Abstract
Cancer-related immunity plays a significant role in the outcome of ovarian cancer, but the exact mechanisms are not fully explored. A retrospective, real-life observational study was conducted including 57 advanced ovarian cancer patients. Immunohistochemistry for CD4+, CD8+, and CD45+ was used for assessing tumor-infiltrating immune cells. Furthermore, an immune-related gene expression assay was performed on 12–10 samples from patients with less than and more than 1-year overall survival (OS), respectively. A higher number of CD4+ (p = 0.0028) and CD45+ (p = 0.0221) immune cells within the tumor microenvironment were associated with longer OS of patients. In a multivariate setting, higher CD4+ T cell infiltration predicted longer OS (p = 0.0392). Twenty-three differentially expressed genes—involved in antigen presentation, costimulatory signaling, matrix remodeling, metastasis formation, and myeloid cell activity—were found when comparing the prognostic groups. It was found that tumor-infiltrating immune cell counts are associated with peculiar gene expression patterns and bear prognostic information in ovarian cancer. SOX11 expression emerged and was validated as a predictive marker for OS.
1. Introduction
Ovarian cancer has an incidence and mortality rate of over 300,000 new cases and 200,000 deaths per year, respectively. It is the 8th most common cancer in females, based on the 2020 GLOBOCAN data [1]. The early symptoms of the disease are not alarming; thus, the majority (over 70%) of ovarian cancer patients are diagnosed at an advanced stage, where the disease has spread throughout the pelvis or elsewhere in the abdomen with or without the involvement of lymph nodes [2,3]. Tumors originating from an epithelial origin are the most common (~95%) and include the following four primary histological subtypes: serous, endometrioid, mucinous, and clear cell ovarian cancer [3].
In the last decades, cancer-related immunity has become a particularly important research area due to its complex relationship with tumor initiation, growth, and progression [4]. Consequently, considerable research interest is focused on the tumor microenvironment (TME). This includes the identification of possible molecular targets, which are usually involved in cancer-related inflammation [5,6]. Similar to other cancer types [7], ovarian cancer can also be characterized by tumor-infiltrating immune cells and cancer-specific cytokines, which have been proven to be promising prognostic markers. However, their exact role in progression and their relationship with the different immune components have not been fully understood [6,8,9,10]. The results of recent studies suggest that the higher number of CD4+, CD8+, and/or CD45+ immune cells in the TME are associated with improved survival in patients with solid tumors [6,11].
Although several studies investigated gene profiles of ovarian cancer patients in various comparisons [11,12,13,14,15,16,17], to our knowledge, no previous study investigated whether the number of tumor-infiltrating immune cells is associated with any gene expression differences. Therefore, a retrospective, real-life observational study (RROS) was conducted, where a detailed analysis of selected ovarian cancer samples was performed to identify a relevant association between the different tumor-infiltrating immune cells and clinicopathological parameters, and their impact on prognosis. Furthermore, the secondary goal of the RROS was to investigate whether there are differences in the various immune functions of those patients with different tumor-infiltrating immune cells, and what gene expression differences could be detected.
2. Results
A total of 57 patients with primary ovarian cancer were included in this RROS. Formalin-fixed and paraffin-embedded (FFPE) samples were obtained from all participants. CD4+, CD8+, and CD45+ tumor-infiltrating immune cells were analyzed in all tumor samples. Furthermore, a subset of 22 samples (12 with poor and 10 with excellent prognosis) was also investigated using NanoString assay-based gene expression profiling. Anamnestic, clinical, and histopathological data of all study subjects are summarized in Table 1. Data of the oncological treatments could be collected for 44 patients (77.2%). The remaining patients were treated at other institution(s) due to their preference. Two and five patients received no and the best supportive treatment only, due to stage I and advanced disease, respectively. Paclitaxel with carboplatin/cisplatin, pegylated liposomal doxorubicin, and gemcitabine + carboplatin with or without bevacizumab were used as the most common first- and second/third-line treatments. Moreover, topotecan, etoposide, and olaparib were used as late-line options.

Table 1.
Anamnestic and clinicopathological data of ovarian cancer patients. Continuous and count data are reported as mean ± standard deviation and the number of observations (percentage), respectively.
2.1. CD4+, CD8+, and CD45+ Immunohistochemical Analysis of Ovarian Tumor Samples
FFPE samples were immunostained for CD4+, CD8+, and CD45+ tumor-infiltrating immune cells, where 9, 15, 17, and 16; 7, 24, 14, and 12; and 3, 10, 10, and 34 samples were stained negatively, weakly, moderately, and strongly for CD4+, CD8+, and CD45+ tumor-infiltrating immune cells, respectively (Figure 1). Of the 57 study participants, 3 (5.3%) were negative for all three immune cells, and 10 and 44 patients had immune cell positivity for 2 and 3 markers, respectively. It was investigated whether any of the clinical and/or histopathological parameters affected the staining categories. It was found that those patients with moderate (59.70 ± 13.21 years) and strong (57.51 ± 16.17 years) CD4+ staining were significantly younger (Kruskal–Wallis p = 0.0355), compared to those with weak staining (70.97 ± 16.00 years; Figure 2A). Those patients with negative and weak CD8+ staining had type 2 diabetes mellitus in their medical history more often than those within the other two categories (negative: 57.14%; weak: 37.50%; moderate: 7.14%; strong: 8.33%; p = 0.0194). Moreover, both hemoglobin (Figure 2B) and hematocrit (Figure 2C) values were tendentiously lower in the strongly stained CD8+ cohort. Furthermore, the length of hospitalization was shorter with the increase of CD45+ leukocyte staining (Kruskal–Wallis p = 0.0346; Figure 2D). No further differences and/or relationships could be justified.
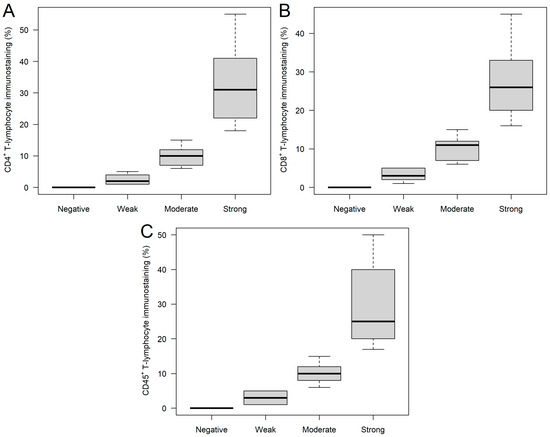
Figure 1.
CD4+ (A), CD8+ (B), and CD45+ (C) immunostaining of ovarian tumor samples. Based on the percentage of stained tumor-infiltrating immune cells, the following categories were created: negative, weak, moderate, and strong staining were found if no (0%), 1–5%, 6–15%, or >15% of the T cells were stained.
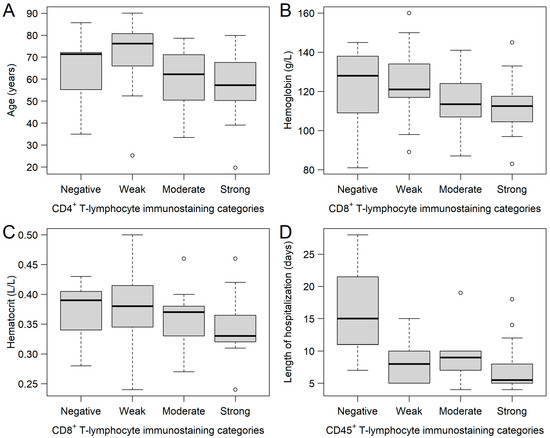
Figure 2.
Relationships between the CD4+, CD8+, and CD45+ immunostaining sub-cohorts and various clinical parameters. (A) Patients with moderate and strong CD4+ T-lymphocyte immunostaining were significantly younger, compared to those with weak staining results. Hemoglobin (B) and hematocrit (C) values of patients were the lowest in those with strong CD8+ staining. Length of hospitalization (D) was the shortest in those patients with strong CD45+ staining. Hollow circles represent outliers (greater/lower 1.5 times the interquartile range above/below the upper/lower quartile).
It was also ©nvestigated whether there is any correlation between the various tumor-infiltrating immune cells and other clinical parameters. No connection could be found between CD4+ and CD8+ (p = 0.9157). A significant connection was observed between CD45+ and CD4+ (Spearman’s rho (ρ): +0.72; p < 0.0001), and between CD45+ and CD8+ (ρ: +0.51; p < 0.0001). Furthermore, negative correlations were found between CD4+ and age (ρ: −0.27; p = 0.0480), CD8+ and hemoglobin (ρ: −0.28; p = 0.0394), and CD8+ and hematocrit (ρ: −0.27; p = 0.0407). No further significant correlations could be justified.
Survival analyses of raw CD4+, CD8+, and CD45+ tumor-infiltrating immune cell percentages revealed that the higher the CD4+ (p = 0.0028) and the CD45+ (p = 0.0221) infiltration within the FFPE samples was, the longer the overall survival (OS) of the patients. However, no such connection could be justified in the case of CD8+ staining (Table 2). When investigating the results in a multivariate setting, where the three immune markers were analyzed together, only the CD4+ results significantly predicted the OS of patients (p = 0.0392; Table 2). In addition to the raw numerical values, we also examined OS in the CD4+, CD8+, and CD45+ sub-cohorts. As above, we could observe that patients with higher staining categories usually had improved OS (Figure 3 and Table 3).

Table 2.
Cox regression model results investigating the univariate and multivariate effects of raw CD4+, CD8+, and CD45+ tumor-infiltrating immune cell percentages over the overall survival of ovarian cancer patients. Interpretation of these data: for every 1% increase, the risk for shorter survival increases/decreases with the HR detailed in the table, e.g., a 0%, 20%, and 80% CD4+ infiltration is associated with a 0.94590 (HR = 1), 0.945920 (HR = 0.3288), and 0.945980 (HR = 0.0117) risk for shorter survival.
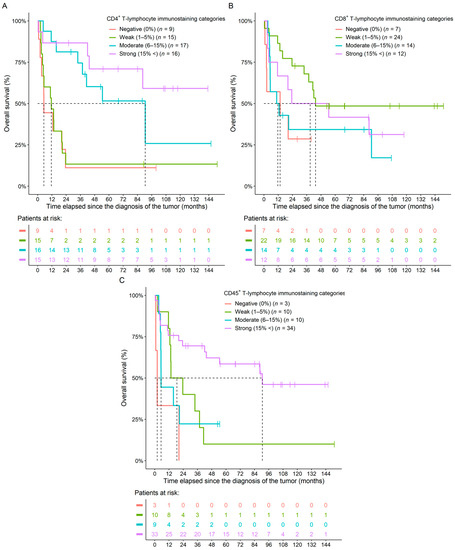
Figure 3.
Naive Kaplan–Meier curves of the CD4+ (A), CD8+ (B), and CD45+ (C) immunostaining categories of ovarian cancer patients. Colors within the bottom tables represent the same cohorts as detailed in the figure legends.

Table 3.
Hazard rates and p-values of univariate survival models investigating the effect of CD4+, CD8+, and CD45+ immunostaining categories over the overall survival of ovarian cancer patients. The corresponding naive Kaplan–Meier curves can be seen in Figure 3.
Furthermore, a second multivariate survival model with additional clinical parameters was also created, where in addition to the CD4+, CD8+, and CD45+ immune cell percentages, age, hemoglobin levels, platelet counts, the duration of hospitalization, histology, and the American Society of Anesthesiologists (ASA) performance scores were also added to the model as explanatory variables. The beneficial effect of higher CD4+ percentages (p = 0.0286) could be observed in this model as well, in addition to the worsening survival effects of higher platelet counts (p = 0.0077), longer hospitalization durations (p = 0.0054), and higher ASA scores (Table S1).
2.2. Immune Panel Gene Expression Analysis
A total of 22 (38.60%) FFPE samples were selected for analysis with the NanoString nCounter® PanCancer IO 360TM Gene Expression Panel. The samples were selected based on the survival times of patients, where 12–10 had an OS of less than or more than 1 year (median survival times: 4.37 months vs. not reached). The clinical characteristics of the two groups were compared. A statistical difference was found in the CD4+ (p = 0.0038) and CD45+ (p = 0.0465) tumor-infiltrating immune cell percentages, platelet count (p = 0.0408), and ASA performance scores (p = 0.0217) of the patients. In all cases, the clinically worse values were associated with the poor prognosis group. The ASA performance scores of the two groups were inversely proportional to each other. Moreover, the length of hospitalization was marginally shorter in the good prognosis group (p = 0.0565; Table S2). Furthermore, the number of patients who received the best supportive care therapy or a single chemotherapy regimen only with a lower number of cycles, was higher in the poor prognosis group. In contrast, in the good prognosis group, several lineages of chemotherapy were characteristic.
Differentially expressed genes (DEGs) were identified using differential expression analysis. Of the 770 tested genes, 23 significant DEGs were found when comparing the prognosis groups (Figure 4). Of those 23 DEGs, 10 and 13 were up- and down-expressed, respectively. The complete list of DEGs, including their functional and cancer-immunity cycle annotations, can be read in Table S3. Gene over-representation was investigated via gene set enrichment analysis (GSEA). Here, 5, 6, 5, and 10 of the 23 DEGS were involved in antigen presentation, costimulatory signaling, matrix remodeling and metastasis formation, and in myeloid cell activity, respectively. GSEA results suggested that, except for matrix remodeling and metastasis, all pathways were downregulated in those ovarian cancer patients with worse survival (Table S4).
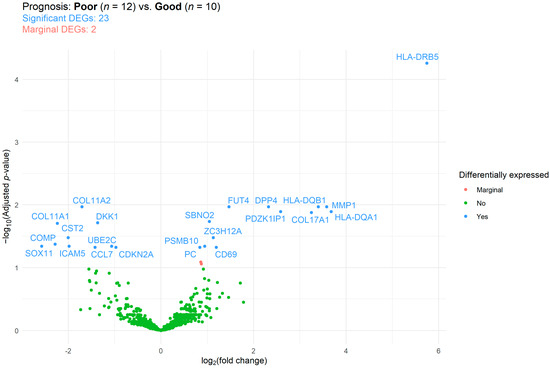
Figure 4.
Differentially expressed genes (DEGs) between those ovarian cancer patients with good and poor prognosis (survival time < 1 and >1 years, respectively). The false discovery rate method was used for p-value adjustment. Reference category: patients with good prognosis.
Expression patterns were further investigated based on the CD4+, CD8+, and CD45+ tumor-infiltrating immune cell categories, ASA, and on tumor extent (T status). It must be noted that due to the number of smaller sample sizes that could be included in the NanoString analyses, some previously independent categories had to be combined: 12, 21, and 12 DEGs were found in the ‘CD4+ 0%’ vs. ‘CD4+ 1–5%’, ‘CD4+ 0%’ vs. ‘CD4+ 5%<’, and ‘CD4+ 1–5%’ vs. ‘CD4+ 5%<’ comparisons, respectively (Table S3, Figures S1–S3). While GSEA revealed no differences in the latter comparison, in the former two, myeloid compartment, matrix remodeling and metastasis, and common signaling pathway functions were down-expressed, and immunometabolism, the killing of cancer cells, and myeloid cell activity functions were up-expressed in those patients with higher CD4+ T cell infiltrations (Table S4). Six and thirteen significant DEGs were found between the negatively/weakly vs. moderately CD8+-stained (Table S3, Figure S4) and the moderately vs. strongly CD8+-stained patient groups (Table S3, Figure S5), respectively. However, no significant DEGS could be identified in the case of the negatively/weakly vs. strongly CD8+-stained cohorts (Table S3, Figure S6). While most DEGs of the first CD8+ comparison affected immune cell adhesion, migration, and localization to tumors, in the second comparison, most DEGs were associated with lymphoid compartment, myeloid cell activity, and immune cell localization to tumors’ functions (Table S4). Finally, 19 significant DEGs were found when comparing the negatively/weakly/moderately vs. strongly CD45+-stained cohorts (Table S3, Figure S7). Of these, the killing of cancer cells, myeloid cell activity, T cell priming and activation, and cell cycle and proliferation functions were up-expressed, and the myeloid compartment function was down-expressed in the strongly stained cohort (Table S4).
When comparing ASA groups I vs. II, I vs. III, and II vs. III, 13, 26, and 37 DEGs were found, respectively. No gene was found that was significant in all three comparisons (Table S3; Figures S8–S10). The following pathways were affected by the ASA score groups. The genes belonging to antigen presentation (ASA I vs. II) and immune cell adhesion and migration (ASA I vs. II) were all up-expressed, and myeloid cell activity (ASA II vs. III) was down-expressed. Half of the genes associated with myeloid compartment (ASA II vs. III) were both down- and up-expressed. Stromal factors were up-expressed in the ASA II group, compared to the other two ASA performance score groups (Table S4). The effect of tumor extent (T status) was also investigated. Here, 41 DEGs were found when T status I and II–III were compared (Figure S11, Table S3), and the down- and up-expression of matrix remodeling and metastasis and common signaling pathways could be observed, respectively (Table S4). A heatmap summarizing all the above-detailed comparisons can be seen in Figure 5 and Figure S12.
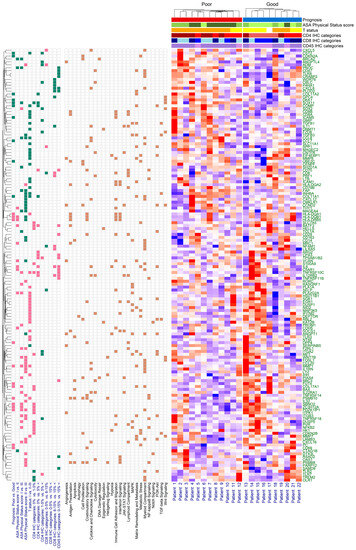
Figure 5.
Heatmap of significantly different gene expressions. The green and pink boxes represent the down-expression and the up-expression of genes, respectively. The brown box shows cancer-immunity cycle enrichment annotation information of the differentially expressed genes. The American Society of Anesthesiologists (ASA) performance scores are represented as: I (light green), II (green), and III (dark green); T status as: I (yellow) and II–III (orange); CD4 IHC categories as: 0% (light red), 1–5% (red), and 5%< (dark red); CD8 IHC categories as: 0–5% (light blue), 6–15% (blue), and 15%< (dark blue); CD45 IHC categories as: 0–15% (light purple) and 15%< (purple).
In all the above-detailed comparisons, a total of 148 significant DEGs were identified, and 101 (68.24%), 27 (18.24%), 12 (8.11%), 6 (4.05%), and 1 (0.68%) of the 148 DEGs were associated with 1, 2, 3, 4, and 5 comparisons, respectively (Table S3). The single gene associated with 5 comparisons was the tumor necrosis factor receptor superfamily member 10c (TNFRSF10C), and those associated with at least 4 comparisons are listed in Table 4. Based on their annotation details, 6 of these 7 DEGs were associated with myeloid cell activity (BATF3, COL11A1, IL6, MMP1, PDZK1IP1, and TNFRSF10C), 4 with common signaling pathways (BATF3, COL11A1, IL6, and SOX11), and 3 each with the killing of cancer cells (IL6, SOX11, and TNFRSF10C) and myeloid compartment pathways (COL11A1, MMP1, and PDZK1IP1). Those 12 DEGs that were associated with 3 comparisons (ADM, CCL8, COL17A1, COMP, CST2, HLA-DQB1, HLA-DRB5, IL12RB2, PLA2G2A, PNOC, SBNO2, TPSAB1/B2, and ZC3H12A) were associated most often with myeloid cell activity (7 of the 12: CCL8, COL17A1, HLA-DQB1, HLA-DRB5, IL12RB2, SBNO2, and ZC3H12A) and with immune cell localization to tumors (6 of the 12: CCL8, HLA-DQB1, HLA-DRB5, IL12RB2, PNOC, and TPSAB1/B2).

Table 4.
Differentially expressed genes that were significantly up- (+) or down-expressed (−) in at least 4 of the comparisons.
2.3. Comparison of NanoString Data with the “Kaplan–Meier Plotter” Web Application
To validate our data, it was investigated whether those DEGs with an FDR-adjusted p < 0.05, and ≥ or ≤±3 log2 fold-change, had the same effects over patient survival as detailed in the Kaplan–Meier Plotter web application (KMplotter [19]: https://kmplot.com/analysis/index.php?p=service&cancer=ovar; access date: 20 July 2023). We examined whether the hazard rates and median survivals obtained from our models were comparable to those observations verified in the KMplotter application [19]. It is notable that the KMplotter database contains only data of selected ovarian cancer patients with serous- and endometrioid-type tumors. Therefore, 13 of the 22 patients remained in this analysis, whose NanoString data were available and who had serous- or endometrioid-type ovarian cancer. To achieve sufficient statistical power in our survival models, the patients were divided into two groups based on the median of the RUVSeq normalized count data for each tested gene. A total of 17 DEGs were found, which met the above conditions (Table 5): COL11A1, COL17A1, COMP, CTAG1B, HLA-DQA1, HLA-DQB1, HLA-DRB5, IL6, ITGB3, LYZ, MAGEC2, MMP1, PDZK1IP1, PLA2G2A, SOX2, SOX11, and TNFRSF18. It was found that the over-expression of SOX11 had a significant effect over patient survival in both datasets (this study: p = 0.0032; KMplotter: p = 0.0008). In addition, similar tendencies were found in the hazard rates and median survival times in the case of COL11A1, COMP and PDZK1IP1; however, their significant effect over survival could only be justified in the literature data [19] (Table 5).

Table 5.
Validation comparisons of the results of the current study, compared to the Kaplan–Meier Plotter web application [19]. p-Values were obtained from log rank tests. It is notable that the Kaplan–Meier Plotter web application contains data on ovarian cancer patients with serous and endometrioid types only. Therefore, from our database, only those patients with similar histology were included.
3. Discussion
Tumor-infiltrating immune cells are characteristic of most solid tumors [20,21], including ovarian cancer [6,8]. Without being exhaustive, the following are known in the literature. CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) have been associated with improved OS and progression-free survival (PFS) in all epithelial ovarian cancers [11,22,23,24,25,26,27], including the high-grade serous [28,29], mucinous [25], clear cell [30], and endometrioid [31] types. CD8+ TILs had been found as a predictive marker for both 5-year, 10-year, and longer survival times [32,33]. Several studies have also investigated the spatial differences in these tumors. In mucinous ovarian carcinomas, CD4+ and CD8+ TILs were expressed at higher levels in the stroma compared to the epithelium [17]. Intraepithelial CD4+ TILs have been associated with an increased PFS and OS in the high-grade serous type [34]. Intra-islet CD4+ TILs of omental metastases were the main risk factors associated with poorer survival in advanced epithelial ovarian cancer, and the highest infiltration was found in the stroma of the omental tissue. CD4+ was significantly elevated in patients with FIGO stages IIIC/IV, compared to those with FIGO stages IIIA/IIIB [27]. Intraepithelial CD8+ TILs and a high CD8+/T-regulatory cell (Treg) ratio were associated with favorable prognosis [34,35]. Based on the results of a meta-analysis, intraepithelial CD8+ TILs have been associated with improved PFS, DFS, and OS [36].
It must be mentioned, however, that the opposite of the above was also reported in several studies. For example, the presence of stromal CD4 + and CD8 + TILs were found to be more characteristic for advanced-stage patients and disease-free survival (DFS), and OS was significantly shorter in the presence of CD8+ TILs [37]. The survival benefit of CD4+ TILs could not be justified in the study of Sato et al. [35]. High CD4+CD25+ Treg infiltration has been associated with higher tumor grades, advanced stage, and suboptimal debulking, but not with survival [26]. In advanced clear cell ovarian cancer, an increased infiltration of CD4+ T cells at the leading edge and stroma was significantly associated with poorer OS [30]. CD8+ TILs were significantly higher in those patients with high-grade tumors, advanced-stage tumors, and omental metastasis, all with shorter DFS and poor prognosis [38]. The ratios of CD8+ TILs to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcomes; moreover, the authors of the study highlighted that the association found that the effector/suppressor ratios may be more important indicators of the disease outcome than individual cell counts [39].
Oncotherapy response has been the subject of significant research and clinical interest. In breast cancer, several studies have shown that a higher presence of TILs, particularly in triple-negative breast cancer (TNBC) and HER2-positive subtypes, is associated with a better response to chemotherapy and targeted therapies. For instance, the “IMpassion130” trial demonstrated improved OS and PFS in patients with advanced TNBC, who received atezolizumab and nab-paclitaxel, especially in those with high levels of TILs [40]. TILs have shown promise as predictive markers in ovarian cancer. Studies have indicated that higher levels of TILs, especially CD8+ T cells, are associated with improved responses to chemotherapy and immunotherapies [41]. These are just a few examples of how TILs have been investigated as predictive biomarkers in various cancer types. The presence and composition of TILs in the tumor microenvironment have been associated with improved responses to a range of oncotherapies, including chemotherapy, targeted therapies, and immunotherapies. However, it is essential to note that the predictive value of TILs may vary depending on the cancer type, stage, and treatment regimen, and ongoing research continues to refine our understanding of their role in predicting therapeutic responses. PARP-inhibitors, for instance, have been utilized mainly in breast and ovarian cancer, and there are no such convincing analyses published about TILs being predictive to date; however, their functional role has been extensively studied [42].
In addition, optimal cytoreduction and p53 mutations have been reported more often in those patients with more evident tumor-infiltrating lymphocyte staining [43]. A large-scale study with 3196 high-grade serous ovarian carcinoma patients revealed that CD8+ TILs were associated with longer patient survival regardless of the extent of the residual disease following cytoreduction, the type of oncological treatment, and the germline BRCA1 pathogenic mutation; however, no associations could be found for BRCA2 mutation carriers [25]. Neoadjuvant chemotherapy can significantly increase the density of CD8+ TILs, and their density has been associated with both PFS and OS [24].
In contrast to CD4+ and CD8+ TILs, CD45+ tumor-infiltrating leukocytes in ovarian cancer are a somewhat less researched area, and in many cases, the naive (CD45RA) and the memory (CD45RO) T cell isoforms are specifically investigated. CD45RO+ tumor-infiltrating leukocytes have been positively associated with survival [44,45]. Those patients with CD45RO+ tumors were also found to have longer survival [45,46], and the combination of CD8+ and CD45RO+ positivity was suggested as a better indicator for prognosis than the two markers alone [46]. Early- and advanced-stage tumors were reported to have different CD45RA+ tumor-infiltrating leukocytes (50% vs. 10.5%, respectively) [45]. Patients from a mesenchymal subtype (derived from immune gene expression subtyping in ovarian cancer) had the lowest tumor purity, a high leukocyte fraction, and a stromal fraction with the highest TGF-β response [47]. Furthermore, in patients with brain metastasis, it has been found that both the primary and brain metastasis samples contained approximately the same amount of CD45RO+ and CD8+ tumor-infiltrating immune cells [48], similar to that of the study investigating the omental metastasis samples detailed above [27].
In the current RROS, we confirmed that most of the included ovarian cancer patients (94.7%) had IHC positivity for CD4+, CD8+, and/or CD45+ immune cells. We were able to justify that strong CD4+ and CD45+ staining was the most advantageous in terms of patient survival. When investigated in a multivariate setting, CD4+ was the only marker of the three that had a significant effect on OS. However, we could not confirm the significant effect of tumor-infiltrating CD8+ T cells on patient survival. Moreover, we also demonstrated that CD4+ TILs were more common in the younger patients, hemoglobin and hematocrit values were lower in those with strong CD8+ staining, and the length of hospital stay was shorter with higher CD45+ staining. Our results, both the survival data and the associations with the other clinical parameters, fit perfectly with what is known from the literature. It should be noted, however, that in majority of the literature data, the presence of CD8+ TILs are generally positively associated with survival and results similar to ours are seen in only a minority of the literature.
A further goal of the current study was to investigate the gene expression profiles of the patients. Previous studies in the literature used several different commercially available gene expression profiling kits. The comparison of our results with previous data was limited to studies using the PanCancer® gene expression profiling kits manufactured by NanoString, supplemented by studies in which similar comparisons were performed. Of the studies using any of the NanoString nCounter® product line-up [12,13,14,15,49,50,51,52,53,54,55,56], the same PanCancer® IO360TM system used in our study was investigated in three [12,15,51]. In the study of James et al. [15], immunologic changes resulting from neoadjuvant chemotherapy exposure were investigated. They found that 10–10 DEGs were up- and down-expressed, and a significant decrease in cell proliferation, DNA damage repair, and epigenetics annotations, and a significant increase in the cytotoxicity and interferon signaling functions, were found. Furthermore, significant changes in the ‘proliferation and stress response’, ‘pro-tumorigenic signaling pathways’, and ‘immunoregulatory pathways’ were identified, all of which suggest that neoadjuvant chemotherapy can induce changes in immunostimulation and immunosuppression [15]. By comparing the significant DEGs of James’ study [15] with those of the current work, 36 of the tested genes were up- or down-expressed in both. The majority of the overlapping DEGs belonged to the ‘myeloid cell activity’ (n = 17), ‘immune cell localization to tumors’ (n = 13), ‘common signaling pathways’ (n = 10), and the ‘myeloid compartment’ (n = 9) annotation categories. Similarly, Jordan et al. [12] also compared pre- and post-neoadjuvant chemotherapy samples. They identified 69 significant DEGs, of which 12 overlapped with our results. Three of the overlapping DEGs belonged to each of the ‘antigen presentation’ (MRC1, SOCS1, UBE2C), ‘cell proliferation’ (CCND2, CENPF, UBE2C), ‘common signaling pathways’ (DUSP1, DUSP5, SOCS1), and ‘immune cell localization to tumors’ (CDH5, CPA3, IFITM2) annotations. Finally, the study of Rocconi et al. [51] investigated the relationship between the gene expression profile and Gemogenovatucel-T (an autologous tumor cell vaccine) treatment. In the current study, we compared patients with improved and poor survival (<12 months and >12 months, respectively), different ASA performance scores, different CD4+, CD8+, and CD45+ staining categories, and different T statuses. A total of 148 significant DEGs were found, of which the ‘myeloid cell activity’ (n = 61), ‘immune cell localization to tumors’ (n = 59), ‘killing of cancer cells’ (n = 37), ‘common signaling pathways’ (n = 35), ‘stromal factors’ (n = 28), ‘T cell priming and activation’ (n = 28), and ‘myeloid compartment’ (n = 27) annotations were the most common. Although not using NanoString, the study of Barnett et al. [26] compared tumors with high and low Treg infiltrations. Using 200 DEGs, the authors predicted high vs. low Treg infiltration tumors with 77% overall accuracy, and the antigen presentation pathway was the most affected of all investigated functional annotations [26]. Validation of the results of our gene expression profiling data was also performed using the KMplotter web application [19], and the same tendencies were justified, which further strengthened our results.
Limitations
The current study has limitations. The sample size of the study was low, and gene expression analysis via NanoString could be performed only for an even lower, select number of patients. The selection of ovarian cancer cases for the NanoString analysis based on the prognosis might be biased by possible associations with other pathological and/or oncological parameters, such as by the correlation with tumor-infiltrating immune cells, subtypes, or whether the treatments of patients with different survival lengths were different. A further limitation was that the study population was not limited to a single ovarian cancer subtype, which increased the heterogeneity of the study. Moreover, the oncological treatment data of patients could not be obtained for every study participant, which might have introduced some bias in the survival analyses.
4. Materials and Methods
4.1. Patients and Study Design
A RROS was conducted with the inclusion of 57 histologically confirmed ovarian cancer patients, who were diagnosed between 2003 and 2017. Patients were operated on at the Department of Obstetrics and Gynecology, Saint Pantaleon Hospital, Dunaujvaros, Hungary. All patients signed a general informed consent form at the time of their operation, in which they all agreed to the anonymous data collection for scientific purposes. The study was approved by the Regional and Institutional Committee of Science and Research Ethics, Semmelweis University (SE TUKEB 133/2015), and the Medical Research Council (ETT-TUKEB 14383/2017). Routine pathological analysis of surgical specimens was performed at the Department of Pathology, Saint Pantaleon Hospital, Dunaujvaros, Hungary, while further analyses, including immunohistochemical and genetic testing, were carried out at the Department of Pathology, Forensic and Insurance Medicine, Semmelweis University, Budapest, Hungary.
4.2. Immunohistochemical Staining of OC Samples
Tissue microarrays (TMA) were composed from the FFPE samples with a systematic core punching algorithm using the Tissue Microarray Builder instrument (Histopathology Ltd., Pécs, Hungary). A total of 57 cores of 2 mm in diameter were taken from the main tumor mass. Immunohistochemical reactions were performed on 4 µm-thick sections cut from TMA blocks mounted on adhesive glass slides (SuperFrost UltraPlus from Gerhard Menzel Ltd., Braunschweig, Germany). We analyzed the following biomarkers in each section: CD4 (helper T cell marker), CD8 (cytotoxic T cell), and CD45 (leukocytes, LCA), in an automated immunostainer (Ventana Benchmark XT, Roche, Tucson, AZ, USA) using the solutions and settings as provided by the manufacturer. For CD45, both CD45RO and CD45RA were detected (Figure 6). The slides were digitalized with a Pannoramic P250beta slide scanner (3DHistech Ltd., Budapest, Hungary), and for the evaluation we used the Pannoramic Viewer with the support of the TMA and the Histoquant modules (3Dhistech Ltd., Budapest, Hungary). Immunoreactions were measured in the lymphocytes/leukocytes regarding all reactions and categorized as follows: negative, weak, moderate, and strong immunostaining were found if no (0%), 1–5%, 6–15%, or >15% of immune cells were stained, respectively. The percentage was determined as the density of infiltration according to the Salgado criteria, in line with the standardization and guidelines of TIL assessment, and adapted to IHC [57] (please see an extended and detailed description in the Supplementary Material). The reactions were quantified and calculated with computer-assisted image analysis by a histopathologist, using the QuPath (version 0.4.0) software environment, resulting in the number of positive cells per annotation, where the size of each annotation corresponded to the core cylinder’s surface of 3.14 mm2 [58].
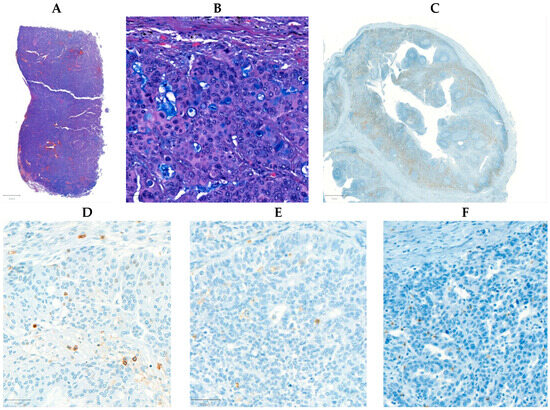
Figure 6.
Microscopic images depicting a high-grade serous ovarian carcinoma. The hematoxylin and eosin-stained tissue is displayed at 0.6× (A) and 10× (B) magnification. A positive control for CD4 taken from the appendix of one of the surgically resected cases at 2× magnification (C). Stains for CD45 (D), CD8 (E), and CD4 (F) at 10× magnification.
4.3. NanoString nCounter PanCancer IO 360 Panel
The RNA of 22 tumors was obtained from FFPE tissue. Five 5-µm thick sections were cut from the FFPE blocks and, by following the manufacturer’s instructions, total RNA was obtained with the High Pure FFPE RNA Isolation Kit (Roche, Basel, Switzerland). RNA concentrations were measured using the Qubit 4 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The RNA samples with adequate concentrations were hybridized to the nCounter® PanCancer IO 360TM Gene Expression Panel (NanoString, Seattle, WA, USA), containing 770 genes, for 16 h using a thermocycler. The samples were transferred to the nCounter Prep Station (NanoString, Seattle, WA, USA) for further processing. The gene expression profiles of the samples were digitalized with the nCounter Digital Analyzer. The results were quantified using nSolver 4.0 Analysis Software (NanoString, Seattle, WA, USA).
4.4. Description of Clinicopathological Data
Pre-, peri-, and post-operative data were collected. Complete blood count and tumor marker measurements were completed at the Central Laboratory of Saint Pantaleon Hospital, Dunaujvaros, Hungary. Age, weight, medical history data, the ASA performance score [18], the type of tumor removal surgery, and results of routine pathological analysis were retrospectively collected from the hospital information system of Saint Pantaleon Hospital, Dunaujvaros, Hungary. Oncological treatment of patients occurred either at the Division of Oncology, Department of Internal Medicine and Oncology, Semmelweis University, Budapest, Hungary, or at another institution not participating in this study, according to the patients’ preferences. Where possible, all details of chemotherapy (medication, number of cycles, etc.) were collected. OS was calculated from the date of diagnosis to the date of death from any cause or until the last follow-up date.
4.5. Data Collection from the Kaplan–Meier Plotter Web Application
The Kaplan–Meier Plotter web application [19] (developed by Győrffy et al. and available online at https://kmplot.com/analysis/index.php?p=service&cancer=ovar; access date: 20 July 2023) is a web application used to discover and validate survival biomarkers in various tumorous diseases, including ovarian cancer. The tool provides a wide variety of gene/mRNA count data, with corresponding survival times and censoring data of the same patients. The OS data were obtained by manually selecting the genes of interest and splitting the measurement data by the median without any further changes to the default settings. From the results presented by the application, the hazard ratio (HR) and its 95% confidence interval (CI), the p-value from the log rank test, and the median OS of the low- and high-expression groups were collected.
4.6. Statistical Analysis
Data were analyzed within the R for Windows environment (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria). Continuous and count data comparisons were performed using the Kruskal–Wallis ANOVA with p-value-adjusted pairwise comparisons using the Wilcoxon rank sum exact test and Fisher exact tests, respectively. Correlation analysis was performed using the Spearman correlation. Cox regression and the log rank test were used to assess survival data (R package survival, version 3.5–5) and plotted using the survminer R package (version 0.4.9). A p-value < 0.05 was considered statistically significant. Continuous, survival, and count data were expressed as the mean ± standard deviation, the HR with a 95% CI, and the number of observations (percentage), respectively.
NanoString data were analyzed using the RUVSeq method [59] (R package RUVSeq, version 1.34.0 [60]). In short, after the normalization of the count data with RUVSeq, differential expression analysis (R package DESeq2, version 1.40.2 [61]) and gene set enrichment analysis (GSEA) were performed. The results were drawn using volcano plots and heatmaps with the ggplot2 (version 3.4.2 [62]) and ComplexHeatmap (version 2.16.0 [63]) R packages, respectively.
5. Conclusions
In our RROS, we found that tumor-infiltrating lymphocyte counts are associated with peculiar gene expression patterns and bear prognostic information in ovarian cancer: CD4+ and CD45+ immune cell infiltration showed significant predictive power for overall survival. The gene SOX11 was identified and validated in an independent dataset as a prognostic marker in ovarian cancer.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241813684/s1.
Author Contributions
Conceptualization, A.J.B., A.M.S. and M.D.; methodology, A.J.B. and A.M.S.; formal analysis, Z.H.; investigation, A.J.B., M.A., J.G., T.M.G., M.H., L.M., D.M. and A.N.; resources, S.B., A.M.S. and M.D.; data curation, A.J.B., Z.H. and M.H.; writing—original draft preparation, A.J.B., Z.H. and A.M.S.; writing—review and editing, M.A., S.B., J.G., T.M.G., M.H., L.M., D.M., A.N. and M.D.; visualization, Z.H.; supervision, S.B., A.M.S. and M.D.; project administration, A.M.S. and M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
The NanoString nCounter® PanCancer IO 360TM Gene Expression Panels were funded by the NVKP_16-1-2016-0004 grant of the National Research and Innovation Fund. The APC was supported by Semmelweis University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional and Institutional Committee of Science and Research Ethics, Semmelweis University (SE TUKEB 133/2015), and the Medical Research Council (ETT-TUKEB 14383/2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to Madar-Dank V (Rutgers University, New Brunswick, NJ, USA) for English proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Burges, A.; Schmalfeldt, B. Ovarian cancer: Diagnosis and treatment. Dtsch. Ärzteblatt Int. 2011, 108, 635–641. [Google Scholar] [CrossRef]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, C.; Zhou, S. Targeting tumor microenvironment in ovarian cancer: Premise and promise. Biochim. Biophys. Acta BBA-Rev. Cancer 2020, 1873, 188361. [Google Scholar] [CrossRef]
- Roxburgh, C.S.; McMillan, D.C. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat. Rev. 2012, 38, 451–466. [Google Scholar] [CrossRef]
- Gavalas, N.G.; Karadimou, A.; Dimopoulos, M.A.; Bamias, A. Immune response in ovarian cancer: How is the immune system involved in prognosis and therapy: Potential for treatment utilization. Clin. Dev. Immunol. 2010, 2010, 791603. [Google Scholar] [CrossRef]
- Singh, M.; Loftus, T.; Webb, E.; Benencia, F. Minireview: Regulatory T Cells and Ovarian Cancer. Immunol. Investig. 2016, 45, 712–720. [Google Scholar] [CrossRef]
- Zou, R.; Jiang, Q.; Jin, T.; Chen, M.; Yao, L.; Ding, H. Pan-cancer analyses and molecular subtypes based on the cancer-associated fibroblast landscape and tumor microenvironment infiltration characterization reveal clinical outcome and immunotherapy response in epithelial ovarian cancer. Front. Immunol. 2022, 13, 956224. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Yang, J.; Wang, Z.; Zhang, Z.; Peng, J.; Wang, Y.; Hong, L. A novel tumor mutational burden-based risk model predicts prognosis and correlates with immune infiltration in ovarian cancer. Front. Immunol. 2022, 13, 943389. [Google Scholar] [CrossRef]
- Jordan, K.R.; Sikora, M.J.; Slansky, J.E.; Minic, A.; Richer, J.K.; Moroney, M.R.; Hu, J.; Wolsky, R.J.; Watson, Z.L.; Yamamoto, T.M.; et al. The Capacity of the Ovarian Cancer Tumor Microenvironment to Integrate Inflammation Signaling Conveys a Shorter Disease-free Interval. Clin. Cancer Res. 2020, 26, 6362–6373. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, M.C.W.; Milne, K.; Pedersen, M.; Hasselager, T.; Olsen, L.R.; Anglesio, M.S.; Borch, T.H.; Kennedy, M.; Briggs, G.; Ledoux, S.; et al. Changes in the Tumor Immune Microenvironment during Disease Progression in Patients with Ovarian Cancer. Cancers 2020, 12, 3828. [Google Scholar] [CrossRef] [PubMed]
- Khalique, S.; Nash, S.; Mansfield, D.; Wampfler, J.; Attygale, A.; Vroobel, K.; Kemp, H.; Buus, R.; Cottom, H.; Roxanis, I.; et al. Quantitative Assessment and Prognostic Associations of the Immune Landscape in Ovarian Clear Cell Carcinoma. Cancers 2021, 13, 3854. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Woodman, M.; De La Cruz, P.; Eurich, K.; Ozsoy, M.A.; Schorl, C.; Hanley, L.C.; Ribeiro, J.R. Adaptive transcriptomic and immune infiltrate responses in the tumor immune microenvironment following neoadjuvant chemotherapy in high grade serous ovarian cancer reveal novel prognostic associations and activation of pro-tumorigenic pathways. Front. Immunol. 2022, 13, 965331. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, X.; Zhang, W.; Fan, J.; Zhou, Y.; Li, W.; Yin, J.; Yang, X.; Guo, E.; Li, X.; et al. Spatial heterogeneity of infiltrating T cells in high-grade serous ovarian cancer revealed by multi-omics analysis. Cell Rep. Med. 2022, 3, 100856. [Google Scholar] [CrossRef]
- Meagher, N.S.; Hamilton, P.; Milne, K.; Thornton, S.; Harris, B.; Weir, A.; Alsop, J.; Bisinoto, C.; Brenton, J.D.; Brooks-Wilson, A.; et al. Profiling the immune landscape in mucinous ovarian carcinoma. Gynecol. Oncol. 2023, 168, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Daabiss, M. American Society of Anaesthesiologists physical status classification. Indian J. Anaesth. 2011, 55, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Gyorffy, B. Discovery and ranking of the most robust prognostic biomarkers in serous ovarian cancer. Geroscience 2023, 45, 1889–1898. [Google Scholar] [CrossRef]
- Kazemi, M.H.; Sadri, M.; Najafi, A.; Rahimi, A.; Baghernejadan, Z.; Khorramdelazad, H.; Falak, R. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front. Immunol. 2022, 13, 1018962. [Google Scholar] [CrossRef]
- Lin, B.; Du, L.; Li, H.; Zhu, X.; Cui, L.; Li, X. Tumor-infiltrating lymphocytes: Warriors fight against tumors powerfully. Biomed. Pharmacother. 2020, 132, 110873. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, L.; Xu, B.; Xiong, Y.; Yang, M.; Rui, X.; Shi, L.; Wu, C.; Jiang, J.; Lu, B. Higher Numbers of T-Bet+ Tumor-Infiltrating Lymphocytes Associate with Better Survival in Human Epithelial Ovarian Cancer. Cell. Physiol. Biochem. 2017, 41, 475–483. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Z.; He, J.; Jin, T.; Han, Y.; Hu, L.; Song, J.; Huang, S. Identification of three molecular subtypes based on immune infiltration in ovarian cancer and its prognostic value. Biosci. Rep. 2020, 40, BSR20201431. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Hua, D.; Li, J.; Zhang, X.; Zhang, Z.; Zhang, B.; Bei, T.; Cui, L.; Chen, S.; Wang, S.; et al. Tumor immune microenvironment changes are associated with response to neoadjuvant chemotherapy and long-term survival benefits in advanced epithelial ovarian cancer: A pilot study. Front. Immunol. 2023, 14, 1022942. [Google Scholar] [CrossRef]
- Ovarian Tumor Tissue Analysis Consortium; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Toloczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.C.; Bean, S.M.; Whitaker, R.S.; Kondoh, E.; Baba, T.; Fujii, S.; Marks, J.R.; Dressman, H.K.; Murphy, S.K.; Berchuck, A. Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol. Oncol. 2010, 116, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Q.; Zhu, Y.; Huang, Y.; Qin, J.; Wu, X.; Zhang, S. Lymphocyte and macrophage infiltration in omental metastases indicates poor prognosis in advance stage epithelial ovarian cancer. J. Int. Med. Res. 2021, 49, 3000605211066245. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Zhang, Q.; Pan, Y.; Lv, Y.; Chen, X.; Zuo, Y.; Hao, D. Clinical significance of the immune microenvironment in ovarian cancer patients. Mol. Omics 2018, 14, 341–351. [Google Scholar] [CrossRef]
- Wang, Q.; Lou, W.; Di, W.; Wu, X. Prognostic value of tumor PD-L1 expression combined with CD8(+) tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int. Immunopharmacol. 2017, 52, 7–14. [Google Scholar] [CrossRef]
- Devlin, M.J.; Miller, R.; Laforets, F.; Kotantaki, P.; Garsed, D.W.; Kristeleit, R.; Bowtell, D.D.; McDermott, J.; Maniati, E.; Balkwill, F.R. The Tumor Microenvironment of Clear-Cell Ovarian Cancer. Cancer Immunol. Res. 2022, 10, 1326–1339. [Google Scholar] [CrossRef]
- Gallego, A.; Berjon, A.; Mendiola, M.; Diez, J.; Castelo, B.; Hernandez, A.; Hardisson, D.; Batlle, J.F.; Garcia, M.J.; Redondo, A. Tumor infiltrating lymphocytes (TILs) in endometrioid and clear cell ovarian carcinoma: Characterization, association with mismatch repair system deficiency and endometriosis, and prognostic implications. J. Clin. Oncol. 2021, 39, e17549. [Google Scholar] [CrossRef]
- Santoiemma, P.P.; Reyes, C.; Wang, L.P.; McLane, M.W.; Feldman, M.D.; Tanyi, J.L.; Powell, D.J., Jr. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol. Oncol. 2016, 143, 120–127. [Google Scholar] [CrossRef]
- Milne, K.; Kobel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.P.; Balmaceda, C.; Bravo, M.L.; Kato, S.; Villarroel, A.; Owen, G.I.; Roa, J.C.; Cuello, M.A.; Ibanez, C. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 151, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Chen, R.; Bai, Y.; Lu, X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017, 8, 15621–15631. [Google Scholar] [CrossRef] [PubMed]
- Arman Karakaya, Y.; Atigan, A.; Guler, O.T.; Demiray, A.G.; Bir, F. The relation of CD3, CD4, CD8 and PD-1 expression with tumor type and prognosis in epithelial ovarian cancers. Ginekol. Pol. 2021, 92, 344–351. [Google Scholar] [CrossRef]
- Yildirim, N.; Akman, L.; Acar, K.; Demir, S.; Ozkan, S.; Alan, N.; Zekioglu, O.; Terek, M.C.; Ozdemir, N.; Ozsaran, A. Do tumor-infiltrating lymphocytes really indicate favorable prognosis in epithelial ovarian cancer? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 55–61. [Google Scholar] [CrossRef]
- Preston, C.C.; Maurer, M.J.; Oberg, A.L.; Visscher, D.W.; Kalli, K.R.; Hartmann, L.C.; Goode, E.L.; Knutson, K.L. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS ONE 2013, 8, e80063. [Google Scholar] [CrossRef]
- Emens, L.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Diéras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Duc, A.N.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bound, N.T.; Vandenberg, C.J.; Kartikasari, A.E.R.; Plebanski, M.; Scott, C.L. Improving PARP inhibitor efficacy in high-grade serous ovarian carcinoma: A focus on the immune system. Front. Genet. 2022, 13, 886170. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.A.; Allison, K.H.; Garcia, R.L.; Gray, H.J.; Goff, B.A.; Swisher, E.M. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: Association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol. Oncol. 2008, 109, 215–219. [Google Scholar] [CrossRef]
- Montfort, A.; Barker-Clarke, R.J.; Piskorz, A.M.; Supernat, A.; Moore, L.; Al-Khalidi, S.; Böhm, S.; Pharoah, P.; McDermott, J.; Balkwill, F.R.; et al. Combining measures of immune infiltration shows additive effect on survival prediction in high-grade serous ovarian carcinoma. Br. J. Cancer 2020, 122, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Zhang, C.; Yang, H.; Qiu, H.; Li, J.; Liu, Y.; Qin, L.; Wang, L.; Hao, S.; et al. Infiltration of dendritic cells and T lymphocytes predicts favorable outcome in epithelial ovarian cancer. Cancer Gene Ther. 2015, 22, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, Q.; Zhang, X.; Yang, M.; Wang, Y.; He, M.; Lu, J.; Liu, H. Spatial cytotoxic and memory T cells in tumor predict superior survival outcomes in patients with high-grade serous ovarian cancer. Cancer Med. 2021, 10, 3905–3918. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Chen, M.; Ma, X. The Immune Subtype Contributes to Distinct Overall Survival for Ovarian Cancer Patients With Platinum-Based Adjuvant Therapy. Front. Immunol. 2022, 13, 872991. [Google Scholar] [CrossRef]
- Gill, C.M.; D’andrea, M.R.; Tomita, S.; Suhner, J.; Umphlett, M.; Zakashansky, K.; Blank, S.V.; Tsankova, N.; Shrivastava, R.K.; Fowkes, M.; et al. Tumor immune microenvironment in brain metastases from gynecologic malignancies. Cancer Immunol. Immunother. 2021, 70, 2951–2960. [Google Scholar] [CrossRef]
- Arend, R.C.; Londoño, A.I.; Montgomery, A.M.; Smith, H.J.; Dobbin, Z.C.; Katre, A.A.; Martinez, A.; Yang, E.S.; Alvarez, R.D.; Huh, W.K.; et al. Molecular Response to Neoadjuvant Chemotherapy in High-Grade Serous Ovarian Carcinoma. Mol. Cancer Res. 2018, 16, 813–824. [Google Scholar] [CrossRef]
- Heong, V.; Tan, T.Z.; Miwa, M.; Ye, J.; Lim, D.; Herrington, C.S.; Iida, Y.; Yano, M.; Yasuda, M.; Ngoi, N.Y.; et al. A multi-ethnic analysis of immune-related gene expression signatures in patients with ovarian clear cell carcinoma. J. Pathol. 2021, 255, 285–295. [Google Scholar] [CrossRef]
- Rocconi, R.P.; Stanbery, L.; da Silva, L.M.; Barrington, R.A.; Aaron, P.; Manning, L.; Horvath, S.; Wallraven, G.; Bognar, E.; Walter, A.; et al. Long-Term Follow-Up of Gemogenovatucel-T (Vigil) Survival and Molecular Signals of Immune Response in Recurrent Ovarian Cancer. Vaccines 2021, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Weberpals, J.I.; Pugh, T.J.; Marco-Casanova, P.; Goss, G.D.; Wright, N.A.; Rath, P.; Torchia, J.; Fortuna, A.; Jones, G.N.; Roudier, M.P.; et al. Tumor genomic, transcriptomic, and immune profiling characterizes differential response to first-line platinum chemotherapy in high grade serous ovarian cancer. Cancer Med. 2021, 10, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Lu, C.; Zhou, H.; Liu, Q.; Yang, J. Differential molecular pathway expression according to chemotherapeutic response in ovarian clear cell carcinoma. BMC Womens Health 2023, 23, 298. [Google Scholar] [CrossRef]
- Mairinger, F.; Bankfalvi, A.; Schmid, K.W.; Mairinger, E.; Mach, P.; Walter, R.F.; Borchert, S.; Kasimir-Bauer, S.; Kimmig, R.; Buderath, P. Digital Immune-Related Gene Expression Signatures In High-Grade Serous Ovarian Carcinoma: Developing Prediction Models For Platinum Response. Cancer Manag. Res. 2019, 11, 9571–9583. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.U.; Kim, A.; Kim, J.Y.; Kim, K.H.; Hwang, C.; Lee, S.J.; Park, W.Y.; Jung, S.; Choi, H.J.; Kim, K. Differences in immune-related gene expressions and tumor-infiltrating lymphocytes according to chemotherapeutic response in ovarian high-grade serous carcinoma. J. Ovarian Res. 2020, 13, 65. [Google Scholar] [CrossRef]
- Jordan, S.E.; Saad, H.; Covarrubias, A.S.; Siemon, J.; Pearson, J.M.; Slomovitz, B.M.; Huang, M.; Pinto, A.; Schlumbrecht, M.; George, S.H. mRNA expression in low grade serous ovarian cancer: Results of a nanoString assay in a diverse population. Gynecol. Oncol. 2020, 159, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Hamilton, A.M.; Furberg, H.; Pietzak, E.; Purdue, M.P.; Troester, M.A.; Hoadley, K.A.; Love, M.I. An approach for normalization and quality control for NanoString RNA expression data. Brief. Bioinform. 2021, 22, bbaa163. [Google Scholar] [CrossRef]
- Risso, D.; Ngai, J.; Speed, T.P.; Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014, 32, 896–902. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Navarro, D.; Pedersen, T.L. ggplot2: Elegant Graphics for Data Analysis (3e). Available online: https://ggplot2-book.org/ (accessed on 1 June 2023).
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).