Transcription Factor Zbtb20 as a Regulator of Malignancy and Its Practical Applications
Abstract
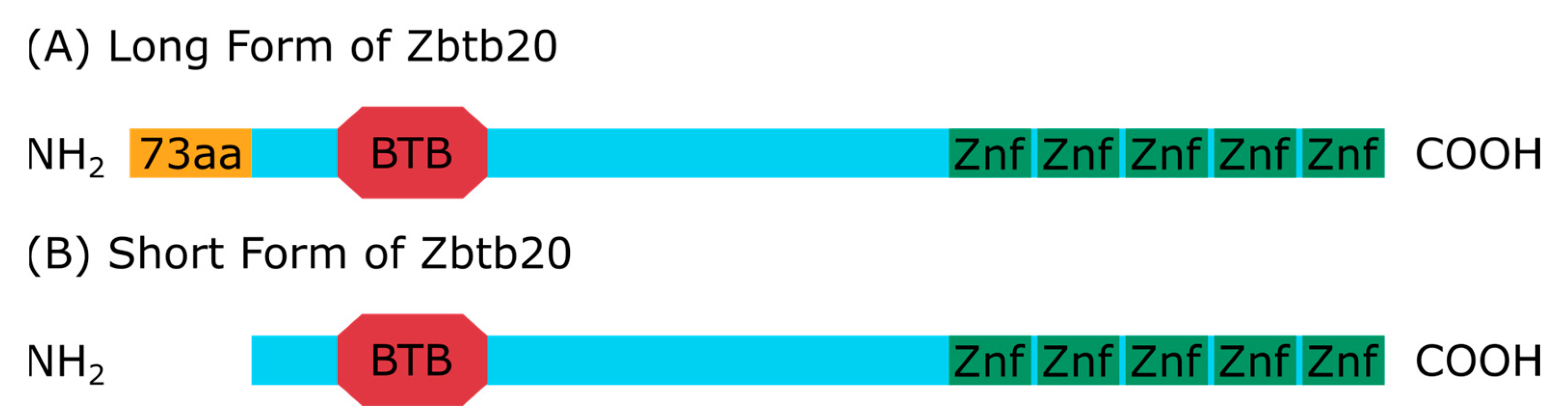
:1. Introduction
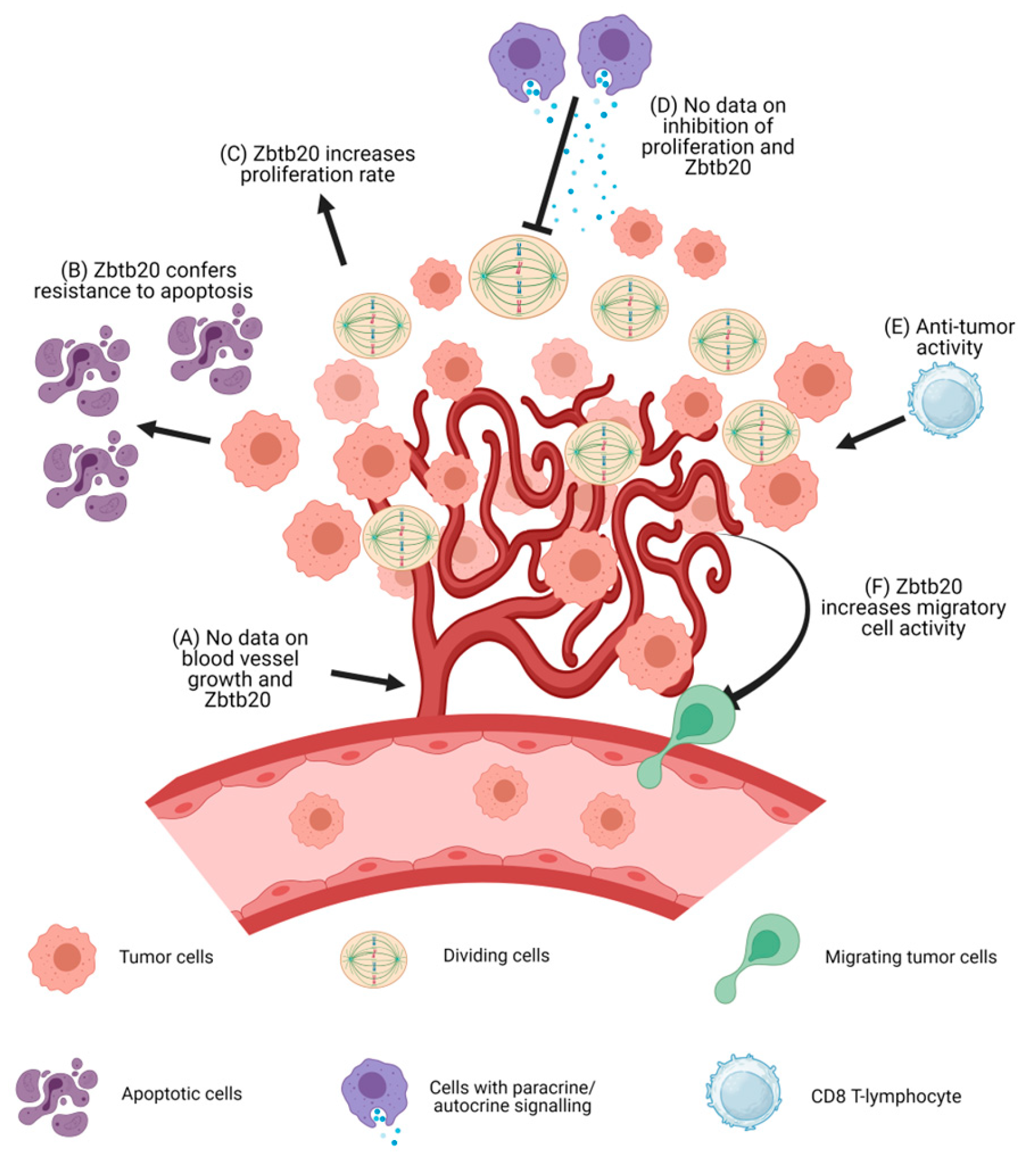
2. Zbtb20 and Malignant Tumors
2.1. Limitless and Self-Sufficient Proliferation, and Resistance to Growth Inhibition
2.2. Evasion of Apoptosis
2.3. Increased Cell Motility Leading to Tissue Invasion
2.4. Chronic Inflammation
2.5. Anti-Tumor Immunity
2.6. Neoangiogenesis
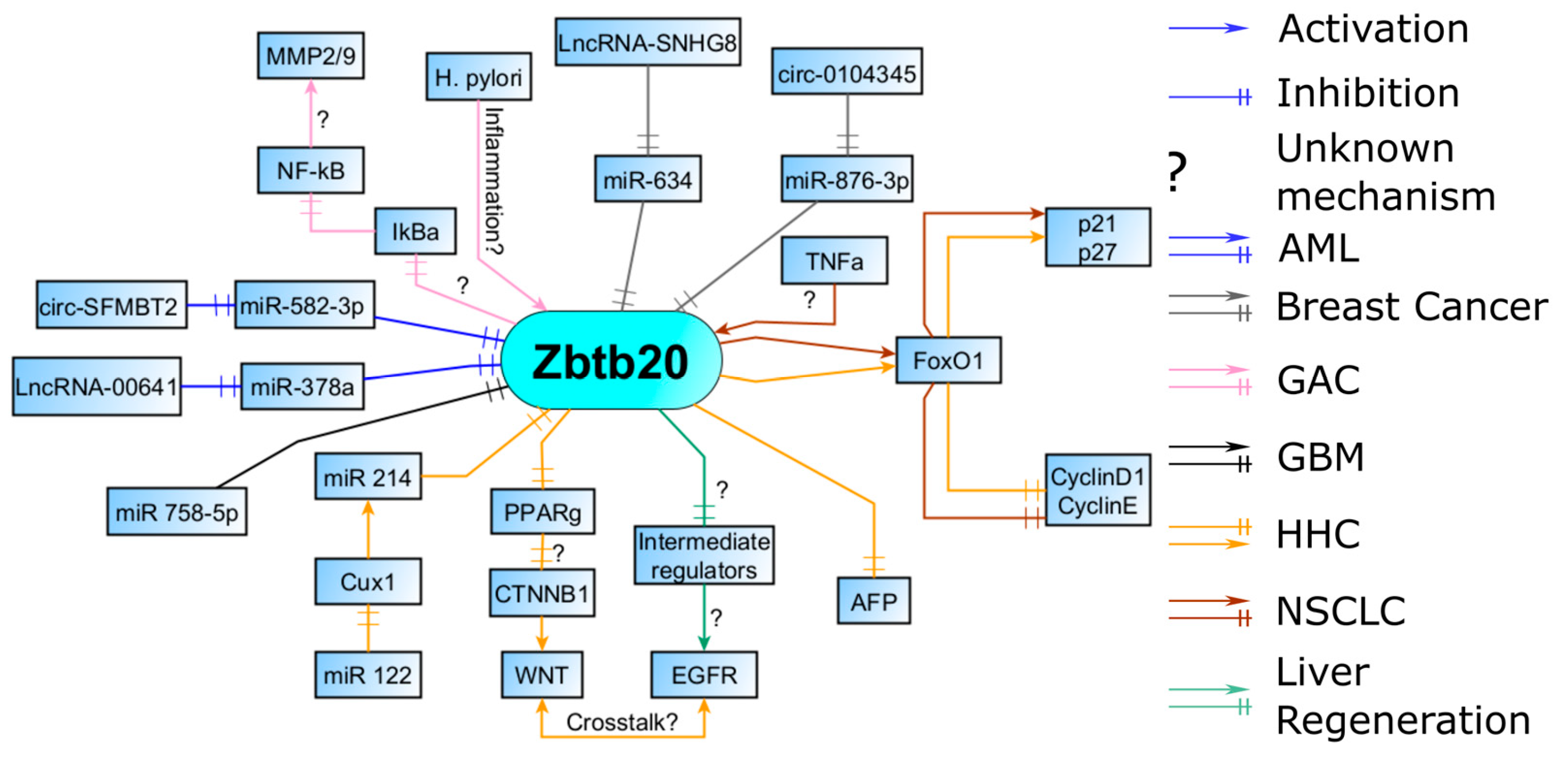
3. Molecular Partners of Zbtb20
3.1. Regulation of Cell Cycle by FOXO1 and Downstream Effectors
3.2. Liver Regeneration and EGFR Induction
3.3. WNT/b-Catenin Pathway
3.4. AFP (Alpha-Fetoprotein) Repression
3.5. Response to Inflammation and Migration
3.6. Interaction with Micro-RNAs, Long Non-Coding RNAs, and Circular RNAs
3.7. Regulation by CUX1
4. Lessons from Other Conditions
4.1. Further Evidence for the Regulation of the EGFR Pathway
4.2. Further Evidence for the Regulation of Inflammation and the NF-kB Pathway
4.3. Further Evidence on the Evasion of Apoptosis
5. A Practical Approach to Zbtb20 Tissue Expression in Malignant Tumors
5.1. Sensitivity
5.2. Specificity
5.3. Differential Expression
6. Future Perspectives and Clinical Application (Discussion)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Han, M.; Zhang, H.; Liu, F.; Pan, Y.; Zhu, J.; Liao, Z.; Chen, X.; Zhang, B. Structures and Biological Functions of Zinc Finger Proteins and Their Roles in Hepatocellular Carcinoma. Biomark. Res. 2022, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wen, D.; Zhang, S.; Jiang, H.; Di, X. The Role of Zinc Finger Proteins in Malignant Tumors. FASEB J. 2023, 37, e23157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, Y.; Qian, K.; Li, L.; Zhang, C.; Fu, X.; Zhang, X.; Chen, H.; Liu, Q.; Cao, S.; et al. A Novel Tumor Suppressor ZBTB1 Regulates Tamoxifen Resistance and Aerobic Glycolysis through Suppressing HER2 Expression in Breast Cancer. J. Biol. Chem. 2020, 295, 14140–14152. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H. Zinc Finger and BTB Domain-Containing Protein 3 Is Essential for the Growth of Cancer Cells. BMB Rep. 2014, 47, 405–410. [Google Scholar] [CrossRef]
- Molloy, M.E.; Lewinska, M.; Williamson, A.K.; Nguyen, T.T.; Kuser-Abali, G.; Gong, L.; Yan, J.; Little, J.B.; Pandolfi, P.P.; Yuan, Z.-M. ZBTB7A Governs Estrogen Receptor Alpha Expression in Breast Cancer. J. Mol. Cell Biol. 2018, 10, 273–284. [Google Scholar] [CrossRef]
- He, J.; Wu, M.; Xiong, L.; Gong, Y.; Yu, R.; Peng, W.; Li, L.; Li, L.; Tian, S.; Wang, Y.; et al. BTB/POZ Zinc Finger Protein ZBTB16 Inhibits Breast Cancer Proliferation and Metastasis through Upregulating ZBTB28 and Antagonizing BCL6/ZBTB27. Clin. Epigenet. 2020, 12, 82. [Google Scholar] [CrossRef]
- Jing, J.; Liu, J.; Wang, Y.; Zhang, M.; Yang, L.; Shi, F.; Liu, P.; She, J. The Role of ZBTB38 in Promoting Migration and Invasive Growth of Bladder Cancer Cells. Oncol. Rep. 2019, 41, 1980–1990. [Google Scholar] [CrossRef]
- Xiang, T.; Tang, J.; Li, L.; Peng, W.; Du, Z.; Wang, X.; Li, Q.; Xu, H.; Xiong, L.; Xu, C.; et al. Tumor Suppressive BTB/POZ Zinc-Finger Protein ZBTB28 Inhibits Oncogenic BCL6/ZBTB27 Signaling to Maintain P53 Transcription in Multiple Carcinogenesis. Theranostics 2019, 9, 8182–8195. [Google Scholar] [CrossRef]
- Zhang, W.; Mi, J.; Li, N.; Sui, L.; Wan, T.; Zhang, J.; Chen, T.; Cao, X. Identification and Characterization of DPZF, a Novel Human BTB/POZ Zinc Finger Protein Sharing Homology to BCL-6. Biochem. Biophys. Res. Commun. 2001, 282, 1067–1073. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc Finger Proteins: New Insights into Structural and Functional Diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Jones, K.A.; Luo, Y.; Dukes-Rimsky, L.; Srivastava, D.P.; Koul-Tewari, R.; Russell, T.A.; Shapiro, L.P.; Srivastava, A.K.; Penzes, P. Neurodevelopmental Disorder-Associated ZBTB20 Gene Variants Affect Dendritic and Synaptic Structure. PLoS ONE 2018, 13, e0203760. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.H.; Tonchev, A.B.; Stoykova, A.; Chowdhury, K. Regulation of Archicortical Arealization by the Transcription Factor Zbtb20. Hippocampus 2012, 22, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Tonchev, A.B.; Tuoc, T.C.; Rosenthal, E.H.; Studer, M.; Stoykova, A. Zbtb20 Modulates the Sequential Generation of Neuronal Layers in Developing Cortex. Mol. Brain 2016, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, H.; Tsai, W.; Zhang, Y.; Du, Y.; Zhong, J.; Szpirer, C.; Zhu, M.; Cao, X.; Barton, M.C.; et al. Zinc Finger Protein ZBTB20 Is a Key Repressor of Alpha-Fetoprotein Gene Transcription in Liver. Proc. Natl. Acad. Sci. USA 2008, 105, 10859–10864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Jiang, X.; Zhang, H.; Lu, Y.; Liu, A.; Ma, X.; Yang, G.; Yang, R.; Shen, H.; Zheng, J.; et al. Zbtb20 Regulates the Terminal Differentiation of Hypertrophic Chondrocytes via Repression of Sox9. Development 2015, 142, 385–393. [Google Scholar] [CrossRef] [PubMed]
- To, J.C.; Chiu, A.P.; Tschida, B.R.; Lo, L.H.; Chiu, C.H.; Li, X.-X.; Kuka, T.P.; Linden, M.A.; Amin, K.; Chan, W.-C.; et al. ZBTB20 Regulates WNT/CTNNB1 Signalling Pathway by Suppressing PPARG during Hepatocellular Carcinoma Tumourigenesis. JHEP Rep. 2021, 3, 100223. [Google Scholar] [CrossRef]
- Nielsen, J.V.; Thomassen, M.; Møllgård, K.; Noraberg, J.; Jensen, N.A. Zbtb20 Defines a Hippocampal Neuronal Identity Through Direct Repression of Genes That Control Projection Neuron Development in the Isocortex. Cereb. Cortex 2014, 24, 1216–1229. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Bähr, M.; Tonchev, A.B.; Stoykova, A. Zbtb20 Regulates Developmental Neurogenesis in the Olfactory Bulb and Gliogenesis after Adult Brain Injury. Mol. Neurobiol. 2019, 56, 567–582. [Google Scholar] [CrossRef]
- Cao, D.; Ma, X.; Cai, J.; Luan, J.; Liu, A.-J.; Yang, R.; Cao, Y.; Zhu, X.; Zhang, H.; Chen, Y.-X.; et al. ZBTB20 Is Required for Anterior Pituitary Development and Lactotrope Specification. Nat. Commun. 2016, 7, 11121. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, J.-H.; Jiang, H.; Wang, K.; Lu, J.-Y.; Jiang, X.; Ma, X.; Chen, Y.-X.; Ren, A.-J.; Zheng, J.; et al. ZBTB20 Regulates EGFR Expression and Hepatocyte Proliferation in Mouse Liver Regeneration. Cell Death Dis. 2018, 9, 462. [Google Scholar] [CrossRef]
- Chevrier, S.; Emslie, D.; Shi, W.; Kratina, T.; Wellard, C.; Karnowski, A.; Erikci, E.; Smyth, G.K.; Chowdhury, K.; Tarlinton, D.; et al. The BTB-ZF Transcription Factor Zbtb20 Is Driven by Irf4 to Promote Plasma Cell Differentiation and Longevity. J. Exp. Med. 2014, 211, 827–840. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhu, J.; Mo, J.; Zhao, H.; Chen, Q. HBV DNA Integrates into Upregulated ZBTB20 in Patients with Hepatocellular Carcinoma. Mol. Med. Rep. 2020, 22, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Huang, Y.; Li, X.; Liu, D.; Chen, J.; Shu, M. Zinc Finger Protein ZBTB20 Is an Independent Prognostic Marker and Promotes Tumor Growth of Human Hepatocellular Carcinoma by Repressing FoxO1. Oncotarget 2016, 7, 14336–14349. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Fernandez de Mattos, S.; van der Horst, A.; Klompmaker, R.; Kops, G.J.P.L.; Lam, E.W.-F.; Burgering, B.M.T.; Medema, R.H. Cell Cycle Inhibition by FoxO Forkhead Transcription Factors Involves Downregulation of Cyclin D. Mol. Cell. Biol. 2002, 22, 7842–7852. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Long Non-Coding RNA LINC00641 Promotes Cell Growth and Migration through Modulating MiR-378a/ZBTB20 Axis in Acute Myeloid Leukemia. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7498–7509. [Google Scholar] [PubMed]
- Zhao, J.; Ren, K.; Tang, J. Zinc Finger Protein ZBTB20 Promotes Cell Proliferation in Non-Small Cell Lung Cancer through Repression of FoxO1. FEBS Lett. 2014, 588, 4536–4542. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-R.; Kim, H.N.; Kweon, S.-S.; Choi, J.-S.; Shim, H.J.; Cho, S.H.; Chung, I.J.; Park, Y.-K.; Kim, S.H.; Choi, Y.-D.; et al. Genetic Variations in the PRKAA1 and ZBTB20 Genes and Gastric Cancer Susceptibility in a Korean Population. Mol. Carcinog. 2013, 52, 155–160. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Hui, X.; Wang, W.; Fang, D.; Ding, L. Mir-758-5p Suppresses Glioblastoma Proliferation, Migration and Invasion by Targeting ZBTB20. Cell. Physiol. Biochem. 2018, 48, 2074–2083. [Google Scholar] [CrossRef]
- Xu, X.; Xie, Q.; Xie, M.; Zeng, Y.; Liu, Q. LncRNA SNHG8 Serves as an Oncogene in Breast Cancer through MiR-634/ZBTB20 Axis. Cancer Manag. Res. 2021, 13, 3017–3028. [Google Scholar] [CrossRef]
- Chang, W.; Shang, Z.; Ming, X.; Wu, J.; Xiao, Y. Circ-SFMBT2 Facilitates the Malignant Growth of Acute Myeloid Leukemia Cells by Modulating MiR-582-3p/ZBTB20 Pathway. Histol. Histopathol. 2022, 37, 13. [Google Scholar] [CrossRef]
- Wu, H.; Wang, A.; Wang, L.; Shi, F.; Lin, F.; Cui, H. A Novel Circ_0104345/MiR-876-3p/ZBTB20 Axis Regulates the Proliferation, Migration, Invasion, and Apoptosis of Breast Cancer Cells. Biochem. Genet. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Zhang, M.; Cheng, L.; Zhang, Y.; Wang, X. ZBTB20 Promotes Cell Migration and Invasion of Gastric Cancer by Inhibiting IκBα to Induce NF-κB Activation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3862–3872. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Teal, E.; Dua-Awereh, M.; Hirshorn, S.T.; Zavros, Y. The Role of Metaplasia during Gastric Regeneration. Am. J. Physiol.-Cell Physiol. 2020, 319, C947–C954. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Blosse, A.; Lehours, P.; Wilson, K.T.; Gobert, A.P. Helicobacter: Inflammation, Immunology, and Vaccines. Helicobacter 2018, 23, e12517. [Google Scholar] [CrossRef]
- Li, N.; Tang, B.; Jia, Y.; Zhu, P.; Zhuang, Y.; Fang, Y.; Li, Q.; Wang, K.; Zhang, W.; Guo, G.; et al. Helicobacter Pylori CagA Protein Negatively Regulates Autophagy and Promotes Inflammatory Response via C-Met-PI3K/Akt-MTOR Signaling Pathway. Front. Cell. Infect. Microbiol. 2017, 7, 417. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Preiss, N.K.; Valenteros, K.B.; Kamal, Y.; Usherwood, Y.-K.; Frost, H.R.; Usherwood, E.J. Zbtb20 Restrains CD8 T Cell Immunometabolism and Restricts Memory Differentiation and Antitumor Immunity. J. Immunol. 2020, 205, 2649–2666. [Google Scholar] [CrossRef]
- Medema, R.H.; Kops, G.J.P.L.; Bos, J.L.; Burgering, B.M.T. AFX-like Forkhead Transcription Factors Mediate Cell-Cycle Regulation by Ras and PKB through P27kip1. Nature 2000, 404, 782–787. [Google Scholar] [CrossRef]
- Zhang, H. ZBTB20 Is a Sequence-Specific Transcriptional Repressor of Alpha-Fetoprotein Gene. Sci. Rep. 2015, 5, 11979. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, C. Convergence between Wnt-β-Catenin and EGFR Signaling in Cancer. Mol. Cancer 2010, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lu, H.; Zhang, L. Serum AFP Levels in Patients Suffering from 47 Different Types of Cancers and Noncancer Diseases. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 199–212. ISBN 978-0-12-817738-9. [Google Scholar]
- Moldogazieva, N.T.; Zavadskiy, S.P.; Sologova, S.S.; Mokhosoev, I.M.; Terentiev, A.A. Predictive Biomarkers for Systemic Therapy of Hepatocellular Carcinoma. Expert Rev. Mol. Diagn. 2021, 21, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T.-Y. Update on the Applications and Limitations of Alpha-Fetoprotein for Hepatocellular Carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tan, Y.; Ren, Y.; Dong, L.; Xie, Z.; Tang, L.; Cao, D.; Zhang, W.; Hu, H.; Wang, H. Zinc Finger Protein ZBTB20 Expression Is Increased in Hepatocellular Carcinoma and Associated with Poor Prognosis. BMC Cancer 2011, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.; Carmody, R. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Chase, A.J.; Baker, A.H.; Newby, A.C. Inhibition of Transcription Factor NF-κB Reduces Matrix Metalloproteinase-1, -3 and -9 Production by Vascular Smooth Muscle Cells. Cardiovasc. Res. 2001, 50, 556–565. [Google Scholar] [CrossRef]
- Li, Y.-F.; Xu, X.-B.; Chen, X.-H.; Wei, G.; He, B.; Wang, J.-D. The Nuclear Factor-κB Pathway Is Involved in Matrix Metalloproteinase-9 Expression in RU486-Induced Endometrium Breakdown in Mice. Hum. Reprod. 2012, 27, 2096–2106. [Google Scholar] [CrossRef]
- Shi, J.; Li, W.; Ding, X. Assessment of the Association between ZBTB20 Rs9841504 Polymorphism and Gastric and Esophageal Cancer Susceptibility: A Meta-Analysis. Int. J. Biol. Mark. 2017, 32, 96–101. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Z.; Wu, C.; Dai, J.; Li, H.; Dong, J.; Wang, M.; Miao, X.; Zhou, Y.; Lu, F.; et al. A Genome-Wide Association Study Identifies New Susceptibility Loci for Non-Cardia Gastric Cancer at 3q13.31 and 5p13.1. Nat. Genet. 2011, 43, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-Y.; Cai, Z.-R.; Liu, J.; Wang, D.-S.; Ju, H.-Q.; Xu, R.-H. Circular RNA: Metabolism, Functions and Interactions with Proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Qiu, B. LncRNA SNHG8 Promotes Cell Migration and Invasion in Breast Cancer Cell through MiR-634/ZBTB20 Axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11639–11649. [Google Scholar] [PubMed]
- Kojima, K.; Takata, A.; Vadnais, C.; Otsuka, M.; Yoshikawa, T.; Akanuma, M.; Kondo, Y.; Kang, Y.J.; Kishikawa, T.; Kato, N.; et al. MicroRNA122 Is a Key Regulator of α-Fetoprotein Expression and Influences the Aggressiveness of Hepatocellular Carcinoma. Nat. Commun. 2011, 2, 338. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Miyaaki, H.; Ichikawa, T. Antitumor Function of MicroRNA-122 against Hepatocellular Carcinoma. J. Gastroenterol. 2014, 49, 589–593. [Google Scholar] [CrossRef]
- Li, F.; Du, M.; Yang, Y.; Wang, Z.; Zhang, H.; Wang, X.; Li, Q. Zinc Finger and BTB Domain-Containing Protein 20 Aggravates Angiotensin II-Induced Cardiac Remodeling via the EGFR-AKT Pathway. J. Mol. Med. 2022, 100, 427–438. [Google Scholar] [CrossRef]
- Weng, M.-Z.; Zhuang, P.-Y.; Hei, Z.-Y.; Lin, P.-Y.; Chen, Z.-S.; Liu, Y.-B.; Quan, Z.-W.; Tang, Z.-H. ZBTB20 Is Involved in Liver Regeneration after Partial Hepatectomy in Mouse. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 48–54. [Google Scholar] [CrossRef]
- Lu, L.; Shi, M.; Qiu, J.; Shi, Z.; Wang, C.; Fu, Y.; Lin, C.; Zhang, L.; Tao, J.; Liu, C.; et al. ZBTB20 Regulates Cardiac Allograft Rejection through NFκB-Mediated Inflammation in Mouse Heart Transplantation. Transplant Immunol. 2022, 74, 101676. [Google Scholar] [CrossRef]
- Tao, J.; Qiu, J.; Lu, L.; Zhang, L.; Fu, Y.; Wang, M.; Han, J.; Shi, M.; Li, L.; Zhao, Z.; et al. ZBTB20 Positively Regulates Oxidative Stress, Mitochondrial Fission, and Inflammatory Responses of Ox-LDL-Induced Macrophages in Atherosclerosis. Oxid. Med. Cell. Longev. 2021, 2021, 5590855. [Google Scholar] [CrossRef]
- Qiu, J.; Peng, P.; Xin, M.; Wen, Z.; Chen, Z.; Lin, S.; Kuang, M.; Fu, Y.; Fang, G.; Li, S.; et al. ZBTB20-Mediated Titanium Particle-Induced Peri-Implant Osteolysis by Promoting Macrophage Inflammatory Responses. Biomater. Sci. 2020, 8, 3147–3163. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, P.; Bao, Y.; Han, Y.; Wang, Y.; Zhang, Q.; Zhan, Z.; Meng, J.; Li, Y.; Li, N.; et al. Zinc Finger Protein ZBTB20 Promotes Toll-like Receptor-Triggered Innate Immune Responses by Repressing IκBα Gene Transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 11097–11102. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhuang, Q.; Li, P.; Zeng, J.; Peng, Y.; Ding, Z.; Cao, H.; Zheng, R.; Wang, W. The Long Non-Coding RNA KLF3-AS1/MiR-10a-3p/ZBTB20 Axis Improves the Degenerative Changes in Human Nucleus Pulposus Cells. Cell Tissue Res. 2023, 393, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Selves, J.; Long-Mira, E.; Mathieu, M.-C.; Rochaix, P.; Ilié, M. Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site. Cancers 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; He, K.; Wang, S.; Chen, W.; Li, H. Expression of Zinc Finger and BTB Domain-Containing 4 in Colorectal Cancer and Its Clinical Significance. Cancer Manag. Res. 2020, 12, 9621–9626. [Google Scholar] [CrossRef] [PubMed]
- McCourt, C.M.; Boyle, D.; James, J.; Salto-Tellez, M. Immunohistochemistry in the Era of Personalised Medicine. J. Clin. Pathol. 2013, 66, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Idikio, H.A. Immunohistochemistry in Diagnostic Surgical Pathology: Contributions of Protein Life-Cycle, Use of Evidence-Based Methods and Data Normalization on Interpretation of Immunohistochemical Stains. Int. J. Clin. Exp. Pathol. 2010, 3, 169. [Google Scholar]
- Painter, J.T.; Clayton, N.P.; Herbert, R.A. Useful Immunohistochemical Markers of Tumor Differentiation. Toxicol. Pathol. 2010, 38, 131–141. [Google Scholar] [CrossRef]
- Kandukuri, S.R.; Lin, F.; Gui, L.; Gong, Y.; Fan, F.; Chen, L.; Cai, G.; Liu, H. Application of Immunohistochemistry in Undifferentiated Neoplasms: A Practical Approach. Arch. Pathol. Lab. Med. 2017, 141, 1014–1032. [Google Scholar] [CrossRef]
- Yokota, S.; Ohnishi, T.; Muroi, M.; Tanamoto, K.; Fujii, N.; Amano, K. Highly-Purified Helicobacter pylori LPS Preparations Induce Weak Inflammatory Reactions and Utilize Toll-like Receptor 2 Complex but Not Toll-like Receptor 4 Complex. FEMS Immunol. Med. Microbiol. 2007, 51, 140–148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanov, D.; Stoyanov, G.S.; Ivanov, M.N.; Spasov, R.H.; Tonchev, A.B. Transcription Factor Zbtb20 as a Regulator of Malignancy and Its Practical Applications. Int. J. Mol. Sci. 2023, 24, 13763. https://doi.org/10.3390/ijms241813763
Stoyanov D, Stoyanov GS, Ivanov MN, Spasov RH, Tonchev AB. Transcription Factor Zbtb20 as a Regulator of Malignancy and Its Practical Applications. International Journal of Molecular Sciences. 2023; 24(18):13763. https://doi.org/10.3390/ijms241813763
Chicago/Turabian StyleStoyanov, Dimo, George S. Stoyanov, Martin N. Ivanov, Radoslav H. Spasov, and Anton B. Tonchev. 2023. "Transcription Factor Zbtb20 as a Regulator of Malignancy and Its Practical Applications" International Journal of Molecular Sciences 24, no. 18: 13763. https://doi.org/10.3390/ijms241813763
APA StyleStoyanov, D., Stoyanov, G. S., Ivanov, M. N., Spasov, R. H., & Tonchev, A. B. (2023). Transcription Factor Zbtb20 as a Regulator of Malignancy and Its Practical Applications. International Journal of Molecular Sciences, 24(18), 13763. https://doi.org/10.3390/ijms241813763







