, 673–678. [Google Scholar] [CrossRef]
- Alao, J.P.; Gamble, S.C.; Stavropoulou, A.V.; Pomeranz, K.M.; Lam, E.W.; Coombes, R.C.; Vigushin, D.M. The cyclin D1 proto-oncogene is sequestered in the cytoplasm of mammalian cancer cell lines. Mol. Cancer 2006, 5, 7. [Google Scholar] [CrossRef]
- Alao, J.P.; Lam, E.W.; Ali, S.; Buluwela, L.; Bordogna, W.; Lockey, P.; Varshochi, R.; Stavropoulou, A.V.; Coombes, R.C.; Vigushin, D.M. Histone deacetylase inhibitor trichostatin A represses estrogen receptor alpha-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clin. Cancer Res. 2004, 10, 8094–8104. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Davies, K.P.; Allen, J.; Zhu, L.; Pestell, R.G.; Zagzag, D.; Kalpana, G.V. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol. Cell. Biol. 2002, 22, 5975–5988. [Google Scholar] [CrossRef]
- MacLeod, R.A.; Dirks, W.G.; Matsuo, Y.; Kaufmann, M.; Milch, H.; Drexler, H.G. Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int. J. Cancer 1999, 83, 555–563. [Google Scholar] [CrossRef]
- Nardone, R.M. Curbing rampant cross-contamination and misidentification of cell lines. BioTechniques 2008, 45, 221–227. [Google Scholar] [CrossRef]
- Nardone, R.M. Eradication of cross-contaminated cell lines: A call for action. Cell Biol. Toxicol. 2007, 23, 367–372. [Google Scholar] [CrossRef]
- Masters, J.R.; Thomson, J.A.; Daly-Burns, B.; Reid, Y.A.; Dirks, W.G.; Packer, P.; Toji, L.H.; Ohno, T.; Tanabe, H.; Arlett, C.F.; et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc. Natl. Acad. Sci. USA 2001, 98, 8012–8017. [Google Scholar] [CrossRef]
- Barallon, R.; Bauer, S.R.; Butler, J.; Capes-Davis, A.; Dirks, W.G.; Elmore, E.; Furtado, M.; Kline, M.C.; Kohara, A.; Los, G.V.; et al. Recommendation of short tandem repeat profiling for authenticating human cell lines, stem cells, and tissues. Vitr. Cell. Dev. Biol. Anim. 2010, 46, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Eltonsy, N.; Gabisi, V.; Li, X.; Russe, K.B.; Mills, G.B.; Stemke-Hale, K. Detection algorithm for the validation of human cell lines. Int. J. Cancer 2012, 131, E1024–E1030. [Google Scholar] [CrossRef]
- Agarwal, N. RGC-5 cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7884. [Google Scholar] [CrossRef][Green Version]
- Clark, A.; Tamm, E.R.; Al-Ubaidi, M.R.; Hollyfield, J.G. On the use of immortalized ocular cell lines in vision research: The unfortunate story of RGC-5. Exp. Eye Res. 2013, 116, 433. [Google Scholar] [CrossRef] [PubMed]
- Sippl, C.; Tamm, E.R. What is the nature of the RGC-5 cell line? Adv. Exp. Med. Biol. 2014, 801, 145–154. [Google Scholar] [CrossRef]
- Hao, M.; Liu, Y.; Chen, P.; Jiang, H.; Kuang, H.Y. Astragaloside IV protects RGC-5 cells against oxidative stress. Neural Regen. Res. 2018, 13, 1081–1086. [Google Scholar] [CrossRef]
- Wei, Z.; Mingxing, W.; Shanxue, L.; Chao, L.; Yanqiu, Z.; Duoduo, X.U.; Jian, W. Buyang Huanwu Tang protects HO-induced RGC-5 cell against oxidative stress and apoptosis reactive oxygen species-mitogen-activated protein kinase signaling pathway. J. Tradit. Chin. Med. 2022, 42, 885–891. [Google Scholar] [CrossRef]
- Park, Y.H.; Snook, J.D.; Zhuang, I.; Shen, G.; Frankfort, B.J. Optimized culture of retinal ganglion cells and amacrine cells from adult mice. PLoS ONE 2020, 15, e0242426. [Google Scholar] [CrossRef]
- Luo, Z.; Chang, K.C.; Wu, S.; Sun, C.; Xia, X.; Nahmou, M.; Bian, M.; Wen, R.R.; Zhu, Y.; Shah, S.; et al. Directly induced human retinal ganglion cells mimic fetal RGCs and are neuroprotective after transplantation in vivo. Stem Cell Rep. 2022, 17, 2690–2703. [Google Scholar] [CrossRef] [PubMed]
- Castillo, B., Jr.; del Cerro, M.; Breakefield, X.O.; Frim, D.M.; Barnstable, C.J.; Dean, D.O.; Bohn, M.C. Retinal ganglion cell survival is promoted by genetically modified astrocytes designed to secrete brain-derived neurotrophic factor (BDNF). Brain Res. 1994, 647, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Barch, M.J.K.T.; Spurbeck, J.L. The AGT Cytogenetics Laboratory Manual; Lippncott-Raven Publishers: Hunters Green Pkwy, MD, USA, 1997. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
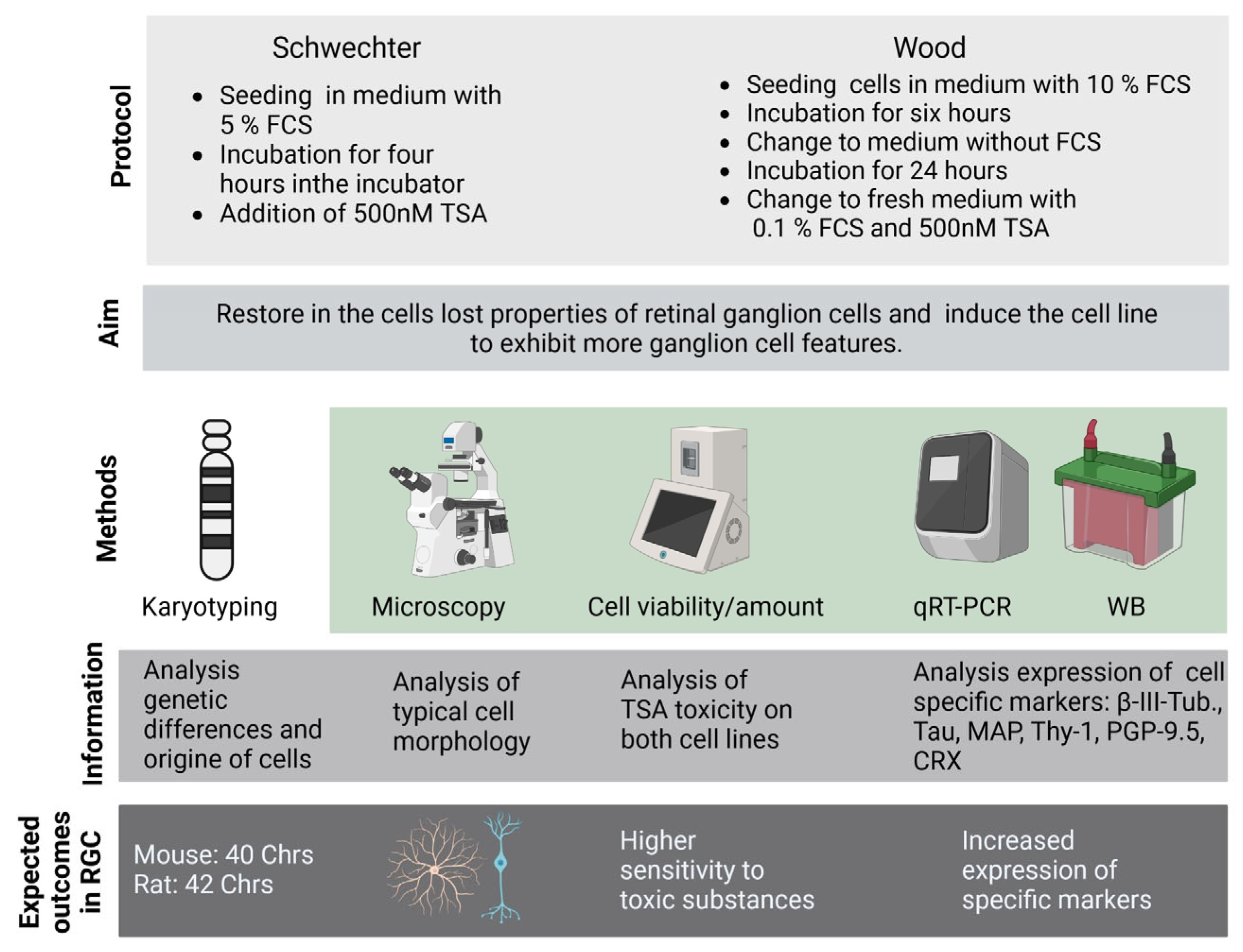


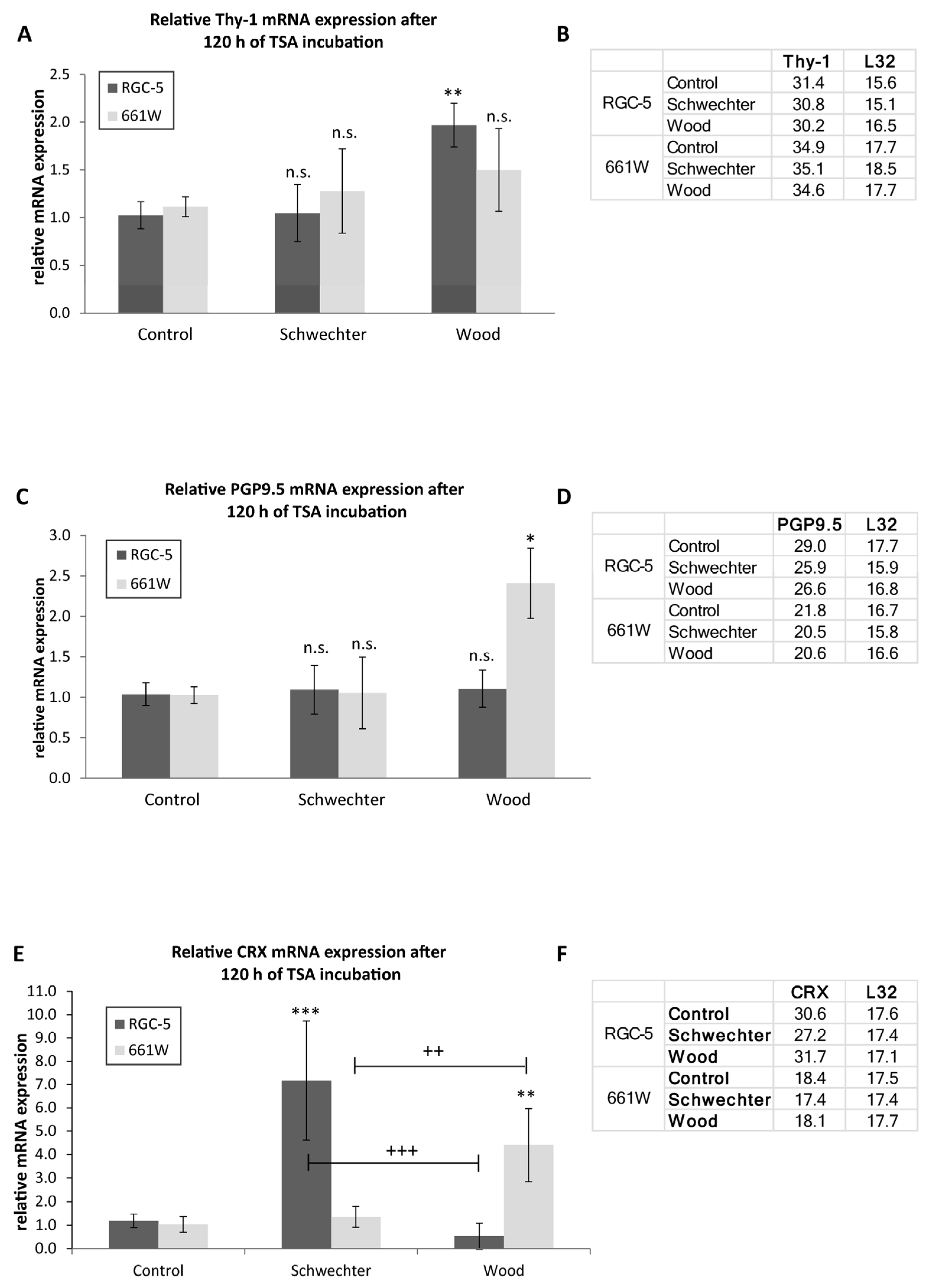
| Protocol | Schwechter et al. [16] | Wood et al. [14] |
|---|---|---|
| Morphology | RGC-5: Differentiation toward a neuronal phenotype after long-term incubation with TSA. 661W: Fewer cells changed morphologies, and neurites were fewer and shorter. Importantly, the majority of cells died during long-term incubation. | Both: Similar trend to that in Schwechter et al. |
| β-III tubulin | RGC-5: In Western blot analysis as well as in the immunohistochemistry, maximum values for long-term incubation (96 h and 120 h) were with TSA. 661W: First, an increase in β-III tubulin expression could be observed. After 72 h, values dropped again. | RGC-5: Maximum increase could be detected after long-term incubation. Increase in expression was stronger than in treatment after Schwechter protocol. 661W: No increase in β-III tubulin expression under long-term incubation. |
| MAP-2, Tau | Both: Expression increased with long-term incubation. | Both: Expression increased. |
| Thy-1 mRNA | Both: No effect | RGC-5: Slight increase after incubation time of 120 h. 661W: No effect |
| PGP9.5 mRNA | Both: No effect | 661W: Twofold increase in mRNA expression after 120 h. |
| CRX mRNA | RGC-5: Sevenfold increase in mRNA expression after 120 h. 661W: No effect | RGC-5: Slight decrease in mRNA expression after 120 h. 661W: Fourfold increase after 120 h. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurst, J.; Attrodt, G.; Bartz-Schmidt, K.-U.; Mau-Holzmann, U.A.; Spitzer, M.S.; Schnichels, S. A Case Study from the Past: “The RGC-5 vs. the 661W Cell Line: Similarities, Differences and Contradictions—Are They Really the Same?”. Int. J. Mol. Sci. 2023, 24, 13801. https://doi.org/10.3390/ijms241813801
Hurst J, Attrodt G, Bartz-Schmidt K-U, Mau-Holzmann UA, Spitzer MS, Schnichels S. A Case Study from the Past: “The RGC-5 vs. the 661W Cell Line: Similarities, Differences and Contradictions—Are They Really the Same?”. International Journal of Molecular Sciences. 2023; 24(18):13801. https://doi.org/10.3390/ijms241813801
Chicago/Turabian StyleHurst, José, Gesine Attrodt, Karl-Ulrich Bartz-Schmidt, Ulrike Angelika Mau-Holzmann, Martin Stephan Spitzer, and Sven Schnichels. 2023. "A Case Study from the Past: “The RGC-5 vs. the 661W Cell Line: Similarities, Differences and Contradictions—Are They Really the Same?”" International Journal of Molecular Sciences 24, no. 18: 13801. https://doi.org/10.3390/ijms241813801
APA StyleHurst, J., Attrodt, G., Bartz-Schmidt, K.-U., Mau-Holzmann, U. A., Spitzer, M. S., & Schnichels, S. (2023). A Case Study from the Past: “The RGC-5 vs. the 661W Cell Line: Similarities, Differences and Contradictions—Are They Really the Same?”. International Journal of Molecular Sciences, 24(18), 13801. https://doi.org/10.3390/ijms241813801