Establishment of a Seven-Gene Signature Associated with CD8+ T Cells through the Utilization of Both Single-Cell and Bulk RNA-Sequencing Techniques in Clear Cell Renal Cell Carcinoma
Abstract
:1. Introduction
2. Results
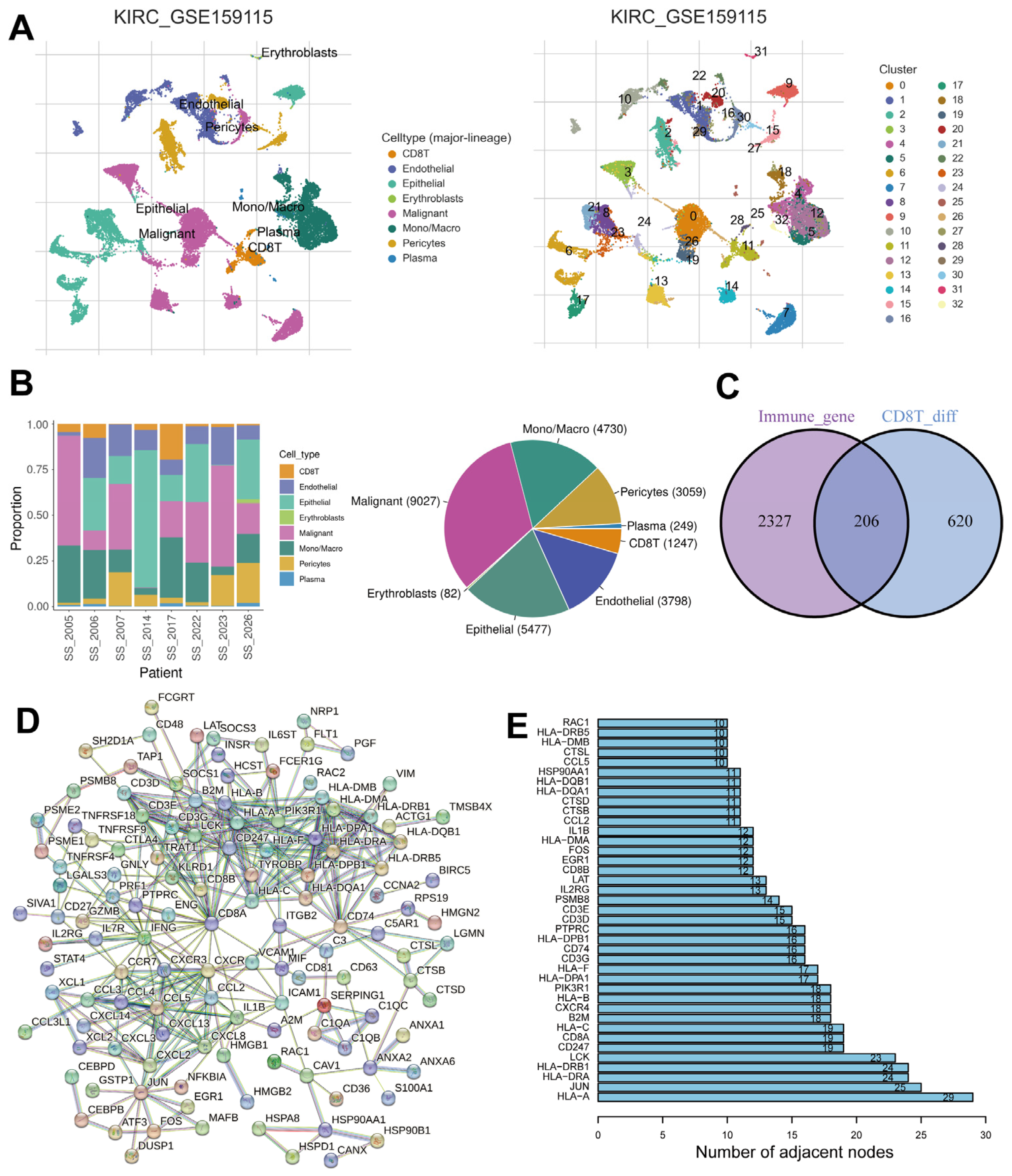
2.1. Identification of Immune- and CD8+ T-Cell-Related DEGs
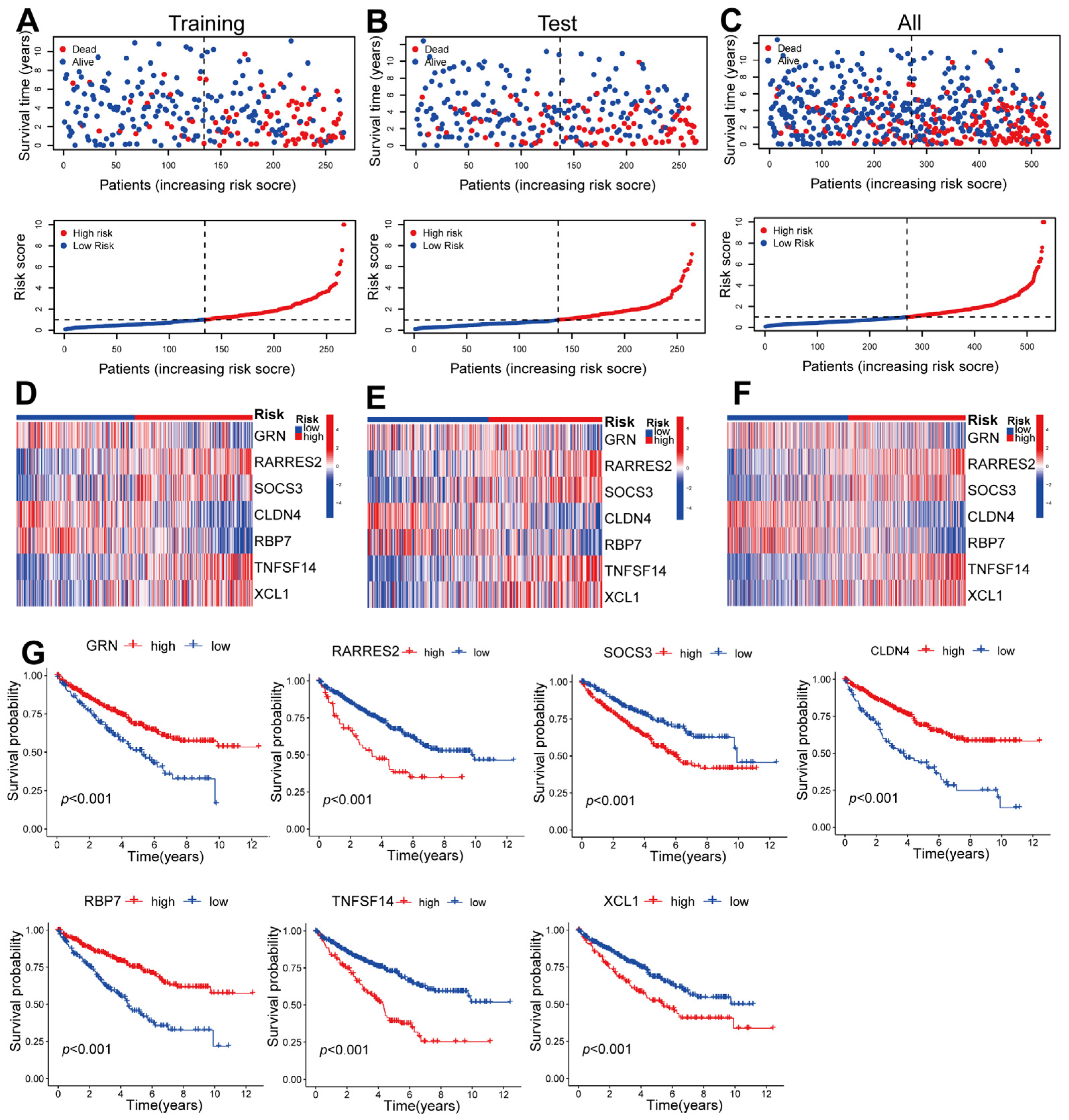
2.2. Construction of a Seven-Gene Signature Related to CD8+ T Cells in ccRCC
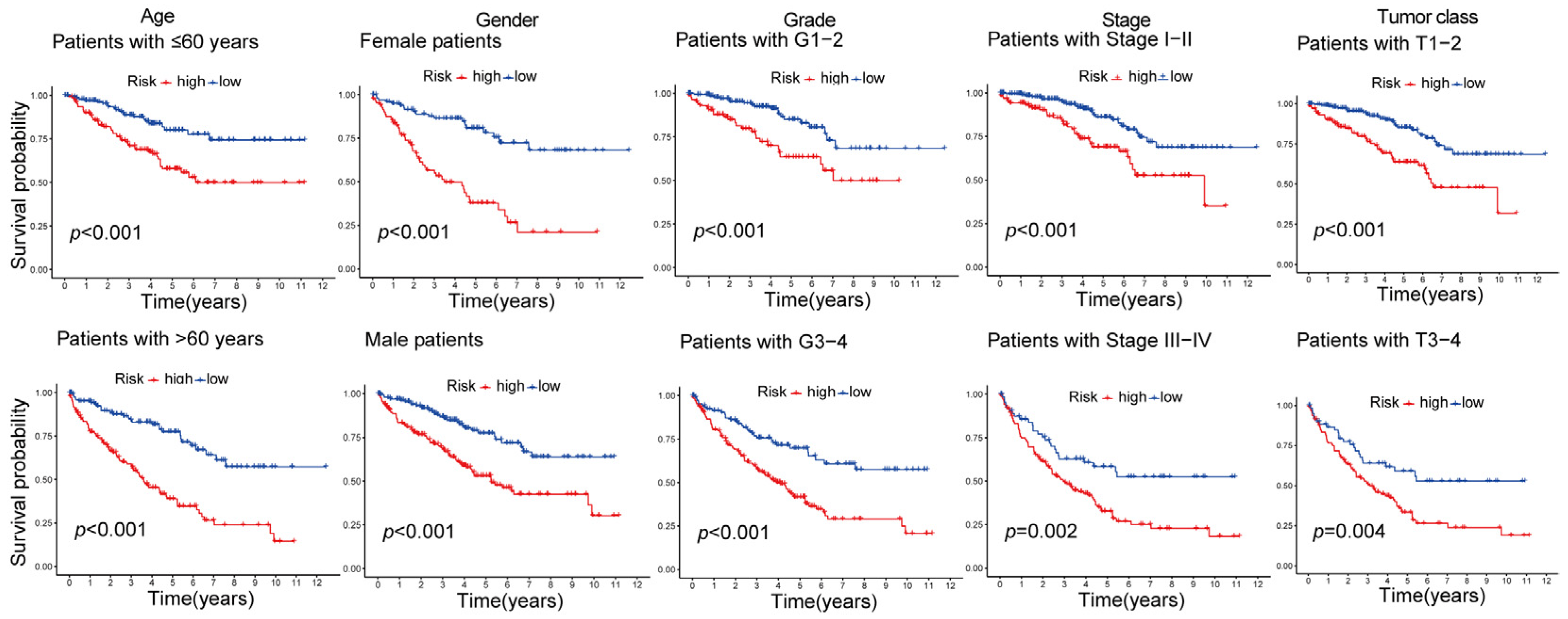
2.3. Validating the Prognosis of CD8+ T-Cell-Associated Genes in Training, Testing, and Overall Datasets
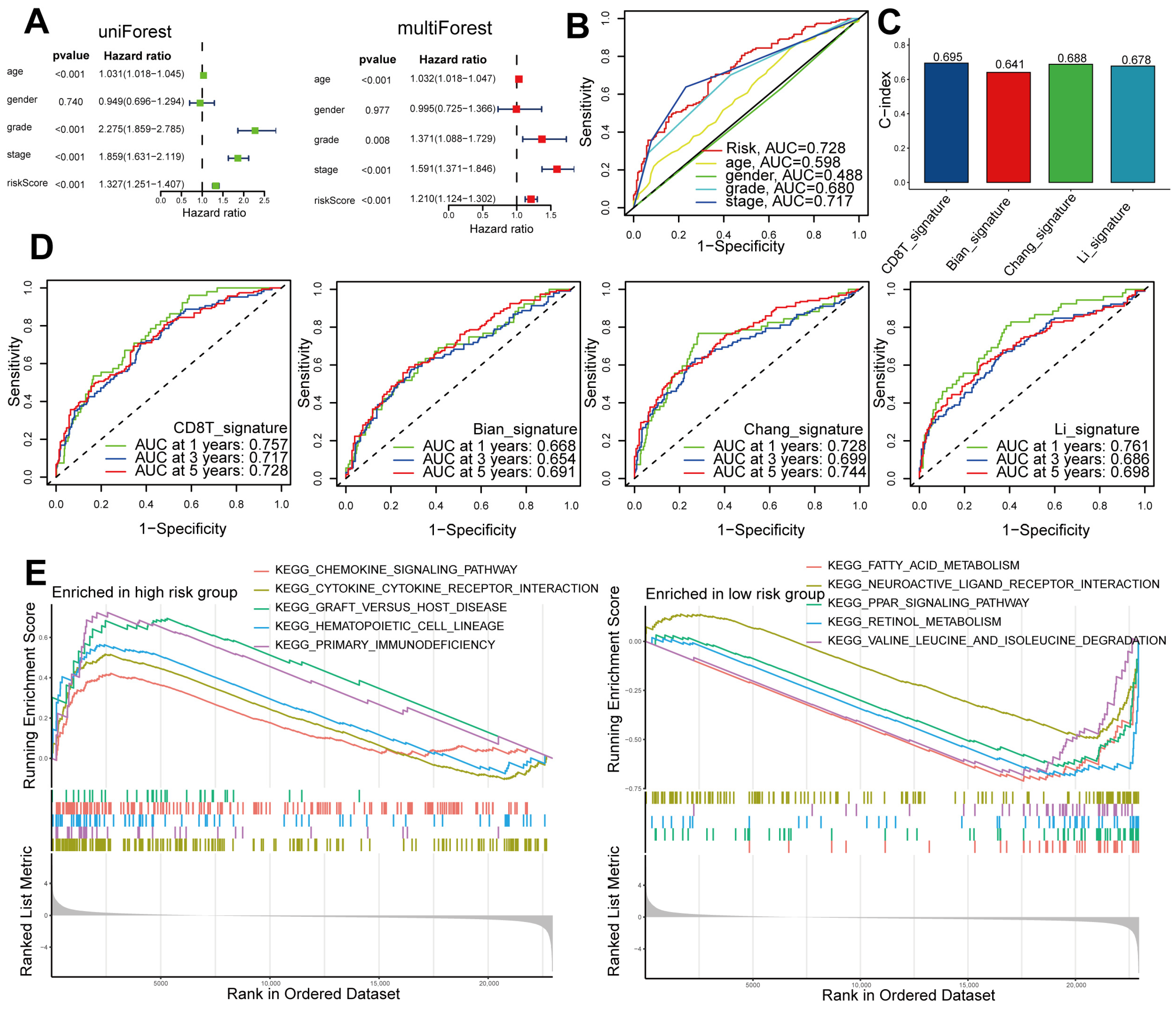
2.4. Independent and Superior Prognostic Ability of the Seven-Gene CD8+ T-Cell-Associated Signature in Patients with ccRCC
2.5. Comparing DEGs Associated with High- and Low-Risk Patients Based on Functional Enrichment
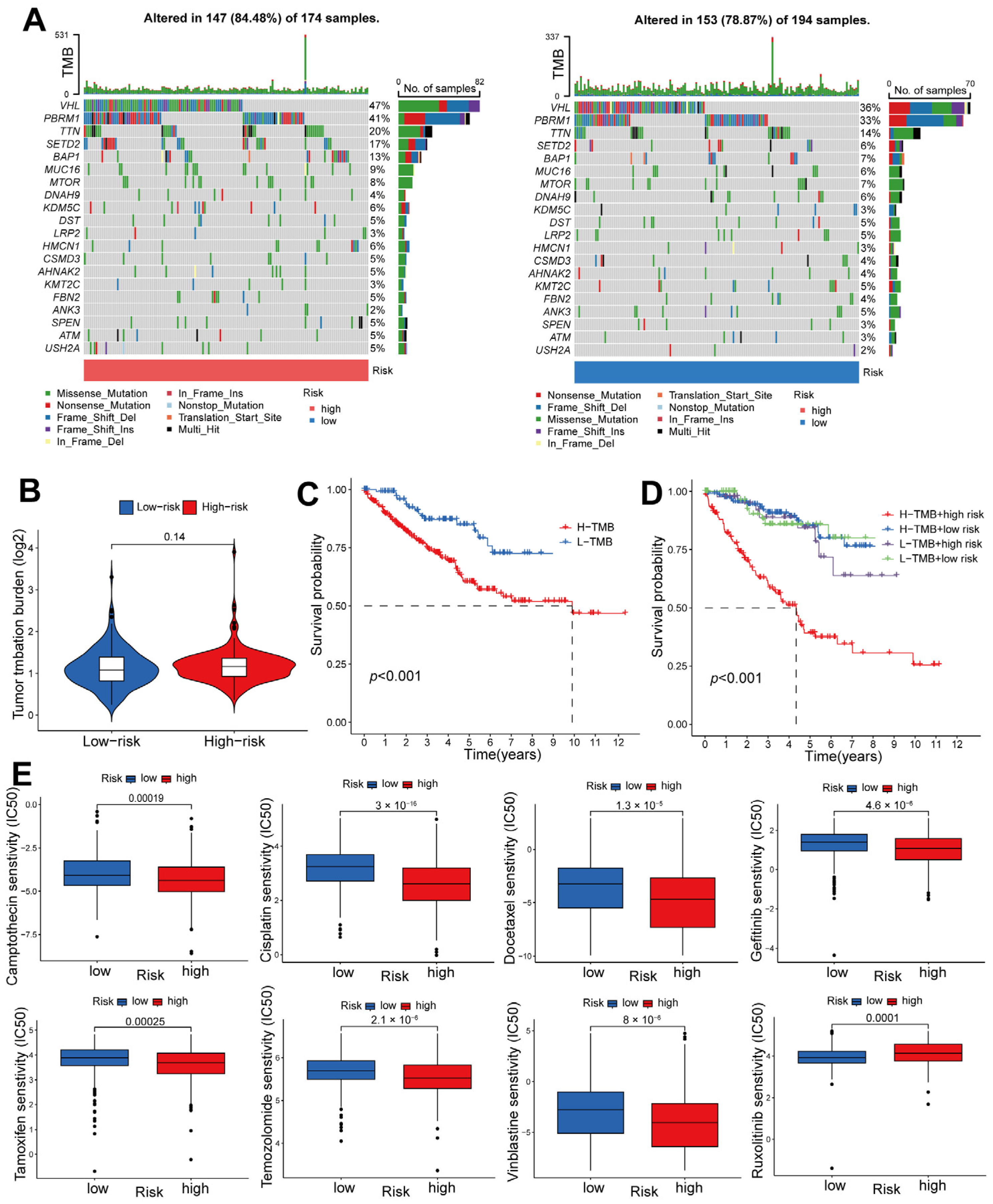
2.6. Risk-Score-Based Characteristics of ccRCC Tumor Mutations and Response to Chemotherapy
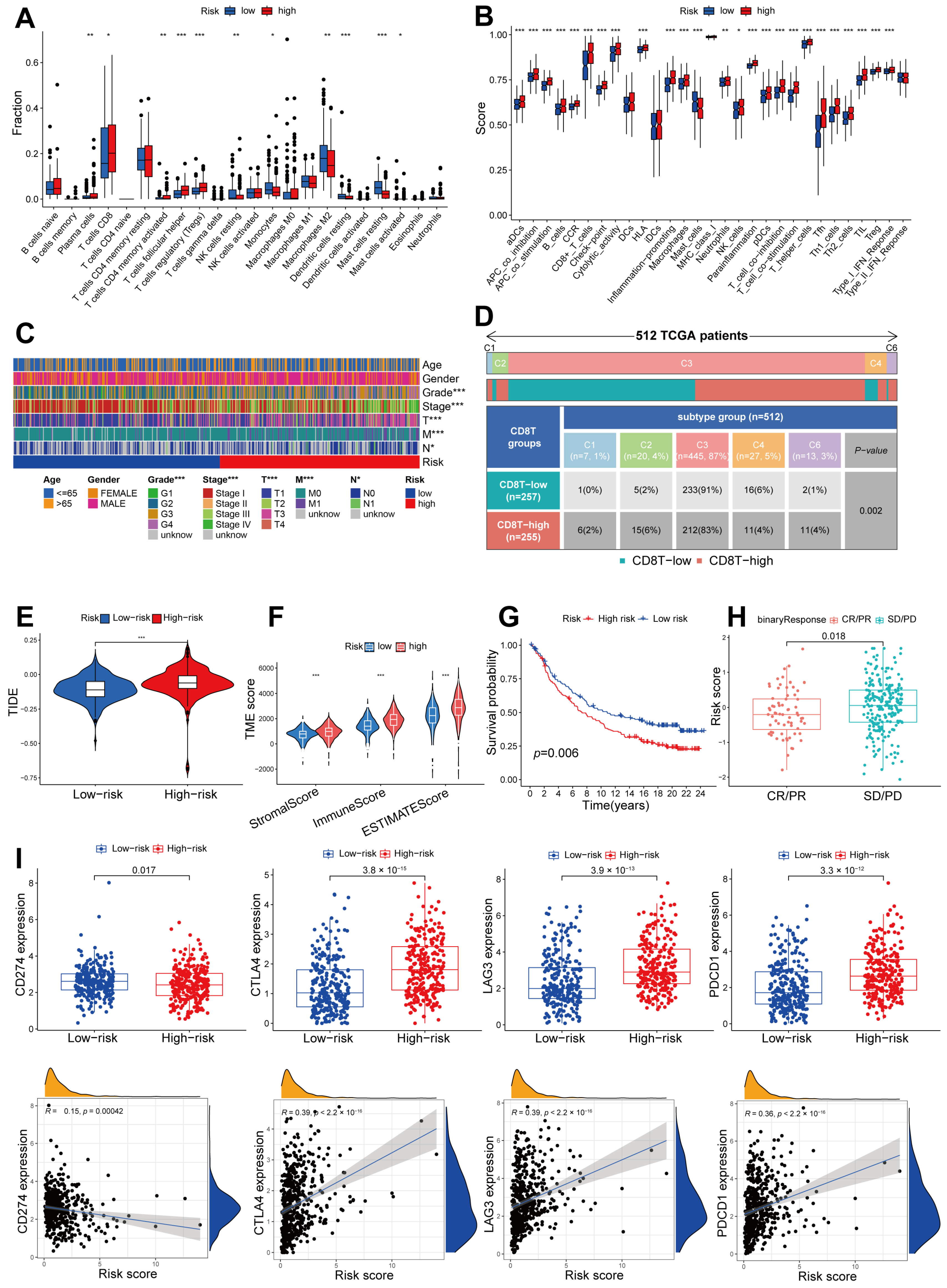
2.7. Immune Infiltration Pattern Variation between Different Risk Groups, and the Potential for Utilizing a Signature Related to CD8+ T Cells in Anti-PD-1/L1 Immunotherapy
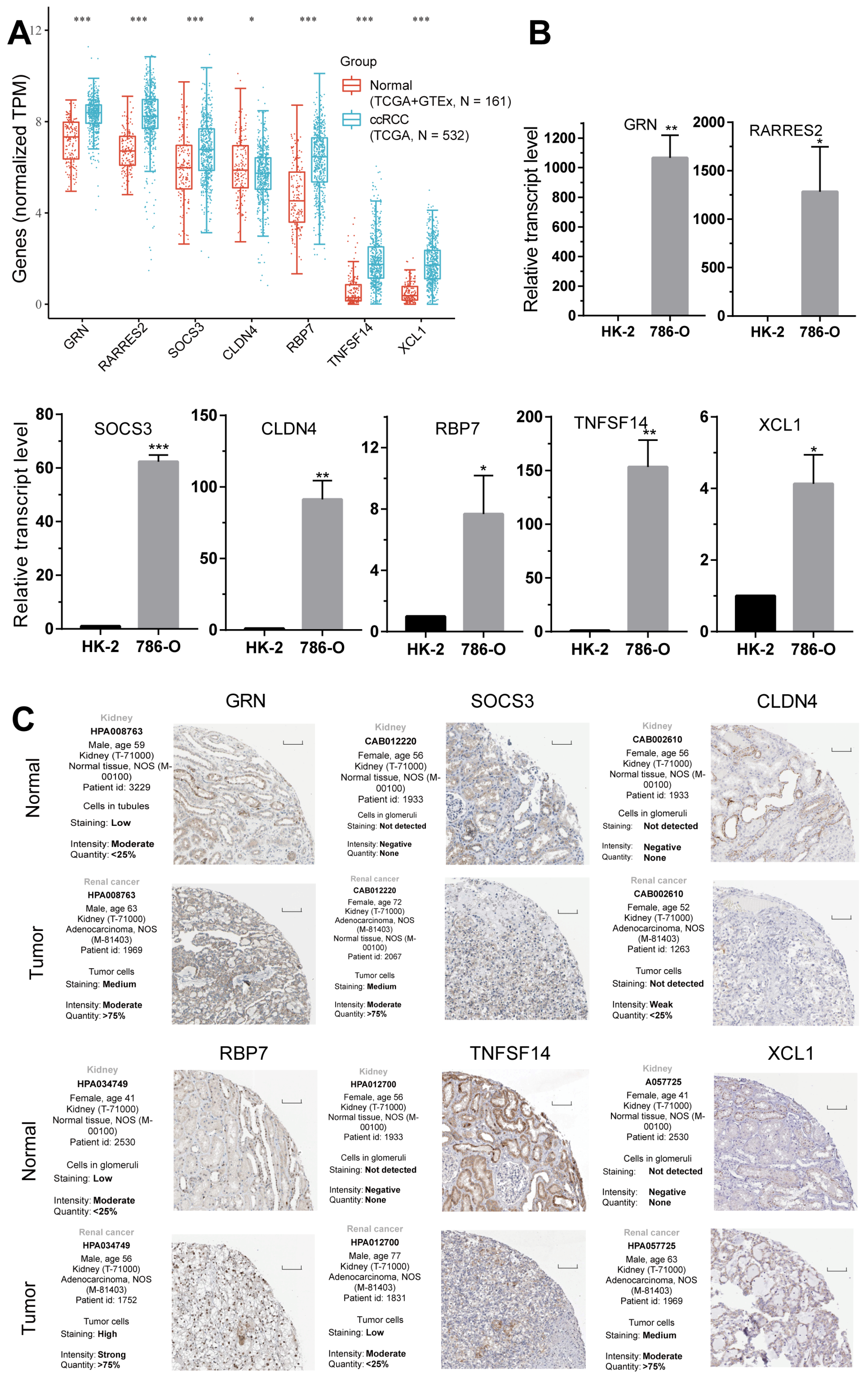
2.8. Validation of the Expression of the Seven CD8+ T-Cell-Related Genes
3. Discussion
4. Materials and Methods
4.1. Data Acquisition
4.2. Construction of a Predictive Signature in TCGA
4.3. Analysis of Functional Enrichment
4.4. Immunotherapeutic Sensitivity Analysis of the CD8+ T-Cell-Related Signature
4.5. qRT-PCR
4.6. Human Protein Atlas (HPA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Wei, P.T.; Lv, W.W.; Han, X.T.; Yang, J.H.; Qin, S.F.; Zhang, Y. MELK is Upregulated in Advanced Clear Cell Renal Cell Carcinoma and Promotes Disease Progression by Phosphorylating PRAS40. Cell Transplant. 2019, 28, 37s–50s. [Google Scholar] [CrossRef]
- Hwang, H.S.; Park, Y.Y.; Shin, S.J.; Go, H.; Park, J.M.; Yoon, S.Y.; Lee, J.L.; Cho, Y.M. Involvement of the TNF-alpha Pathway in TKI Resistance and Suggestion of TNFR1 as a Predictive Biomarker for TKI Responsiveness in Clear Cell Renal Cell Carcinoma. J. Korean Med. Sci. 2020, 35, e31. [Google Scholar] [CrossRef]
- Lopez-Fernandez, E.; Lopez, J.I. The Impact of Tumor Eco-Evolution in Renal Cell Carcinoma Sampling. Cancers 2018, 10, 485. [Google Scholar] [CrossRef]
- Sun, S.S.; Fu, Y.; Lin, J.Y. Upregulation of MYBL2 independently predicts a poorer prognosis in patients with clear cell renal cell carcinoma. Oncol. Lett. 2020, 19, 2765–2772. [Google Scholar] [CrossRef]
- Dou, Q.; Gao, S.; Gan, H.; Kang, Z.; Zhang, H.; Yang, Y.; Tong, H. A Metastasis-Related lncRNA Signature Correlates with the Prognosis in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 692535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ren, H.; Ge, L.; Zhang, W.; Song, F.; Huang, P. A review on the role of long non-coding RNA and microRNA network in clear cell renal cell carcinoma and its tumor microenvironment. Cancer Cell Int. 2023, 23, 16. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Dang, C.; Zhang, H.; Wang, X.; Yin, J.; Jia, R.; Zhang, Y. The Early Diagnostic and Prognostic Value of BIRC5 in Clear-Cell Renal Cell Carcinoma Based on the Cancer Genome Atlas Data. Urol. Int. 2022, 106, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, L.; Jordao, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Kimmelman, A.C.; DePinho, R.A. Metabolic Codependencies in the Tumor Microenvironment. Cancer Discov. 2021, 11, 1067–1081. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.; Tabernacki, T.; Kobyra, J.; Roberts, P.; Chiappinelli, K.B. Combining epigenetic and immune therapy to overcome cancer resistance. Semin. Cancer Biol. 2020, 65, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Brech, D.; Herbstritt, A.S.; Diederich, S.; Straub, T.; Kokolakis, E.; Irmler, M.; Beckers, J.; Buttner, F.A.; Schaeffeler, E.; Winter, S.; et al. Dendritic Cells or Macrophages? The Microenvironment of Human Clear Cell Renal Cell Carcinoma Imprints a Mosaic Myeloid Subtype Associated with Patient Survival. Cells 2022, 11, 3289. [Google Scholar] [CrossRef]
- Braun, D.A.; Street, K.; Burke, K.P.; Cookmeyer, D.L.; Denize, T.; Pedersen, C.B.; Gohil, S.H.; Schindler, N.; Pomerance, L.; Hirsch, L.; et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell 2021, 39, 632–648.e8. [Google Scholar] [CrossRef]
- Xue, B.; Guo, W.M.; Jia, J.D.; Kadeerhan, G.; Liu, H.P.; Bai, T.; Shao, Y.; Wang, D.W. MUC20 as a novel prognostic biomarker in ccRCC correlating with tumor immune microenvironment modulation. Am. J. Cancer Res. 2022, 12, 695–712. [Google Scholar]
- Lin, J.; Yu, M.; Xu, X.; Wang, Y.; Xing, H.; An, J.; Yang, J.; Tang, C.; Sun, D.; Zhu, Y. Identification of biomarkers related to CD8(+) T cell infiltration with gene co-expression network in clear cell renal cell carcinoma. Aging 2020, 12, 3694–3712. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, R.; Zhang, L.; Wei, Y.; Zhang, R.; Cai, W.; Hu, W. LINC00887 Fosters Development of Clear Cell Renal Cell Carcinoma via Inhibiting CD8+ T Cell Immune Infiltration. Comput. Math. Methods Med. 2022, 2022, 2582474. [Google Scholar] [CrossRef]
- Bian, Z.L.; Fan, R.; Xie, L.M. A Novel Cuproptosis-Related Prognostic Gene Signature and Validation of Differential Expression in Clear Cell Renal Cell Carcinoma. Genes 2022, 13, 851. [Google Scholar] [CrossRef]
- Chang, K.L.; Yuan, C.; Liu, X.G. Ferroptosis-Related Gene Signature Accurately Predicts Survival Outcomes in Patients with Clear-Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 649347. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Li, Y.Q.; Lyu, Y.F.; Tan, L.Y.; Zheng, X.Y.; Jiang, H.W.; Wen, H.; Feng, C.C. Development of a Phagocytosis-Dependent Gene Signature to Predict Prognosis and Response to Checkpoint Inhibition in Clear-Cell Renal Cell Carcinoma. Front. Immunol. 2022, 13, 853088. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Z.; Kapoor, A.; Gu, Y.; Chow, M.J.; Peng, J.Y.; Major, P.; Tang, D.M. Construction of a Novel Multigene Panel Potently Predicting Poor Prognosis in Patients with Clear Cell Renal Cell Carcinoma. Cancers 2020, 12, 3471. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Zhang, R.; Chen, B.; Huang, J.; Li, C.; Zhang, W.; Wang, Y.; Gao, Y.; Zheng, J.; et al. A New Prognostic Risk Signature of Eight Ferroptosis-Related Genes in the Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 700084. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, W.; Jiang, D.; Xiong, H.; Liao, G.; Yang, X.; Ma, H.; Li, J.; Qiu, M.; Li, B.; et al. Systematic Chromatin Accessibility Analysis Based on Different Immunological Subtypes of Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 575425. [Google Scholar] [CrossRef]
- Bannoud, N.; Dalotto-Moreno, T.; Kindgard, L.; Garcia, P.A.; Blidner, A.G.; Marino, K.V.; Rabinovich, G.A.; Croci, D.O. Hypoxia Supports Differentiation of Terminally Exhausted CD8 T Cells. Front. Immunol. 2021, 12, 660944. [Google Scholar] [CrossRef]
- Tummler, C.; Snapkov, I.; Wickstrom, M.; Moens, U.; Ljungblad, L.; Maria Elfman, L.H.; Winberg, J.O.; Kogner, P.; Johnsen, J.I.; Sveinbjornsson, B. Inhibition of chemerin/CMKLR1 axis in neuroblastoma cells reduces clonogenicity and cell viability in vitro and impairs tumor growth in vivo. Oncotarget 2017, 8, 95135–95151. [Google Scholar] [CrossRef] [PubMed]
- Pachynski, R.K.; Wang, P.; Salazar, N.; Zheng, Y.Y.; Nease, L.; Rosalez, J.; Leong, W.I.; Virdi, G.; Rennier, K.; Shin, W.J.; et al. Chemerin Suppresses Breast Cancer Growth by Recruiting Immune Effector Cells into the Tumor Microenvironment. Front. Immunol. 2019, 10, 983. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ito, M.; Chikuma, S.; Akanuma, T.; Nakatsukasa, H. Negative Regulation of Cytokine Signaling in Immunity. Cold Spring Harb. Perspect. Biol. 2018, 10, a028571. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Lu, S.; Wu, Z.; Zhou, L.; Cheng, Z.; Bai, Y.; Zhao, J.; Zhang, Q.; Mao, H. Quantitative assessment of gene promoter methylation in non-small cell lung cancer using methylation-sensitive high-resolution melting. Oncol. Lett. 2018, 15, 7639–7648. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.D.; Wang, Y.; Chlewicki, L.K.; Zhang, Y.; Guo, J.Y.; Liang, W.; Wang, J.Y.; Wang, X.X.; Fu, Y.X. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade. Cancer Cell 2016, 30, 500. [Google Scholar] [CrossRef]
- Sabet, M.N.; Asl, M.M.; Esfeh, M.K.; Nasrabadi, N.; Shakarami, M.; Alani, B.; Alimolaie, A.; Azhdari, S.; Cheraghi, E. Mesenchymal stem cells as professional actors in gastrointestinal cancer therapy: From Naive to genetically modified. Iran. J. Basic. Med. Sci. 2021, 24, 561–576. [Google Scholar] [CrossRef]
- Shan, M.J.; Wang, Y.B. Viewing keloids within the immune microenvironment. Am. J. Transl. Res. 2022, 14, 718–727. [Google Scholar] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Zhang, J. 8-Gene signature related to CD8(+) T cell infiltration by integrating single-cell and bulk RNA-sequencing in head and neck squamous cell carcinoma. Front. Genet. 2022, 13, 938611. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.L.; Qi, J.; Peng, D.; Guan, B.; Zhang, J.Y.; Li, Z.W.; Zhang, H.X.; Li, T.; Shi, Y.; et al. Increased chromosomal instability characterizes metastatic renal cell carcinoma. Transl. Oncol. 2021, 14, 100929. [Google Scholar] [CrossRef]
- Li, S.J.; Rodriguez, J.; Li, W.Y.; Bullova, P.; Fell, S.M.; Surova, O.; Westerlund, I.; Topcic, D.; Bergsland, M.; Stenman, A.; et al. EglN3 hydroxylase stabilizes BIM-EL linking VHL type 2C mutations to pheochromocytoma pathogenesis and chemotherapy resistance. Proc. Natl. Acad. Sci. USA 2019, 116, 16997–17006. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Duan, Y.; Yue, K.; Ye, B.; Chen, P.; Zhang, J.; He, Q.; Wu, Y.; Lai, Q.; Li, H.; Wu, Y.; et al. LncRNA MALAT1 promotes growth and metastasis of head and neck squamous cell carcinoma by repressing VHL through a non-canonical function of EZH2. Cell Death Dis. 2023, 14, 149. [Google Scholar] [CrossRef]
- Menasche, B.L.; Davis, E.M.; Wang, S.; Ouyang, Y.; Li, S.; Yu, H.; Shen, J. PBRM1 and the glycosylphosphatidylinositol biosynthetic pathway promote tumor killing mediated by MHC-unrestricted cytotoxic lymphocytes. Sci. Adv. 2020, 6, eabc3243. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sahin, M.; Wakamatsu, T.; Inoue-Yamauchi, A.; Zhao, W.; Han, S.; Nargund, A.M.; Yang, S.; Lyu, Y.; Hsieh, J.J.; et al. SETD2 regulates chromatin accessibility and transcription to suppress lung tumorigenesis. J. Clin. Investig. 2023, 8, e154120. [Google Scholar] [CrossRef]
- Pang, Y.P.; Wang, Y.S.; Zhou, X.Y.; Ni, Z.; Chen, W.J.; Liu, Y.; Du, W.L. Cuproptosis-Related LncRNA-Based Prediction of the Prognosis and Immunotherapy Response in Papillary Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 1464. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Zheng, Y. Identification of a tissue resident memory CD8 T cell-related risk score signature for colorectal cancer, the association with TME landscapes and therapeutic responses. Front. Genet. 2022, 13, 1088230. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Geeleher, P.; Cox, N.; Huang, R.S. pRRophetic: An R Package for Prediction of Clinical Chemotherapeutic Response from Tumor Gene Expression Levels. PLoS ONE 2014, 9, e107468. [Google Scholar] [CrossRef] [PubMed]
- Mayakonda, A.; Lin, D.C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef]
- Jiang, P.; Gu, S.; Pan, D.; Fu, J.; Sahu, A.; Hu, X.; Li, Z.; Traugh, N.; Bu, X.; Li, B.; et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 2018, 24, 1550–1558. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, M. Integrated bioinformatic analysis identified a novel prognostic pan-programmed cell death signature for bladder cancer. Front. Immunol. 2022, 13, 1030097. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, Y.; Wang, X.; Cui, X.; Cheng, X.; Fu, Q.; Song, Y.; Yu, P.; Liu, Y.; Yu, Y. Hydrogen Sulfide Attenuates High-Fat Diet-Induced Obesity: Involvement of mTOR/IKK/NF-kappaB Signaling Pathway. Mol. Neurobiol. 2022, 59, 6903–6917. [Google Scholar] [CrossRef]


| Covariates | Total (n = 533) | Test (n = 266) | Train (n = 267) | p Value |
|---|---|---|---|---|
| Age | 0.5443 | |||
| ≤65 | 349 (65.48%) | 178 (66.92%) | 171 (64.04%) | |
| >65 | 184 (34.52%) | 88 (33.08%) | 96 (35.96%) | |
| Gender | 0.6735 | |||
| Female | 188 (35.27%) | 91 (34.21%) | 97 (36.33%) | |
| Male | 345 (64.73%) | 175 (65.79%) | 170 (63.67%) | |
| Grade | 0.4805 | |||
| G1 | 14 (2.63%) | 7 (2.63%) | 7 (2.62%) | |
| G2 | 229 (42.96%) | 107 (40.23%) | 122 (45.69%) | |
| G3 | 206 (38.65%) | 106 (39.85%) | 100 (37.45%) | |
| G4 | 76 (14.26%) | 43 (16.17%) | 33 (12.36%) | |
| Unknown | 8 (1.5%) | 3 (1.13%) | 5 (1.87%) | |
| Stage | 0.1334 | |||
| Stage I | 267 (50.09%) | 128 (48.12%) | 139 (52.06%) | |
| Stage II | 57 (10.69%) | 34 (12.78%) | 23 (8.61%) | |
| Stage III | 123 (23.08%) | 56 (21.05%) | 67 (25.09%) | |
| Stage IV | 83 (15.57%) | 48 (18.05%) | 35 (13.11%) | |
| Unknown | 3 (0.56%) | 0 (0%) | 3 (1.12%) | |
| T | 0.2643 | |||
| T1 | 273 (51.22%) | 130 (48.87%) | 143 (53.56%) | |
| T2 | 69 (12.95%) | 41 (15.41%) | 28 (10.49%) | |
| T3 | 180 (33.77%) | 88 (33.08%) | 92 (34.46%) | |
| T4 | 11 (2.06%) | 7 (2.63%) | 4 (1.5%) | |
| M | 0.4739 | |||
| M0 | 422 (79.17%) | 208 (78.2%) | 214 (80.15%) | |
| M1 | 79 (14.82%) | 43 (16.17%) | 36 (13.48%) | |
| Unknown | 32 (6%) | 15 (5.64%) | 17 (6.37%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhou, X.; Xie, Y.; Wu, J.; Li, T.; Yu, T.; Pang, Y.; Du, W. Establishment of a Seven-Gene Signature Associated with CD8+ T Cells through the Utilization of Both Single-Cell and Bulk RNA-Sequencing Techniques in Clear Cell Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 13729. https://doi.org/10.3390/ijms241813729
Chen Y, Zhou X, Xie Y, Wu J, Li T, Yu T, Pang Y, Du W. Establishment of a Seven-Gene Signature Associated with CD8+ T Cells through the Utilization of Both Single-Cell and Bulk RNA-Sequencing Techniques in Clear Cell Renal Cell Carcinoma. International Journal of Molecular Sciences. 2023; 24(18):13729. https://doi.org/10.3390/ijms241813729
Chicago/Turabian StyleChen, Yubin, Xinyu Zhou, Yanwei Xie, Jianan Wu, Tingting Li, Tian Yu, Yipeng Pang, and Wenlong Du. 2023. "Establishment of a Seven-Gene Signature Associated with CD8+ T Cells through the Utilization of Both Single-Cell and Bulk RNA-Sequencing Techniques in Clear Cell Renal Cell Carcinoma" International Journal of Molecular Sciences 24, no. 18: 13729. https://doi.org/10.3390/ijms241813729
APA StyleChen, Y., Zhou, X., Xie, Y., Wu, J., Li, T., Yu, T., Pang, Y., & Du, W. (2023). Establishment of a Seven-Gene Signature Associated with CD8+ T Cells through the Utilization of Both Single-Cell and Bulk RNA-Sequencing Techniques in Clear Cell Renal Cell Carcinoma. International Journal of Molecular Sciences, 24(18), 13729. https://doi.org/10.3390/ijms241813729






