The Germinal Origin of Salivary and Lacrimal Glands and the Contributions of Neural Crest Cell-Derived Epithelium to Tissue Regeneration
Abstract
1. Introduction
2. Results
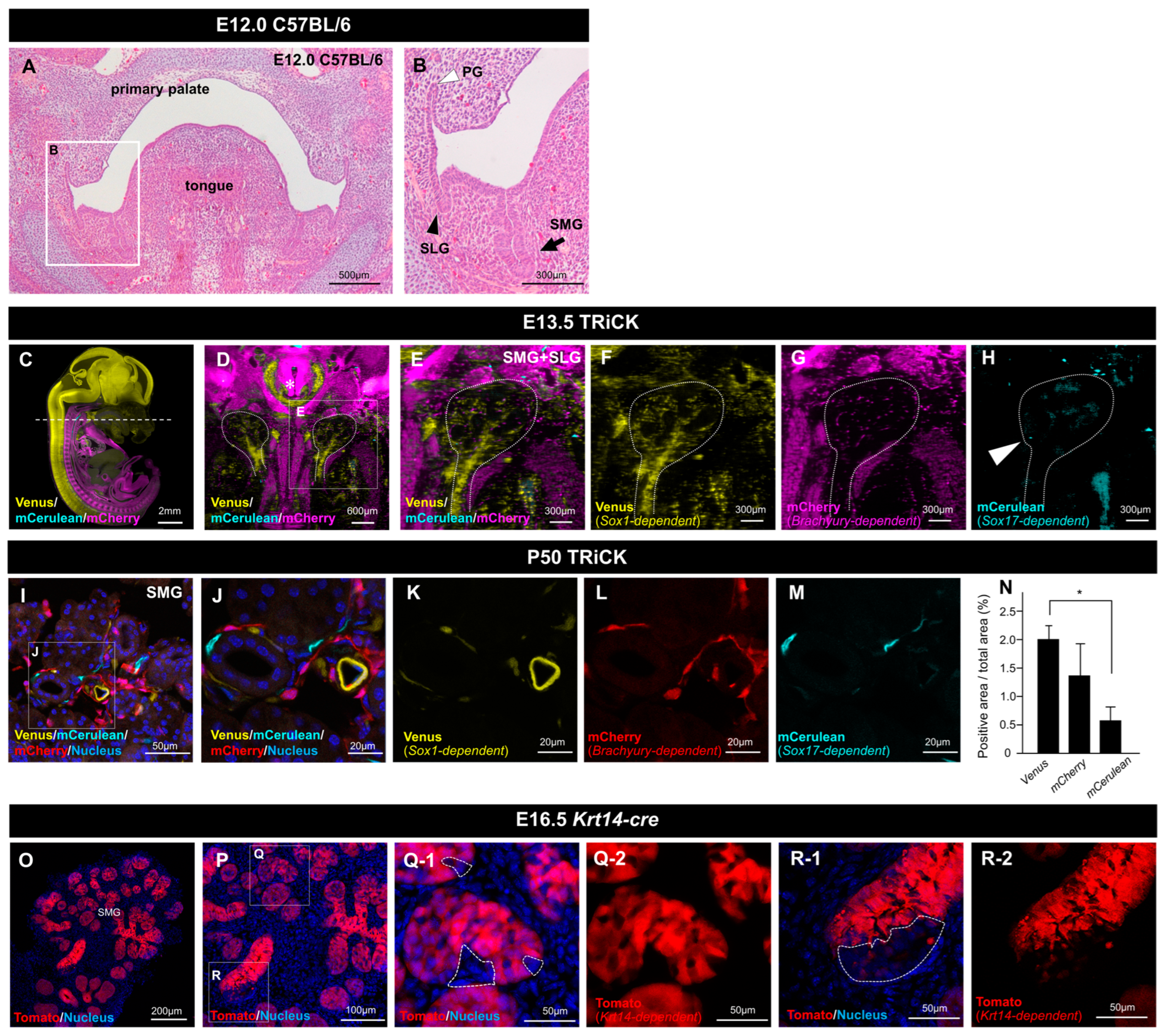
2.1. Analysis of Embryonic and Adult Salivary Glands of TRiCK Mice
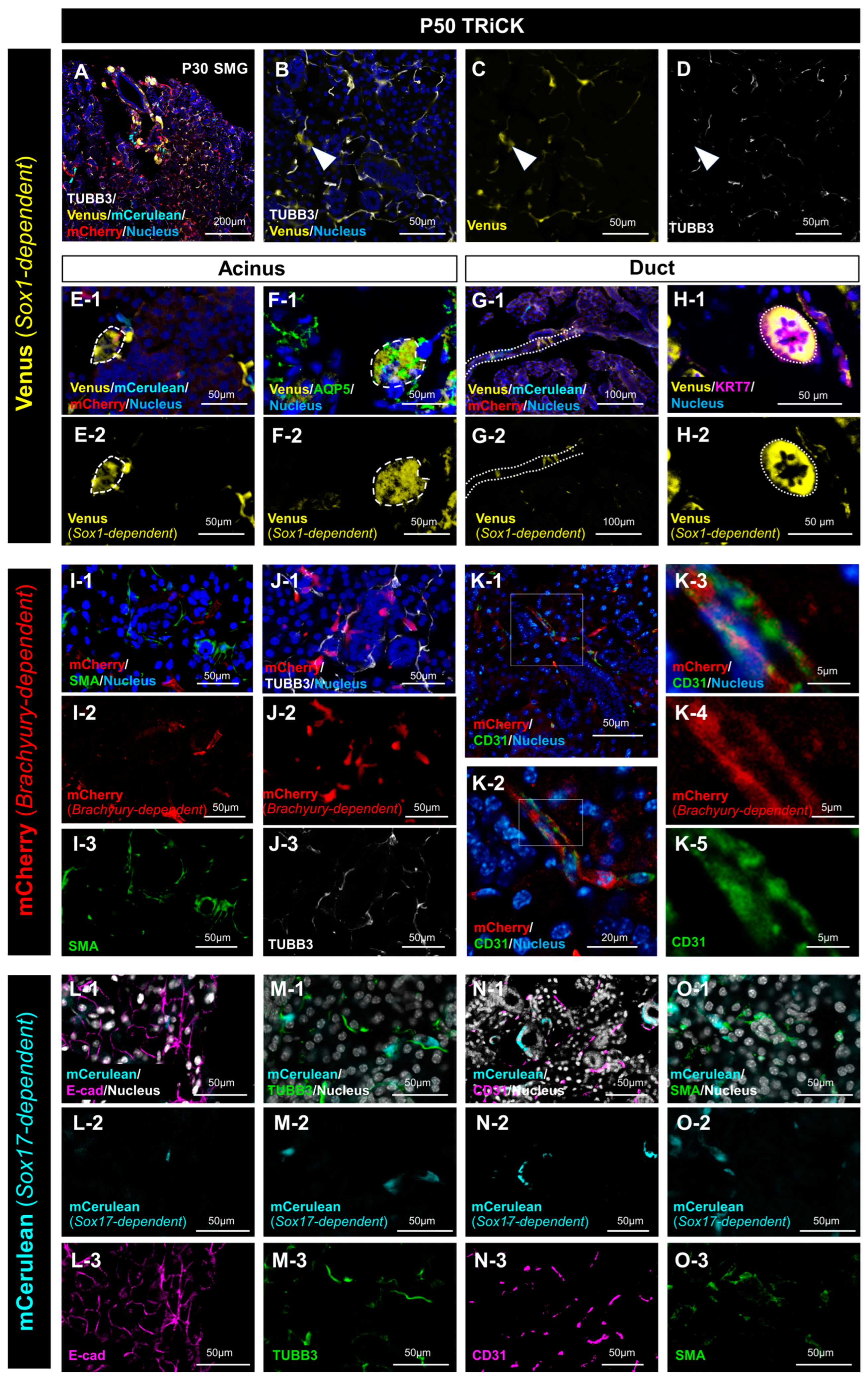
2.1.1. Analysis of Salivary Gland Tissue Derived from Sox1-Positive Cells
2.1.2. Analysis of Salivary Gland Tissue Derived from T/Brachyury-Positive Cells
2.1.3. Analysis of Salivary Gland Tissue Derived from Sox17-Positive Cells
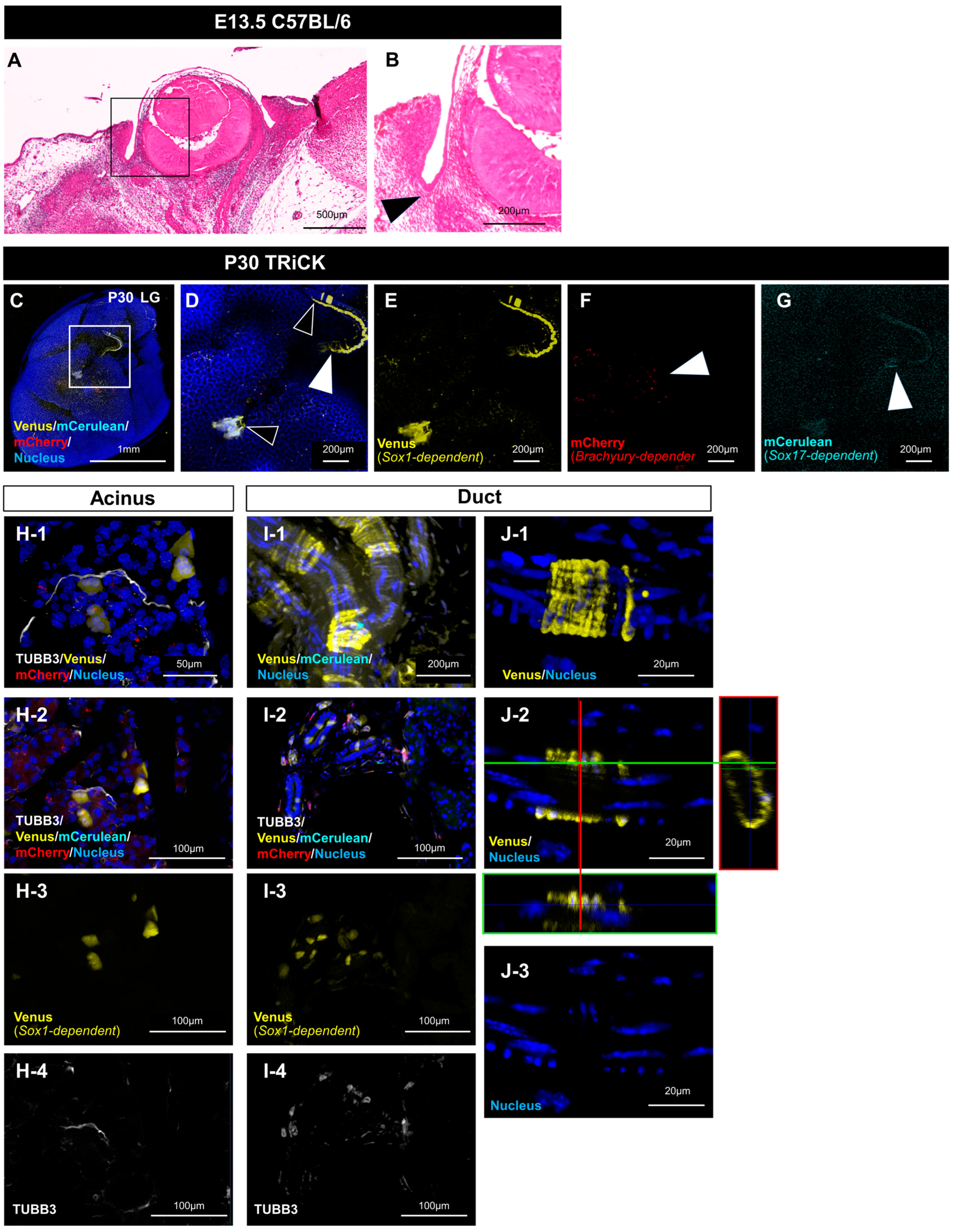
2.2. Analysis of LG Tissue Showed Involvement of All Three Germ Layers
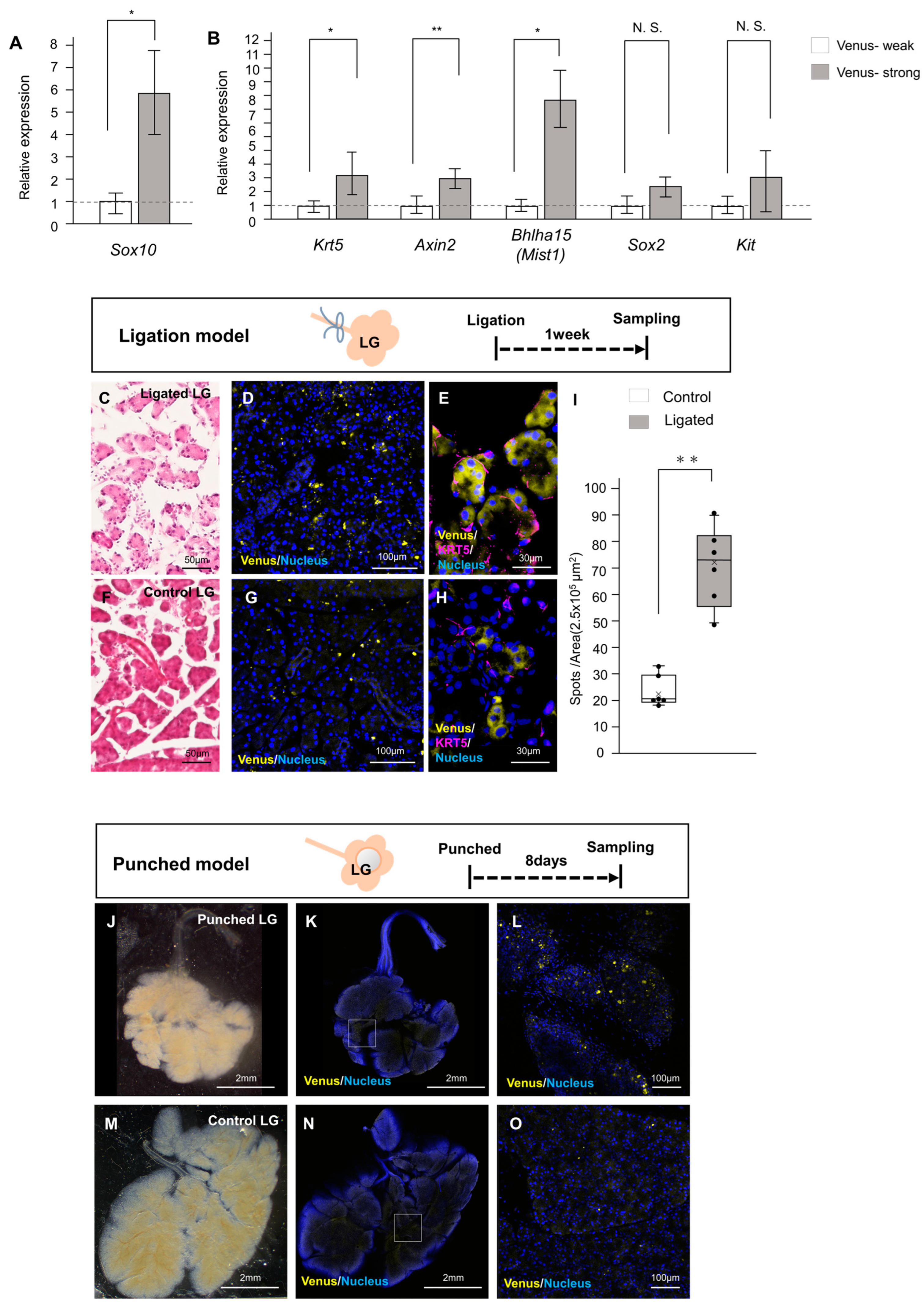
2.3. Involvement of Neural Crest-Derived Epithelial Cells in Tissue Repair
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Mouse Injury Model of Duct Ligation
4.3. Tissue Punch Wounded Mouse Model
4.4. Tissue cleaning
4.5. Cell Sorting
4.6. RT-qPCR
4.7. Immunohistochemistry (IHC)
4.8. Hematoxylin-Eosin (HE) Staining and PAS Staining
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, H.A.; Ober, E.A.; Roeser, T.; Stainier, D.Y.R. Formation of the Digestive System in Zebrafish. I. Liver Morphogenesis. Dev. Biol. 2003, 253, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Wilson, S.; Stock, D.W. The Genetic Basis of Modularity in the Development and Evolution of the Vertebrate Dentition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1633–1653. [Google Scholar] [CrossRef]
- Snyder, R.C. Comparative Anatomy: The Vertebrate Body. Science 1962, 138, 888. [Google Scholar] [CrossRef]
- Hisatomi, Y.; Okumura, K.; Nakamura, K.; Matsumoto, S.; Satoh, A.; Nagano, K.; Yamamoto, T.; Endo, F. Flow Cytometric Isolation of Endodermal Progenitors from Mouse Salivary Gland Differentiate into Hepatic and Pancreatic Lineages. Hepatology 2004, 39, 667–675. [Google Scholar] [CrossRef]
- Adams, A.E. An Experimental Study of the Development of the Mouth in the Amphibian Embryo. J. Exp. Zool. 1924, 40, 311–379. [Google Scholar] [CrossRef]
- Reif, W.E. Evolution of Dermal Skeleton and Dentition in Vertebrates Evolutionary Biology. Evol. Biol. 1982, 15, 287–368. [Google Scholar]
- Rothova, M.; Thompson, H.; Lickert, H.; Tucker, A.S. Lineage Tracing of the Endoderm during Oral Development. Dev. Dyn. 2012, 241, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Nordgarden, H.; Storhaug, K.; Lyngstadaas, S.F.; Jensen, J.L. Salivary Gland Function in Persons with Ectodermal Dysplasias. Eur. J. Oral Sci. 2003, 111, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Fukumoto, E.; Yoshizaki, K.; Iwamoto, T.; Yamada, A.; Tanaka, K.; Suzuki, H.; Aizawa, S.; Arakaki, M.; Yuasa, K.; et al. Platelet-Derived Growth Factor Receptor Regulates Salivary Gland Morphogenesis via Fibroblast Growth Factor Expression. J. Biol. Chem. 2008, 283, 23139–23149. [Google Scholar] [CrossRef] [PubMed]
- Soukup, V.; Epperlein, H.H.; Horácek, I.; Cerny, R. Dual Epithelial Origin of Vertebrate Oral Teeth. Nature 2008, 455, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Siddam, A.D.; Barnum, C.E.; Janga, S.C.; Lachke, S.A. RNA-Binding Proteins in Eye Development and Disease: Implication of Conserved RNA Granule Components. Wiley Interdiscip. Rev. RNA 2016, 7, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Zyrianova, T.; Basova, L.V.; Makarenkova, H. Isolation of Myoepithelial Cells from Adult Murine Lacrimal and Submandibular Glands. J. Vis. Exp. 2019, 148, e59602. [Google Scholar] [CrossRef]
- Serizawa, T.; Isotani, A.; Matsumura, T.; Nakanishi, K.; Nonaka, S.; Shibata, S.; Ikawa, M.; Okano, H. Developmental Analyses of Mouse Embryos and Adults Using a Non-Overlapping Tracing System for All Three Germ Layers. Development 2019, 146, 174938. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Motohashi, T.; Aoki, H.; Tezuka, K.I.; Watanabe, N.; Wakaoka, T.; Era, T.; Kunisada, T. Dual Origin of Melanocytes Defined by Sox1 Expression and Their Region-Specific Distribution in Mammalian Skin. Dev. Growth Differ. 2013, 55, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, C.; Biechele, S.; Zhu, Q.; Song, L.; Lanner, F.; Jing, N.; Rossant, J. Location of Transient Ectodermal Progenitor Potential in Mouse Development. Development 2013, 140, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Keuls, R.A.; Oh, Y.S.; Patel, I.; Parchem, R.J. Post-Transcriptional Regulation in Cranial Neural Crest Cells Expands Developmental Potential. Proc. Natl. Acad. Sci. USA 2023, 120, e2212578120. [Google Scholar] [CrossRef]
- Le Gouar, M.; Guillou, A.; Vervoort, M. Expression of a SoxB and a Wnt2/13 Gene during the Development of the Mollusc Patella Vulgata. Dev. Genes Evol. 2004, 214, 250–256. [Google Scholar] [CrossRef]
- Herrmann, B.G.; Labeit, S.; Poustka, A.; Kingt, T.R.; Lehrach, H. Cloning of the T Gene Required in Mesoderm Formation in the Mouse. Nature 1990, 343, 617–622. [Google Scholar] [CrossRef]
- Matsuno, K.; Mae, S.-I.; Okada, C.; Nakamura, M.; Watanabe, A.; Toyoda, T.; Uchida, E.; Osafune, K. Redefining Definitive Endoderm Subtypes by Robust Induction of Human Induced Pluripotent Stem Cells. Differentiation 2016, 92, 281–290. [Google Scholar] [CrossRef]
- Nowotschin, S.; Hadjantonakis, A.K.; Campbell, K. The Endoderm: A Divergent Cell Lineage with Many Commonalities. Development 2019, 146, dev150920. [Google Scholar] [CrossRef]
- Byrne, C.; Tainsky, M.; Fuchs, E. Programming Gene Expression in Developing Epidermis. Development 1994, 120, 2369–2383. [Google Scholar] [CrossRef] [PubMed]
- Tadeu, A.M.; Horsley, V. Notch signaling represses p63 expression in the developing surface ectoderm. Development 2013, 140, 3777–3786. [Google Scholar] [CrossRef]
- Dassule, H.R.; Lewis, P.; Bei, M.; Maas, R.; McMahon, A.P. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 2000, 127, 4775–4785. [Google Scholar] [CrossRef] [PubMed]
- Suter, D.M.; Tirefort, D.; Julien, S.; Krause, K.-H. A Sox1 to Pax6 Switch Drives Neuroectoderm to Radial Glia Progression During Differentiation of Mouse Embryonic Stem Cells. Stem Cells 2009, 27, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Colleoni, S.; Giannelli, S.G.; Brunetti, D.; Colombo, E.; Lagutina, I.; Galli, C.; Broccoli, V. Direct Derivation of Neural Rosettes from Cloned Bovine Blastocysts: A Model of Early Neurulation Events and Neural Crest Specification In Vitro. Stem Cells 2006, 24, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Ding, X.; Wang, A.; Li, S. Neural Crest-like Stem Cells for Tissue Regeneration. Stem Cells Transl. Med. 2021, 10, 681–693. [Google Scholar] [CrossRef]
- Ninche, N.; Kwak, M.; Ghazizadeh, S. Diverse Epithelial Cell Populations Contribute to the Regeneration of Secretory Units in Injured Salivary Glands. Development 2020, 147, 192807. [Google Scholar] [CrossRef]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2+ Adult Stem and Progenitor Cells Are Important for Tissue Regeneration and Survival of Mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef]
- Ono Minagi, H.; Usami, Y.; Sakai, M.; Sakai, T. Morphological Differences between Regenerating Salivary Glands after Sali-vary Gland Duct Ligation and Embryonic Salivary Glands. Ann. Anat. 2020, 229, 151482. [Google Scholar] [CrossRef]
- Farmer, D.T.; Nathan, S.; Finley, J.K.; Shengyang Yu, K.; Emmerson, E.; Byrnes, L.E.; Sneddon, J.B.; McManus, M.T.; Tward, A.D.; Knox, S.M. Defining Epithelial Cell Dynamics and Lineage Relationships in the Developing Lacrimal Gland. Development 2017, 144, 2517–2528. [Google Scholar] [CrossRef]
- Bei, M. Molecular Genetics of Tooth Development. Curr. Opin. Genet. Dev. 2009, 19, 504–510. [Google Scholar] [CrossRef]
- Huysseune, A.; Cerny, R.; Witten, P.E. The Conundrum of Pharyngeal Teeth Origin: The Role of Germ Layers, Pouches, and Gill Slits. Biol. Rev. 2022, 97, 414–447. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.; Nassif, A.; Asselin, A.; Taïhi, I.; Fohrer-Ting, H.; Klein, C.; Gogly, B.; Berdal, A.; Robert, B.; Fournier, B.P. Involvement of Neural Crest and Paraxial Mesoderm in Oral Mucosal Development and Healing. Biomaterials 2018, 172, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Mittwoch, U. Different Gene Expressions on the Left and the Right: A Genotype/Phenotype Mismatch in Need of Attention. Ann. Hum. Genet. 2008, 72, 2–9. [Google Scholar] [CrossRef]
- Ras, F.; Habets, L.L.M.H.; Van Ginkel, F.C.; Prahl-Andersen, B. Facial Left-Right Dominance in Cleft Lip and Palate: Three-Dimension Evaluation. Cleft Palate Craniofacial J. 1994, 31, 461–465. [Google Scholar] [CrossRef]
- Kuony, A.; Michon, F. Epithelial Markers ASMA, Krt14, and Krt19 Unveil Elements of Murine Lacrimal Gland Morphogenesis and Maturation. Front. Physiol. 2017, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Kouskoura, T.; Kozlova, A.; Alexiou, M.; Blumer, S.; Zouvelou, V.; Katsaros, C.; Chiquet, M.; Mitsiadis, T.A.; Graf, D. The Etiology of Cleft Palate Formation in BMP7-Deficient Mice. PLoS ONE 2013, 8, e59463. [Google Scholar] [CrossRef]
- Troy, T.-C.; Turksen, K. Commitment of Embryonic Stem Cells to an Epidermal Cell Fate and Differentiation in Vitro. Dev. Dyn. 2005, 232, 293–300. [Google Scholar] [CrossRef]
- Shalom-Feuerstein, R.; Lena, A.M.; Zhou, H.; De La Forest Divonne, S.; Van Bokhoven, H.; Candi, E.; Melino, G.; Aberdam, D. Δnp63 Is an Ectodermal Gatekeeper of Epidermal Morphogenesis. Cell Death Differ. 2011, 18, 887–896. [Google Scholar] [CrossRef]
- Ono Minagi, H.; Sarper, S.E.; Kurosaka, H.; Kuremoto, K.; Taniuchi, I.; Sakai, T.; Yamashiro, T. Runx1 Mediates the Development of the Granular Convoluted Tubules in the Submandibular Glands. PLoS ONE 2017, 12, e184395. [Google Scholar] [CrossRef]
- Widera, D.; Zander, C.; Heidbreder, M.; Kasperek, Y.; Noll, T.; Seitz, O.; Saldamli, B.; Sudhoff, H.; Sader, R.; Kaltschmidt, C.; et al. Adult Palatum as a Novel Source of Neural Crest-Related Stem Cells. Stem Cells 2009, 27, 1899–1910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brandl, C.; Florian, C.; Driemel, O.; Weber, B.H.F.; Morsczeck, C. Identification of Neural Crest-Derived Stem Cell-like Cells from the Corneal Limbus of Juvenile Mice. Exp. Eye Res. 2009, 89, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sipp, D.; Enomoto, H. Tissue Interactions in Neural Crest Cell Development and Disease. Science 2013, 341, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Trainor, P.A. Neural Crest Stem Cells: Discovery, Properties and Potential for Therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef]
- Perera, S.N.; Kerosuo, L. On the Road Again: Establishment and Maintenance of Stemness in the Neural Crest from Embryo to Adulthood. Stem Cells 2021, 39, 7–25. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzawa, T.; Yamada, A.; Yamaguchi, T.; Mishima, K.; Osumi, N.; Maki, K.; Kamijo, R. Identification of Gene Expression Profile of Neural Crest-Derived Cells Isolated from Submandibular Glands of Adult Mice. Biochem. Biophys. Res. Commun. 2014, 446, 481–486. [Google Scholar] [CrossRef]
- Khan, S.; Fitch, S.; Knox, S.; Arora, R. Exocrine Gland Structure-Function Relationships. Development 2022, 149, 197657. [Google Scholar] [CrossRef]
- Patel, R.; Shahane, A. The Epidemiology of Sjogren’s Syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [CrossRef]
- Tawfik, H.A.; Dutton, J.J. Embryologic and Fetal Development of the Human Orbit. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 1172. [Google Scholar] [CrossRef]
- Hayashi, Y.; Liu, C.-Y.; Jester, J.J.; Hayashi, M.; Wang, I.-J.; Funderburgh, J.L.; Saika, S.; Roughley, P.J.; Whei-Cheng Kao, C.; Whei-Yang Kao, W. Excess Biglycan Causes Eyelid Malformation by Perturbing Muscle Development and TGF-α Signaling. Dev. Biol. 2005, 277, 222–234. [Google Scholar] [CrossRef]
- Fonseca, I.; Moura Nunes, J.F.; Soares, J. Expression of CD44 Isoforms in Normal Salivary Gland Tissue: An Immunohistochemical and Ultrastructural Study. Histochem. Cell Biol. 2000, 114, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Maria, A.M.; Cai, Y.; Tran, S.D. Cell Surface Markers CD44 and CD166 Localized Specific Populations of Salivary Acinar Cells. Oral Dis. 2012, 18, 162–168. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono-Minagi, H.; Nohno, T.; Serizawa, T.; Usami, Y.; Sakai, T.; Okano, H.; Ohuchi, H. The Germinal Origin of Salivary and Lacrimal Glands and the Contributions of Neural Crest Cell-Derived Epithelium to Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 13692. https://doi.org/10.3390/ijms241813692
Ono-Minagi H, Nohno T, Serizawa T, Usami Y, Sakai T, Okano H, Ohuchi H. The Germinal Origin of Salivary and Lacrimal Glands and the Contributions of Neural Crest Cell-Derived Epithelium to Tissue Regeneration. International Journal of Molecular Sciences. 2023; 24(18):13692. https://doi.org/10.3390/ijms241813692
Chicago/Turabian StyleOno-Minagi, Hitomi, Tsutomu Nohno, Takashi Serizawa, Yu Usami, Takayoshi Sakai, Hideyuki Okano, and Hideyo Ohuchi. 2023. "The Germinal Origin of Salivary and Lacrimal Glands and the Contributions of Neural Crest Cell-Derived Epithelium to Tissue Regeneration" International Journal of Molecular Sciences 24, no. 18: 13692. https://doi.org/10.3390/ijms241813692
APA StyleOno-Minagi, H., Nohno, T., Serizawa, T., Usami, Y., Sakai, T., Okano, H., & Ohuchi, H. (2023). The Germinal Origin of Salivary and Lacrimal Glands and the Contributions of Neural Crest Cell-Derived Epithelium to Tissue Regeneration. International Journal of Molecular Sciences, 24(18), 13692. https://doi.org/10.3390/ijms241813692








