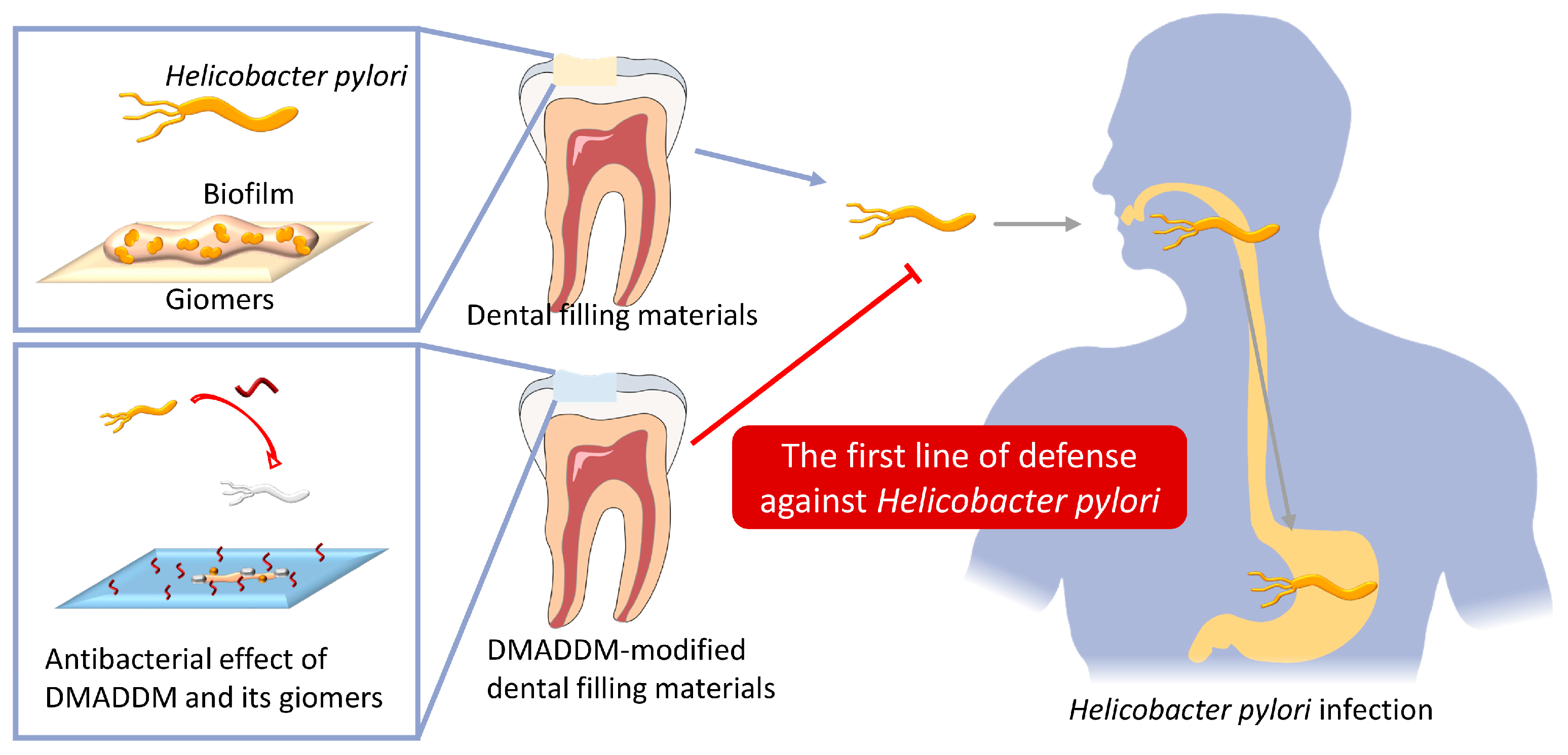
Dimethylaminododecyl Methacrylate-Incorporated Dental Materials Could Be the First Line of Defense against Helicobacter pylori
Abstract
1. Introduction
2. Results
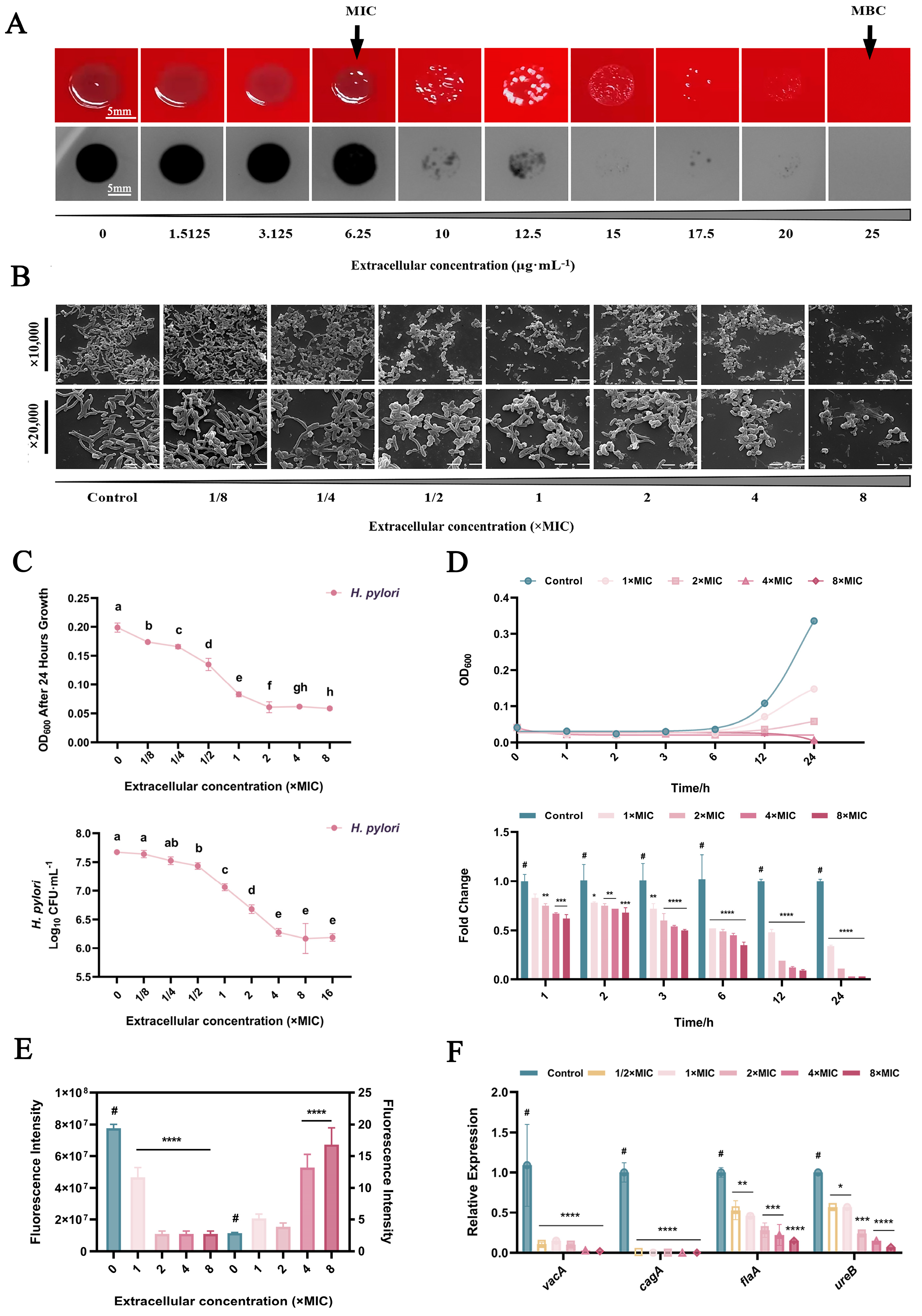
2.1. Inhibition of H. pylori in Planktonic Form
2.2. Inhibition of H. pylori Biofilms
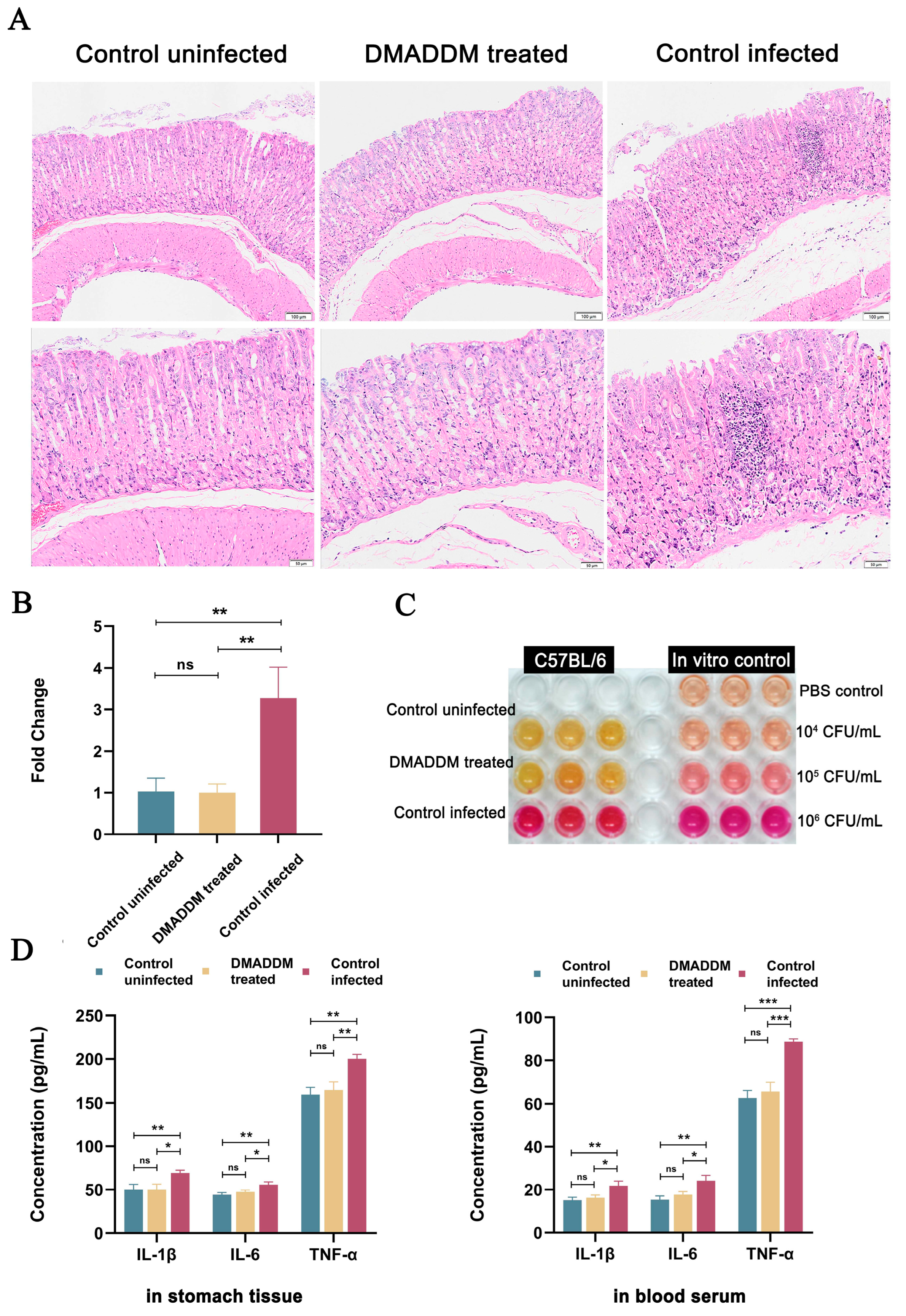
2.3. Antibacterial Effects of Pretreatment In Vivo
3. Discussion
4. Materials and Methods
4.1. Synthesis of DMADDM and DMADDM–Modified Giomers
4.2. H. pylori Strains and Biofilm Cultivation
4.3. Antimicrobial Activity Assay
4.4. Morphology Changing Assay
4.5. Bacterial Killing Assay
4.6. Viability Test
4.7. Virulence Gene Expression Assay
4.8. Biofilm Inhibiting Assay
4.9. Antibiofilm Assays of DMADDM–Modified Giomers
4.10. In Vivo Inhibition Efficacy of DMADDM–Modified Giomers against H. pylori Infection
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e1115. [Google Scholar] [CrossRef]
- Quaglia, N.C.; Dambrosio, A. Helicobacter pylori: A foodborne pathogen? World J. Gastroenterol. 2018, 24, 3472–3487. [Google Scholar] [CrossRef]
- Yuan, C.; Adeloye, D.; Luk, T.T.; Huang, L.; He, Y.; Xu, Y.; Ye, X.; Yi, Q.; Song, P.; Rudan, I. The global prevalence of and factors associated with Helicobacter pylori infection in children: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2022, 6, 185–194. [Google Scholar] [CrossRef]
- Nouraie, M.; Latifi-Navid, S.; Rezvan, H.; Radmard, A.R.; Maghsudlu, M.; Zaer-Rezaii, H.; Amini, S.; Siavoshi, F.; Malekzadeh, R. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter 2009, 14, 40–46. [Google Scholar] [CrossRef]
- Rowland, M.; Imrie, C.; Bourke, B.; Drumm, B. How should Helicobacter pylori infected children be managed? Gut 1999, 45 (Suppl. 1), I36–I39. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Wang, J.; Liao, B.; Cheng, L.; Ren, B. The interactions between oral-gut axis microbiota and Helicobacter pylori. Front. Cell. Infect. Microbiol. 2022, 12, 914418. [Google Scholar] [CrossRef]
- Yee, J.K. Helicobacter pylori colonization of the oral cavity: A milestone discovery. World J. Gastroenterol. 2016, 22, 641–648. [Google Scholar] [CrossRef]
- Kadota, T.; Hamada, M.; Nomura, R.; Ogaya, Y.; Okawa, R.; Uzawa, N.; Nakano, K. Distribution of Helicobacter pylori and Periodontopathic Bacterial Species in the Oral Cavity. Biomedicines 2020, 8, 161. [Google Scholar] [CrossRef]
- Kazanowska-Dygdała, M.; Duś, I.; Radwan-Oczko, M. The presence of Helicobacter pylori in oral cavities of patients with leukoplakia and oral lichen planus. J. Appl. Oral Sci. 2016, 24, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.; Huovinen, S.; Liutu, M.; Uksila, J.; Leino, R. Peptic ulcer and Helicobacter pylori in patients with lichen planus. Acta Derm. Venereol. 2000, 80, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Ren, B.; Zhou, X.; Cheng, L. Helicobacter pylori in the Oral Cavity: Current Evidence and Potential Survival Strategies. Int. J. Mol. Sci. 2022, 23, 13646. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.K.C. Are the view of Helicobacter pylori colonized in the oral cavity an illusion? Exp. Mol. Med. 2017, 49, e397. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382.e1317. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, J.H.; Li, X.Y.; Zhu, Y.; Graham, D.Y.; Lu, N.H. Systematic review with meta-analysis: The global recurrence rate of Helicobacter pylori. Aliment. Pharmacol. Ther. 2017, 46, 773–779. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, H.Q.; Ma, C.; Zhang, A.B.; Feng, H.; Zhang, D.; Liu, C. The association between chronic periodontitis and oral Helicobacter pylori: A meta-analysis. PLoS ONE 2019, 14, e0225247. [Google Scholar] [CrossRef]
- Ansari, S.A.; Iqbal, M.U.N.; Khan, T.A.; Kazmi, S.U. Association of oral Helicobacter pylori with gastric complications. Life Sci. 2018, 205, 125–130. [Google Scholar] [CrossRef]
- Colman, S.; Dhaese, S.; Stove, V.; De Waele, J.J.; Verstraete, A.G. Pilot study of oral fluid and plasma meropenem and piperacillin concentrations in the intensive care unit. Clin. Chim. Acta 2021, 523, 72–76. [Google Scholar] [CrossRef] [PubMed]
- AlSaeed, T.; Nosrat, A.; Melo, M.A.; Wang, P.; Romberg, E.; Xu, H.; Fouad, A.F. Antibacterial Efficacy and Discoloration Potential of Endodontic Topical Antibiotics. J. Endod. 2018, 44, 1110–1114. [Google Scholar] [CrossRef]
- Kashyap, D.; Baral, B.; Verma, T.P.; Sonkar, C.; Chatterji, D.; Jain, A.K.; Jha, H.C. Oral rinses in growth inhibition and treatment of Helicobacter pylori infection. BMC Microbiol. 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Zhu, C.G.; Zhou, X.; Wang, H.; Han, Q.; Ren, B.; Cheng, L. Anti-bacterial and anti-microbial aging effects of resin-based sealant modified by quaternary ammonium monomers. J. Dent. 2021, 112, 103767. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, M.; Ren, B.; Wu, T.; Xu, H.H.K.; Liu, Y.; Peng, X.; Yang, G.; Weir, M.D.; Zhang, S.; et al. The anti-caries effects of dental adhesive resin influenced by the position of functional groups in quaternary ammonium monomers. Dent. Mater. 2018, 34, 400–411. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Ma, Y.; Hu, Y.; Wu, Y.; Lan, F.; Weir, M.D.; Li, M.; Ren, B.; Oates, T.W.; et al. Two-staged time-dependent materials for the prevention of implant-related infections. Acta Biomater. 2020, 101, 128–140. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, L.; Weir, M.D.; Lin, N.J.; Lin-Gibson, S.; Zhou, X.D.; Xu, H.H. Primer containing dimethylaminododecyl methacrylate kills bacteria impregnated in human dentin blocks. Int. J. Oral. Sci. 2016, 8, 239–245. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Zhou, Y.; Zhou, X.; Liao, B.; Xu, H.H.K.; Chu, C.H.; Cheng, L.; Ren, B. Dimethylaminododecyl methacrylate inhibits Candida albicans and oropharyngeal candidiasis in a pH-dependent manner. Appl. Microbiol. Biotechnol. 2020, 104, 3585–3595. [Google Scholar] [CrossRef]
- Zhang, K.; Ren, B.; Zhou, X.; Xu, H.H.; Chen, Y.; Han, Q.; Li, B.; Weir, M.D.; Li, M.; Feng, M.; et al. Effect of Antimicrobial Denture Base Resin on Multi-Species Biofilm Formation. Int. J. Mol. Sci. 2016, 17, 1033. [Google Scholar] [CrossRef]
- Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.K.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacteria and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, 2013. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Ren, B.; Li, H.; Weir, M.D.; Zhou, X.; Oates, T.W.; Cheng, L.; Xu, H.H.K. Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species? Dent. Mater. 2017, 33, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Ren, B.; Li, X.; Wang, L.; Zhou, H.; Weir, M.D.; Zhou, X.; Masri, R.M.; Oates, T.W.; et al. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018, 8, 5509. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cheng, L.; Zhou, X.; Xu, H.H.; Weir, M.D.; Meyer, M.; Maurer, H.; Li, Q.; Hannig, M.; Rupf, S. In situ antibiofilm effect of glass-ionomer cement containing dimethylaminododecyl methacrylate. Dent. Mater. 2015, 31, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, B.; Cheng, L.; Xu, H.H.K.; Li, H.; Huang, Y.; Zhang, Q.; Zhou, X.; Liang, J.; Zou, J. Novel Giomers Incorporated with Antibacterial Quaternary Ammonium Monomers to Inhibit Secondary Caries. Pathogens 2022, 11, 578. [Google Scholar] [CrossRef]
- Ho, S.A.; Hoyle, J.A.; Lewis, F.A.; Secker, A.D.; Cross, D.; Mapstone, N.P.; Dixon, M.F.; Wyatt, J.I.; Tompkins, D.S.; Taylor, G.R.; et al. Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. J. Clin. Microbiol. 1991, 29, 2543–2549. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Silva, H.; Oliveira, R.; Almeida, C.; Azevedo, N.F.; Vieira, M.J. Morphological transition of Helicobacter pylori adapted to water. Future Microbiol. 2017, 12, 1167–1179. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; Mileti, A.; Paolillo, R.; Giorgio, F.; Principi, M.; Di Leo, A. The Puzzle of Coccoid Forms of Helicobacter pylori: Beyond Basic Science. Antibiotics 2020, 9, 293. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Paluch, E.; Karwańska, M.; Wieliczko, A.; Gościniak, G. Myricetin as an Antivirulence Compound Interfering with a Morphological Transformation into Coccoid Forms and Potentiating Activity of Antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef]
- Kadkhodaei, S.; Siavoshi, F.; Akbari Noghabi, K. Mucoid and coccoid Helicobacter pylori with fast growth and antibiotic resistance. Helicobacter 2020, 25, e12678. [Google Scholar] [CrossRef]
- Shi, Z.; Li, X.; Fan, X.; Xu, J.; Liu, Q.; Wu, Z.; Pan, D. PMA-qPCR method for the selective quantitation of viable lactic acid bacteria in fermented milk. Front. Microbiol. 2022, 13, 984506. [Google Scholar] [CrossRef]
- Miotto, M.; Barretta, C.; Ossai, S.O.; da Silva, H.S.; Kist, A.; Vieira, C.R.W.; Parveen, S. Optimization of a propidium monoazide-qPCR method for Escherichia coli quantification in raw seafood. Int. J. Food Microbiol. 2020, 318, 108467. [Google Scholar] [CrossRef] [PubMed]
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371, eaba0862. [Google Scholar] [CrossRef] [PubMed]
- Povolotsky, T.L.; Keren-Paz, A.; Kolodkin-Gal, I. Metabolic Microenvironments Drive Microbial Differentiation and Antibiotic Resistance. Trends Genet. 2021, 37, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J. The biology of cag in the Helicobacter pylori-human interaction. Gastroenterology 2005, 128, 1512–1515. [Google Scholar] [CrossRef]
- Bridge, D.R.; Merrell, D.S. Polymorphism in the Helicobacter pylori CagA and VacA toxins and disease. Gut Microbes 2013, 4, 101–117. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Ottemann, K.M. Helicobacter pylori Biofilm Involves a Multigene Stress-Biased Response, Including a Structural Role for Flagella. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Mobley, H.L. The role of Helicobacter pylori urease in the pathogenesis of gastritis and peptic ulceration. Aliment. Pharmacol. Ther. 1996, 10 (Suppl. 1), 57–64. [Google Scholar] [CrossRef]
- Feng, J.; Cheng, L.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Li, Q.; Hannig, M.; Rupf, S. Effects of water aging on the mechanical and anti-biofilm properties of glass-ionomer cement containing dimethylaminododecyl methacrylate. Dent. Mater. 2019, 35, 434–443. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Liao, T.; Debowski, A.W.; Nilsson, H.O.; Fulurija, A.; Haslam, S.M.; Mulloy, B.; Dell, A.; Stubbs, K.A.; et al. The redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. PLoS Pathog. 2017, 13, e1006280. [Google Scholar] [CrossRef]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, 10–1128. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Hindler, J.F. New consensus guidelines from the Clinical and Laboratory Standards Institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin. Infect. Dis. 2007, 44, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Moore, J.H.; Kolling, G.L.; McGrath, J.S.; Papin, J.A.; Swami, N.S. Minimum Bactericidal Concentration of Ciprofloxacin to Pseudomonas aeruginosa Determined Rapidly Based on Pyocyanin Secretion. Sens. Actuators B Chem. 2020, 312, 127936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, H.J.; Zhao, Q.; Shi, Y.; Chai, A.; Li, B. First Report of Fusarium oxysporum Causing Wilt on Coriander in China. Plant Dis. 2021, 105, 4164. [Google Scholar] [CrossRef] [PubMed]
- Flores-Treviño, C.E.; Urrutia-Baca, V.H.; Gómez-Flores, R.; De La Garza-Ramos, M.A.; Sánchez-Chaparro, M.M.; Garza-Elizondo, M.A. Molecular detection of Helicobacter pylori based on the presence of cagA and vacA virulence genes in dental plaque from patients with periodontitis. J. Dent. Sci. 2019, 14, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Baca, V.H.; Escamilla-García, E.; de la Garza-Ramos, M.A.; Tamez-Guerra, P.; Gomez-Flores, R.; Urbina-Ríos, C.S. In Vitro Antimicrobial Activity and Downregulation of Virulence Gene Expression on Helicobacter pylori by Reuterin. Probiotics Antimicrob. Proteins 2018, 10, 168–175. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Castillo, M.; Chavez, I.; Barreda, F.; Suarez, N.; Nieves, J.; Bernabe, L.A.; Valdivia, D.; Ruiz, E.; Dias-Neto, E.; et al. Prevalence of Helicobacter pylori Infection, Its Virulent Genotypes, and Epstein-Barr Virus in Peruvian Patients With Chronic Gastritis and Gastric Cancer. J. Glob. Oncol. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Elizalde, J.I.; Mendez, A.; Gomez, J.; del Rivero, M.; Gironella, M.; Closa, D.; Quintero, E.; Pique, J.M. Gastric mucosal blood flow changes in Helicobacter pylori infection and NSAID-induced gastric injury. Helicobacter 2003, 8, 124–131. [Google Scholar] [CrossRef]
- Tang, L.; Tang, B.; Lei, Y.; Yang, M.; Wang, S.; Hu, S.; Xie, Z.; Liu, Y.; Vlodavsky, I.; Yang, S. Helicobacter pylori-Induced Heparanase Promotes, H. pylori Colonization and Gastritis. Front. Immunol. 2021, 12, 675747. [Google Scholar] [CrossRef]

| Gene | 5′–3′ Sequence | Product Size (bp) |
|---|---|---|
| vacA [54] | Fwd: CCTACTGAGAATGGTGGCAATA Rvs: GTTCTTCACGAGAGCGTAGTT | 87 |
| cagA [54] | Fwd: GACCGACTCGATCAAATAGCA Rvs: TTAGCTGAAAGCCCTACCTTAC | 113 |
| flaA [55] | Fwd: CAGTATAGATGGTCGTGGGATTG Rvs: GAGAGAAAGCCTTCCGTAGTTAG | 127 |
| ureB [56] | Fwd: CAAAATCGCTGGCATTGGT Rvs: CTTCACCGGCTAAGGCTTCA | 100 |
| 16S rRNA 1, [57] | Fwd: CTCATTGCGAAGGCGACCT Rvs: TCTAATCCTGTTTGCTCCCCA | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Shan, T.; Ren, B.; Zhang, L.; Xu, H.H.K.; Wang, N.; Zhou, X.; Li, H.; Cheng, L. Dimethylaminododecyl Methacrylate-Incorporated Dental Materials Could Be the First Line of Defense against Helicobacter pylori. Int. J. Mol. Sci. 2023, 24, 13644. https://doi.org/10.3390/ijms241713644
Chen X, Shan T, Ren B, Zhang L, Xu HHK, Wang N, Zhou X, Li H, Cheng L. Dimethylaminododecyl Methacrylate-Incorporated Dental Materials Could Be the First Line of Defense against Helicobacter pylori. International Journal of Molecular Sciences. 2023; 24(17):13644. https://doi.org/10.3390/ijms241713644
Chicago/Turabian StyleChen, Xi, Tiantian Shan, Biao Ren, Lin Zhang, Hockin H. K. Xu, Nanxi Wang, Xuedong Zhou, Hong Li, and Lei Cheng. 2023. "Dimethylaminododecyl Methacrylate-Incorporated Dental Materials Could Be the First Line of Defense against Helicobacter pylori" International Journal of Molecular Sciences 24, no. 17: 13644. https://doi.org/10.3390/ijms241713644
APA StyleChen, X., Shan, T., Ren, B., Zhang, L., Xu, H. H. K., Wang, N., Zhou, X., Li, H., & Cheng, L. (2023). Dimethylaminododecyl Methacrylate-Incorporated Dental Materials Could Be the First Line of Defense against Helicobacter pylori. International Journal of Molecular Sciences, 24(17), 13644. https://doi.org/10.3390/ijms241713644








