1. Introduction
Anterior cruciate ligament (ACL) injuries are a common sports-related injury affecting over 100,000 patients each year, and reconstruction is often necessary to restore knee stability due to the poor healing capacity of the ACL [
1,
2]. The estimated failure rate of reconstructed tendon grafts is approximate 10–15% due to poor graft healing at the tendon–bone enthesis, thereby leading to requirement of a second revision surgery [
3]. The enhancement of graft osteointegration into bone tunnels is a promising therapeutic strategy to improve the long-term healing quality in patients after reconstruction [
4]. Increasing evidence provides proof of the basic principle that promotion of the neovascularization at the interface between the tendon graft and bone tunnels favors the support of sufficient oxygen and nutrients to the cells [
5,
6], thereby contributing to more bony ingrowth. However, as the proangiogenic factors also induce inflammatory responses, they may exert dual-phase effects on the tendon–bone healing.
For instance, blocking vascular endothelial growth factor (VEGF), one commonly used angiogenic factor, significantly reduced angiogenesis, graft maturation and biomechanical strength following ACL reconstruction [
6], and injection of intra-tunnel VEGF accelerated the graft healing process [
7]. Of note, as one pro-inflammatory cytokine, VEGF may induce osteoclastogenesis of the osteoclast precursors, which could thereby promote the secretion of PDGF-BB favoring type H (CD31
hiEMCN
hi) vessel formation [
8]. However, the over-expression of VEGF impeded improvements in tensile strength [
6]. Similarly, exogenous VEGF delivery in sheep with ACL reconstruction also increased newly formed vessels while inducing negative mechanical effects [
9]. The biphasic model of VEGF may be ascribed to its pro-angiogenic and pro-inflammatory effects, thus resulting in excessive infiltration of fibroblasts in the long-term intervention [
10].
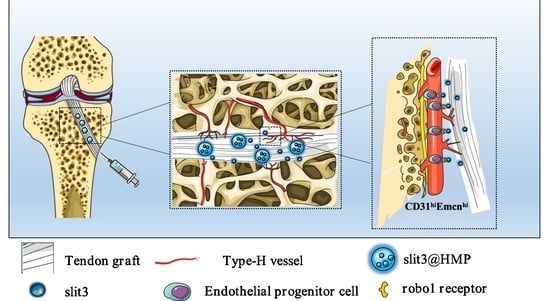
Recently, it has been reported that the type H vessel favors bone regeneration through modulation of angiogenesis–osteogenesis coupling effects [
11,
12]. Type H blood vessels are mainly distributed in the metaphysis, periosteum, and endosteum [
13]. In ACL reconstruction, the bone tunnel will pass through the metaphysis region with abundant type H vessels, indicating that CD31
hiEmcn
hi endothelium may be involved in the angiogenesis during tendon–bone healing. Slit guidance ligand 3 (slit3), derived from osteoblasts or osteoclasts [
14], is coupled to anabolic bone formation via activation of ERK mitogen-activated protein kinase and Hippo signaling through Robo family receptors, thereby enhancing type H vessel formation [
15]. Although platelet-derived growth factor BB (PDGF-BB), mainly secreted by mononuclear preosteoclasts, can also induce type H vessel formation during bone remodeling [
8], it constitutes as a key pro-inflammatory cytokine in concert with transforming growth factor beta (TGFβ) to induce fibrosis [
16]. Of note, the extensive deposition of fibrous connective tissue initiated by macrophage activation impairs tendon–bone healing [
17]. Therefore, the delivery of slit3 to bone tunnels may be a better therapeutic option beneficial for angiogenesis relative to the PDGF-BB administration. To our best knowledge, this is the first report on the strategy involving the modulation of a specific vessel subtype, the CD31
hiEMCN
hi-expressing type H vessel, for enhancement of the graft osteointegration. We raised the hypothesis that the delivery of slit3 could accelerate and promote the osseous ingrowth towards the TBI through increasing type H vessels at the graft enthesis. Accordingly, we encapsulated slit3 or its neutralizing antibody in the micro-hydrogels, for an extended release, prior to injection into the bone tunnels of mice with ACL reconstruction. The immunofluorescence staining, radiographic measurement, gait analysis, and biomechanical testing were applied to assess the treatment outcomes and verify if slit3 affects the graft healing via modulation of type H vessel formation.
3. Materials and Methods
All the operations were performed with the approval by the local Animal Care and Use committee (SYSU-IACUC-2022-001534). The detailed information is described as follows.
3.1. Study Design
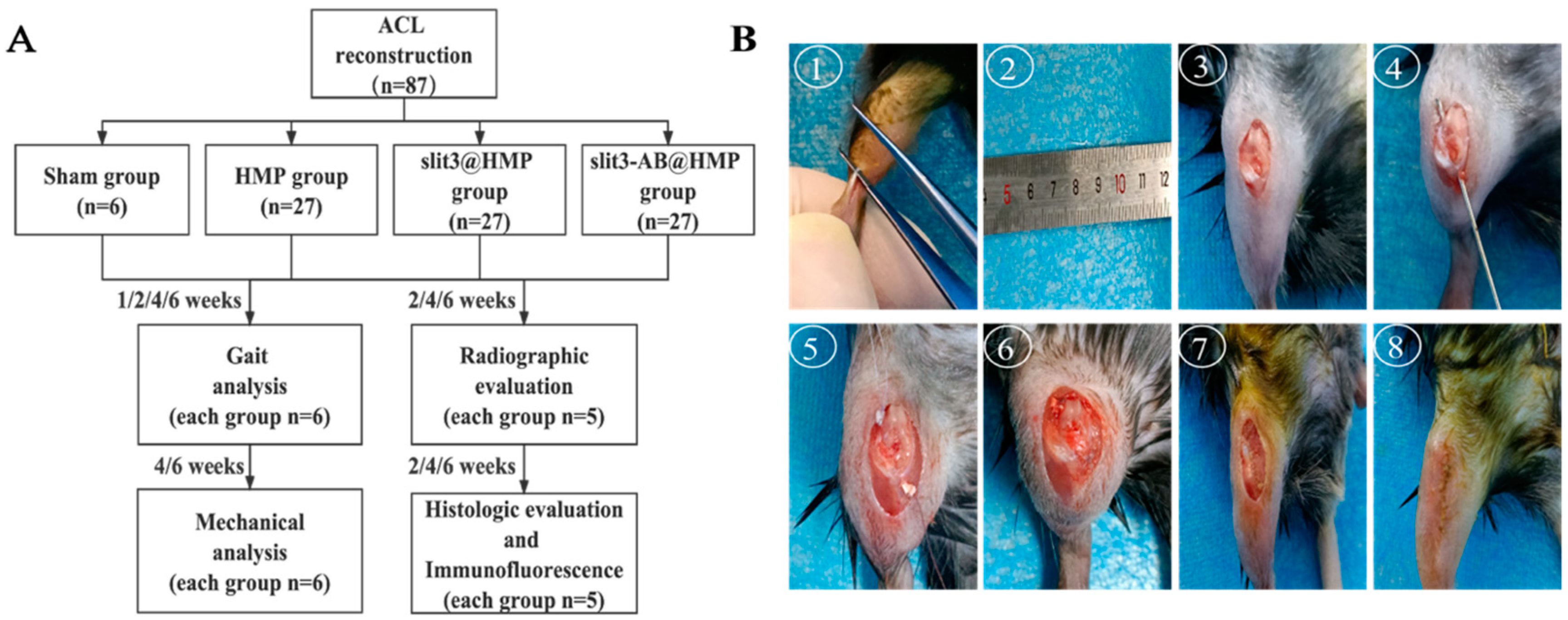
Eighty-seven 10-week-old male C57BL/C mice were purchased and housed in a specific-pathogen-free animal facility. They were divided into 3 groups for ACL reconstruction, which were treated with hydrogel microparticles (HMP) alone, slit3 loaded HMP (slit3@HMP), and slit3 neutralizing antibody (slit3-AB@HMP), respectively (n = 27 for each group). After surgery, the mice in the above three groups and the sham group were used for gait analysis at 1, 2, 4, and 6 weeks (n = 6/group/time point), general histological and immunofluorescence analyses at 2, 4, and 6 weeks post-operation (n = 5/group/time point), micro-CT scanning and assessment at 2, 4, and 6 weeks after surgery (n = 5/group/time point), and biomechanical testing for knee joint laxity displacement and load to failure at 4 and 6 weeks post-surgery (n = 6/group/time point). The flowchart of the experiment are shown in
Figure 1.
3.2. Preparation of HMPs
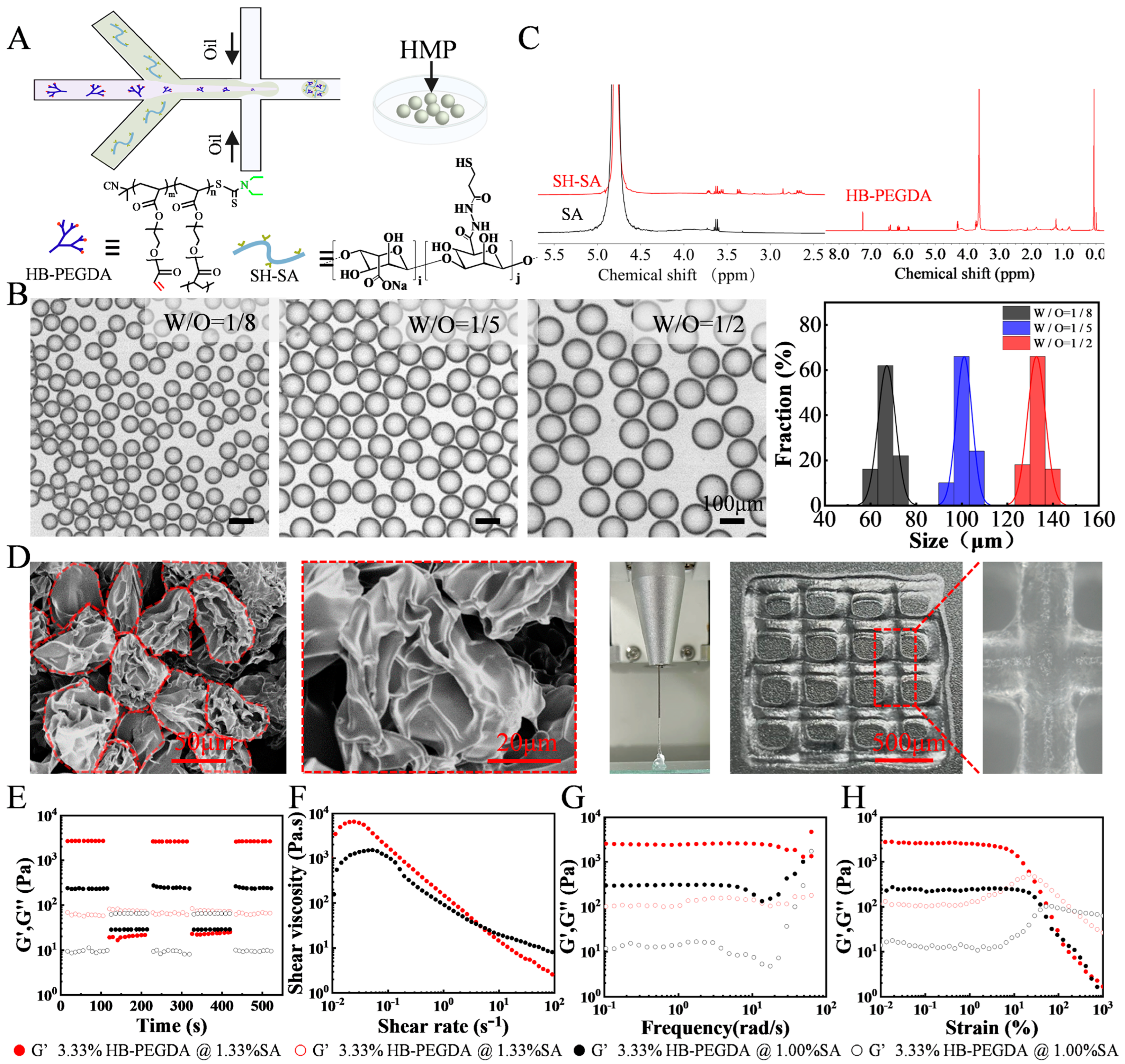
To generate droplets, a microfluidic water-in-oil segmentation system was utilized. In a typical experimental configuration, the water phase consisted of an aqueous dispersion containing 1% (
w/
v) thiolated sodium alginate (SH-SA), 3.33% (
w/
v) vinyl-terminated hyperbranched (polyethylene glycol) diacrylate (HB-PEGDA), and 3.52 × 10
9 infectious units per milliliter (IFU/mL) of adenovirus. The oil phase, on the other hand, consisted of 3M™ Fluorinert™ FC-40 supplemented with 2 wt% Pico-SurfTM. The resulting droplets were collected in an Eppendorf tube and subsequently transferred to a drying oven, where the HMPs were cured at a temperature of 37 °C for a minimum of 1 h. To complete the process, the HMPs underwent purification by introducing Novec 7500 oil containing 20% v/v 1H, 1H, 2H, 2H-perfluoro-1-octanol (PFO) to eliminate any remaining surfactants. Following this, the HMPs were allowed to swell and equilibrate with buffer for a minimum of 2 h at 37 °C prior to their utilization. Fully swollen and equilibrated building block HMPs were pelleted at 8000 rpm for five minutes, and the excess buffer was removed to give the HMPs. All the catalog and company details of the materials used in this study are shown in
Table S1.
3.3. Characterization of HMPs
The chemical structure and composition of the polymers for preparing HMPs were verified by 1H Nuclear Magnetic Resonance Spectroscopy (NMR) (HAAKE RheoStress 1, Thermo Scientific, Massachusetts, USA). Particle size of HMPs was visualized using the ICX41 (SOPTOP ICX41 Sunny Optical Technology (Group) Co., Ltd., Ningbo, China) inverted optical microscope and a scanning electron microscope (SEM, S-4800 field, Hitachi, Tokyo, Japan). The rheological behaviors of the resulting HMPs was tested at 25 °C using a rheometer (HAAKE RheoStress 1, Thermo Scientific, Massachusetts, USA) with a stainless steel parallel plate rotor with a diameter of 8 mm. A dynamic oscillatory strain amplitude sweep measurement was performed by varying the strain percentage from 0.01% to 1000% at a frequency of 1 Hz. Additionally, oscillatory frequency sweeps were conducted, ranging from 0.1 to 100 rad/s at a fixed strain amplitude of 1%. Furthermore, strain-cycle experiments were carried out to assess the recovery properties of the HMPs. These experiments involved subjecting the samples to cycles of high strain (γ = 1000%) followed by low-amplitude strain (γ = 0.1%) until the monitoring resumed, allowing for the investigation of the hydrogels’ recovery behavior after failure at high strain. Moreover, flow curves were obtained through strain-rate-controlled measurements, covering a range of shear rates from 10−2 to 102 s−1. These experiments provided valuable insights into the viscoelastic and flow properties of the HMPs.
3.4. In Vitro Cytotoxicity Assessment and Stability Testing of slit3 Loaded in HMPs
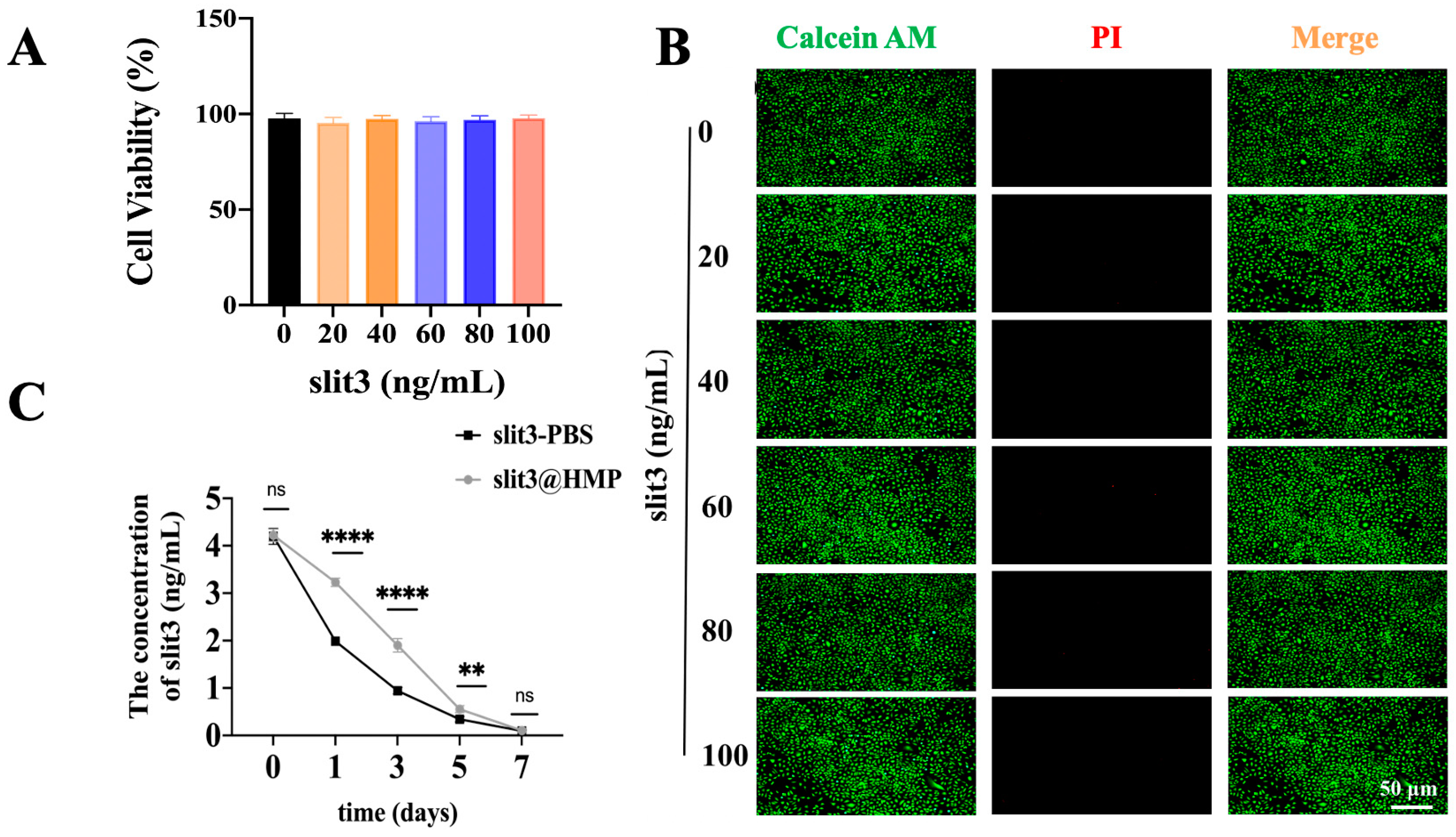
Human umbilical vein endothelial cells (HUVECs) were cultured in Minimum Essential Medium α (MEM α, Gibco, 12571071,Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco,10099141, Carlsbad, CA, USA) and 1% penicillin–streptomycin at 37 °C in a humidified atmosphere containing 5% CO2 in an incubator (Esco Micro Pte. Ltd., CCL-170B-8, Singapore). HUVECs were seeded in the wells of 96-well culture plates at a density of 1.0 × 104 cells per well and cultured overnight for adhesion. The HMPs loaded with a series of ascending concentrations of slit3 were then added into wells for direct co-culture with HUVECs. After 48 h, the medium was removed and then replaced with 110 μL fresh culture medium containing 10 μL cell counting kit-8 (CCK8) reagents prior to incubation in a dark environment for 4 h at 37 °C. Afterwards, 80 μL CCK8-containing medium was pipetted from each well and transferred into a new 96-well plate. The absorbance at the wavelength of 450 nm was measured on a microplate reader (ALLSHENG, AMR-100, Hangzhou, China).
In addition, the cytocompatibility of slit3@HMP was assessed by Live/Dead staining assay. The HUVECs were seeded in a 48-well plate at a density of 104 cells per well and cultured overnight prior to the addition of different doses of slit3@HMP solution. After 24 h, the viability of cells was analyzed using the Live/Dead assay kit (Solarbio, CA1630, Beijing, China). The images were taken using a fluorescent microscope (MSHOT, MF52-M, Guangzhou, China). Therefore, the slit3@HMP were placed into the dialysis bag (JielePu, MD10-100000, Changsha, China) for measurement of the remained slit3 at different time points by the Elisa kit (Cloud-Clone, SED353M, Wuhan, China). The slit3 alone was set as the control group.
3.5. Surgical Procedure
The mice were anesthetized through intraperitoneal injection of 1% sodium pentobarbital and 10% chloral hydrate. After anesthesia, a 5–7 mm incision was made in the back of the calf to expose the Achilles tendon. Then, an 8-0 suture (R831, Jinhuan, China) was used to fix the two ends of the gastrocnemius tendon prior to isolation from the Achilles tendon. Afterwards, an incision was made adjacent to the patellar tendon to allow the patellar bone dislocation to facilitate the creation of the bone tunnel of 0.5 mm in diameter using a bone drill along the footprints of original ACL through transtibial technique. A certain concentration of HMP (2 × 10
4 HMPs in 1 mL PBS) was manually mixed with human recombinant slit3 or slit3-AB protein (100 μg/mL in HMP-PBS solution) for 1 h on ice for surgical use [
19]. After that, 10 μL HMP, slit3@HMP, or slit3-AB@HMP was injected into the pre-drilled bone tunnels prior to the placement of the isolated tendon graft. Both of the graft ends were then sutured tightly with surrounding soft tissues around tibial entrance and femoral exit. Of note, initial graft tensioning is important for tendon–bone healing according to both animal [
20] and clinical [
21] studies. In our study, a constant force of approximately 0.1 N was applied to keep the graft tension during the surgery. Finally, the patellar bone was relocated to facilitate a layer-by-layer closure of incisions with absorbable sutures (Ethicon 8-0, New Brunswick, NJ, USA). The surgical procedures and experimental design were showed in
Figure 8.
Analgesics including meloxicam and buprenorphine (0.1 mg per kg body weight, twice daily for 3 days) were injected for pain relief. We maintained all animals in the Animal Facility of the Sun Yat-Sen University. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University, Guangzhou, China.
3.6. Tissue Collection
For those samples for radiographic assessment, histological and immunofluorescence analyses, the mice were anesthetized with sodium pentobarbital and then fixed with a mixed solution composed of 4% paraformaldehyde and 0.1% sodium heparin (MACKLIN, 9041-08-01, Shanghai, China) through transcardiac perfusion. Afterwards, the femur–tendon graft–tibia complexes (FTGTC) were harvested and then fixed in 4% paraformaldehyde for 48 h prior to decalcification and tissue embedding.
For those samples for biomechanical testing, the mice were euthanized with overdose of anesthetics and then FTGTC was wrapped with saline gauze and stored at −80 °C.
3.7. Radiographic Evaluation of Peri-Tunnel Bone Tissue by Micro-Computed Tomography (Micro-CT)
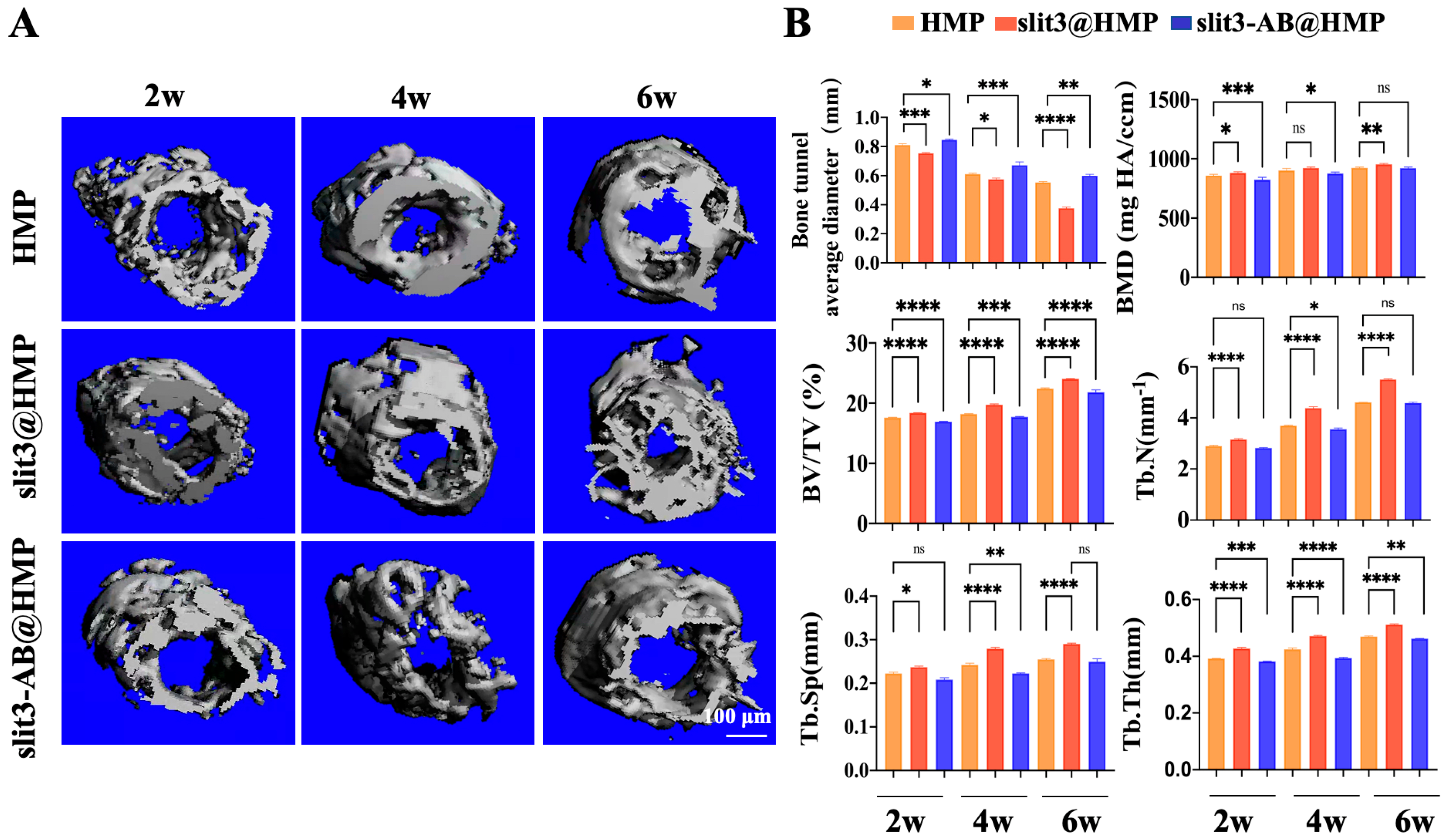
The knee joint of mice was fixed in the scanning tube and scanned with the voltage, current, integration time, and isotropic voxel size set as 70 kV, 114 μA, 230 ms, and 9 μm, respectively (SCANCO μCT100, Bassersdorf, Switzerland). The bone volume/total volume fraction (BV/TV), bone mineral density (BMD), number of trabecular bone (Tb.N), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) of the peri-tunnel bone tissue covered by a circle 0.5 mm in diameter in bone tunnels in the tibia were measured. Five samples in each group at each time point were used for statistical analysis.
3.8. Histological Evaluation
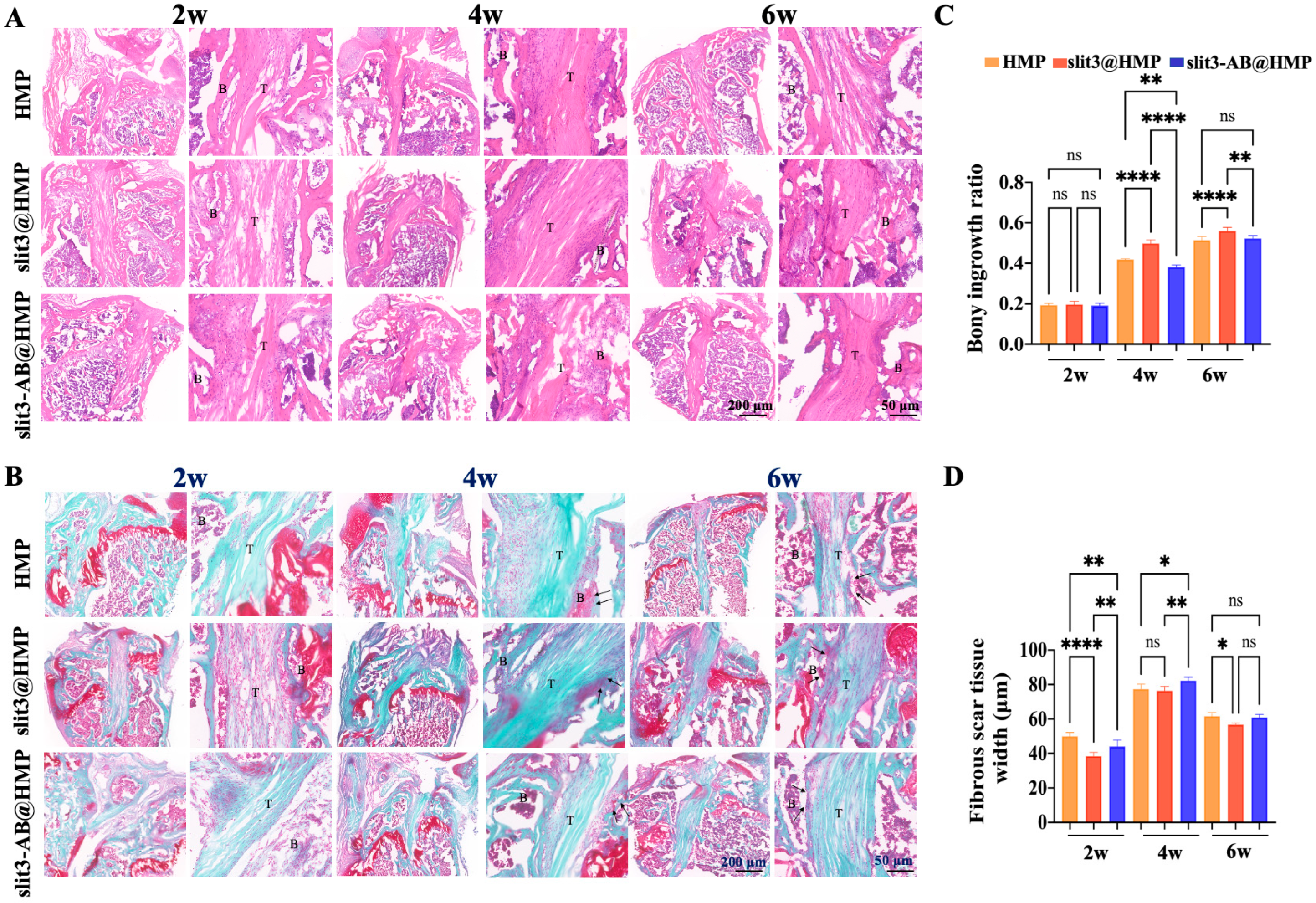
After micro-CT scanning, the FTGTC were decalcified with 10% ethylenediaminetetraacetic acid (EDTA). After decalcification, the samples were dehydrated serially in ascending concentrations of sucrose solution ranging from 10% to 30% for 48 h. The dehydrated samples were then embedded in optimum cutting temperature compound (OCT) for sections (10 μm) along the longitudinal direction of bone tunnels. Hematoxylin and Eosin (H&E) staining was performed to identify and measure the fibrous scar tissue, which is a kind of irregularly arranged fibrous tissue consisting of numerous spindle-shaped cells [
22], at the TBI. In addition, the bony ingrowth at the TBI was assessed by Safranin O/Fast Green (SO/FG) staining. The images were captured and then analyzed by two blinded researchers using ImageJ (NIH, Bethesda, MD, USA). The microscopic images were acquired using a microscope (BDS400, CQOPTEC, Chongqing, China).
3.9. Immunofluorescence Staining
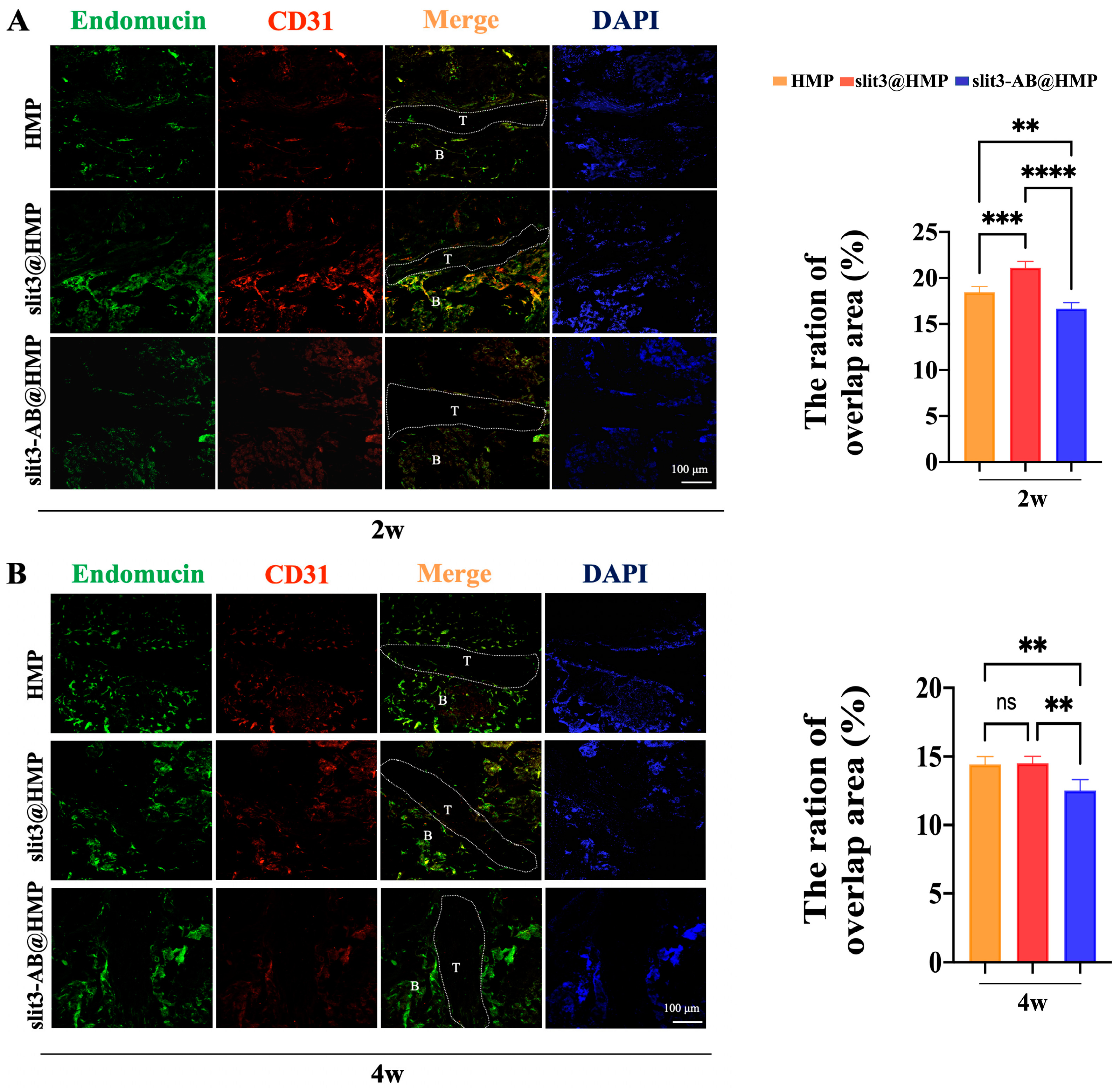
The tissue sections were transferred directly into antigen retrieval solution (sodium citrate, pH 6.0) and then blocked with a mixture of 3% goat serum, 1% bovine serum albumin (BSA), and 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 1 h at room temperature. Subsequently, the sections (20 μm) were probed overnight with the primary antibodies at 4 °C and then incubated with the secondary antibodies for 1 h at room temperature. The cell nuclei were counterstained with DAPI and the images were recorded using confocal laser scanning microscope (FV3000, Olympus, Tokyo, Japan). The following primary antibodies were used: Endomucin (sc-65495, SANTA CRUZ, Dallas, TX, USA) and CD31 (ab28364, Abcam, Cambridge, UK). The secondary antibodies used included anti-rat Alexa Fluor 488 (4416, Cell Signaling, Boston, MA, USA) and anti-rabbit Alexa Fluor 649 (BS10034, Bioworld, Dallas, TX, USA).
3.10. Biomechanical Testing
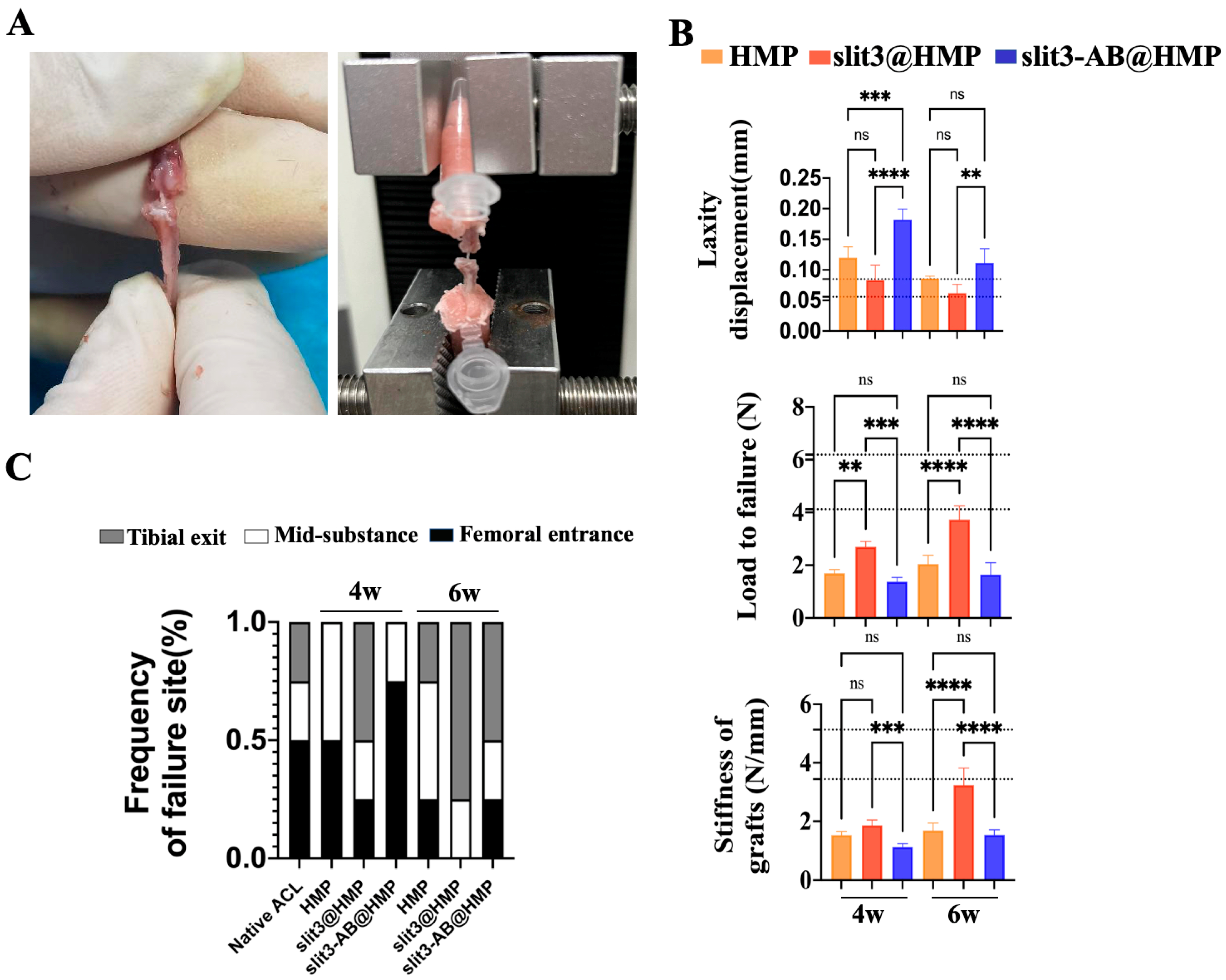
The frozen FTGTC were thawed at the room temperature for measurement of dynamic laxity and maximum load to failure. All the soft tissue but the tendon grafts were carefully removed. Then, the two ends of FTGTC were fixed by denture base resins in a 0.5 mL centrifuge tube prior to fixation in jigs. After that, the assessment of dynamic joint laxity was performed with the knee in full extension at a speed of 2 mm/min in a cyclic loading model between 0.1 N and 1 N on a mechanical testing machine (Zhiqu, ZQ-990LB, Dongguan, China). After 10 cycles, the resultant displacement was recorded. After the laxity test, the tensile test for the maximal load to failure was conducted at a speed of 2 mm/min until an abrupt drop in loading curve. The maximal load and the model of failure were recorded. The stiffness was calculated at linear region of the load-displacement curve.
3.11. Gait Analysis
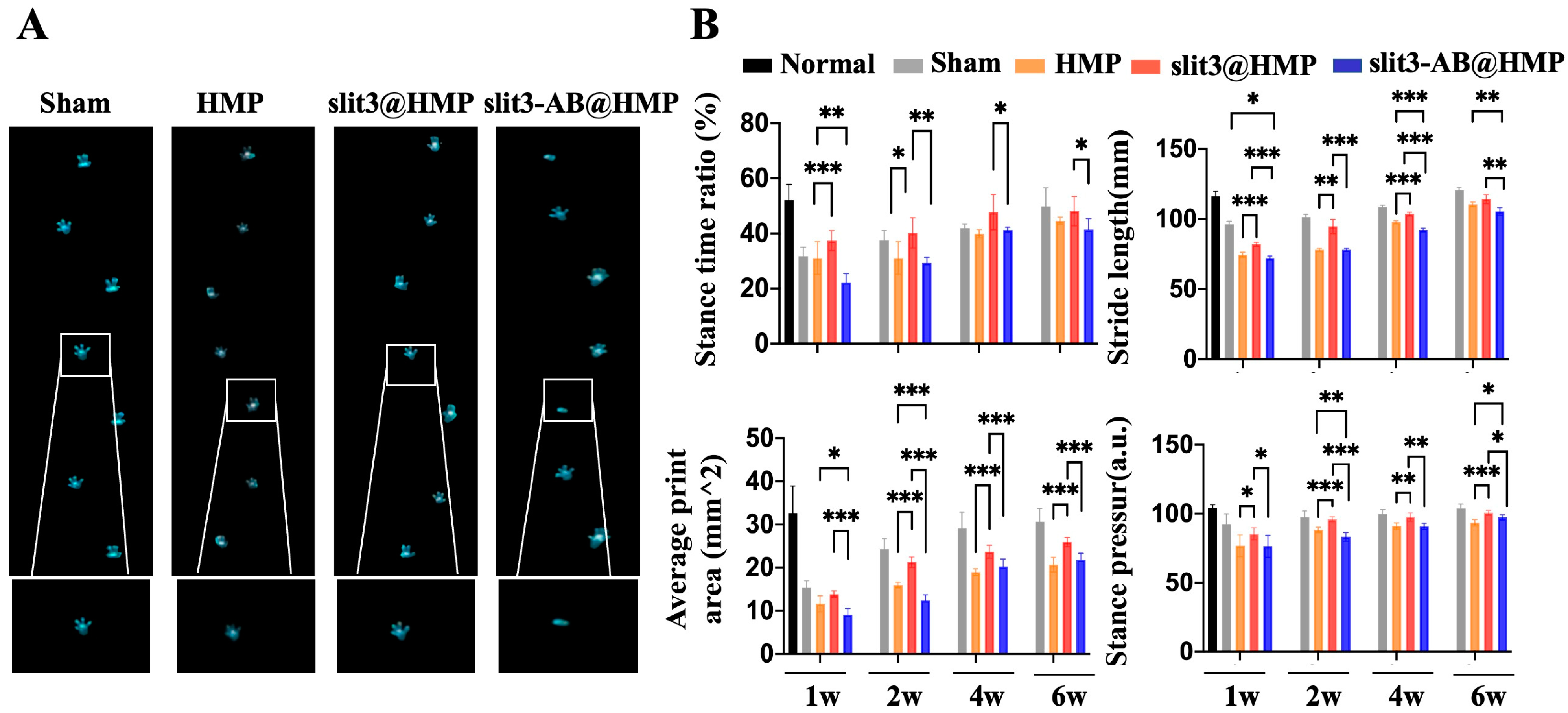
The gait performance of mice in the sham group, the HMP-treated group, the slit3@HMP-treated group, and the slit3-AB@HMP-treated group at 1, 2, 4, and 6 weeks post-surgery were analyzed. The gait behavior of the mice without surgery was also recorded for comparison. The mice were placed on a flat glass runway (40 × 8 cm) and allowed to walk freely. After the mice were adapted to walk, a high-speed camera was used to record the animal footprints under the runway to collect data on Stance time ratio, Stride length, Average print area, and Stance pressure by the gait analysis software Runway Scan (CleverSys, Reston, VA, USA).
3.12. Statistical Analysis
The data are expressed as mean ± standard deviation (mean ± SD). SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for data analysis, and Prism Version 8 (GraphPad Software) was applied for image drawing. The independent t-test was used for comparison of data between two groups, and one-way analysis of variance (ANOVA) with Bonferroni post hoc test was used for multiple comparisons in over two groups. p < 0.05 was considered statistically significant and the statistical significance was declared as (*) at p < 0.05, (**) at p < 0.01, (***) at p < 0.001, and (****) at p < 0.0001. Animal surgeries and data analysis were blindly performed by different investigators.
4. Discussion
This is the first study to investigate the effects of slit3 treatment on tendon–bone healing through modulation of type H vessel formation at the TBI in mice models with ACL reconstruction. Increased CD31hiEmcnhi endothelium was observed at the TBI after slit3 treatment while the opposite result was detected when the slit3-AB was applied. Concomitantly, the slit3 treatment favored the bony ingrowth towards the TBI, contributing to improved gait performance and load to failure with less laxity displacement. The results indicated that the local injection of slit3 may be a promising approach to accelerate and enhance the graft osteointegration into bone tunnels in patients with ACL reconstruction.
The TBI structure is highly complex and heterogeneous, making it challenging for the graft osteointegration into bone tunnels. Osteogenesis is strictly connected to angiogenesis and jointly contributes to new bone formation, so it is critical to understand the crosstalk between endothelium and osteoprogenitors. In addition to promoted osseous growth into the TBI, less fibrous scar tissue is expected for satisfactory graft healing. Therefore, some typical angiogenic factors, such as VEGF and PDGF-BB, may not be recommended for modulation of angiogenesis at the TBI due to their potential involvement in pro-inflammatory responses [
23]. Most recently, osteoblast- and osteoclast-derived slit3 has been found to be a novel angiogenic factor favoring type H vessel formation via activation of slit3/Roundabout guidance receptor 1 (Robo1)-dependent signaling in the endothelium progenitor cells [
13]. More importantly, the enhancement of type H vessel formation facilitates the osteogenic differentiation of surrounding enriched osteoprogenitors. Simultaneously, the slit3 secreted by the increased osteoblasts further favors type H vessel formation, thereby contributing to the onset of coupling effects between angiogenesis and osteogenesis.
Generally, type H vessels have been identified in specific locations, mainly in metaphysis near the growth plate and both the periosteum and endosteum of the diaphysis [
24]. In terms of ACL reconstruction, the pre-drilled bone tunnels through the metaphysis around the growth plate may support type H vessel formation at the TBI. Consistent to our hypothesis, there was type H vessel formation at the TBI in the HMP group at 2 weeks after surgery. In this study, as a result of the short half-life in the physiological environment of slit3 [
25], we considered to deliver slit3 through in situ injection of the slit3-loaded HMPs, aiming to improve the structural stability of slit3 for extended release. As supported by the release behavior in
Figure 2C, the slit3 loaded in HMPs exhibited much better stability relative to the slit3 alone in the simulated solution. Encouragingly, the slit3 treatment remarkably increased the type H vessel formation while the slit3-AB decreased the CD31
hiEmcn
hi signal. Apparently, the modulation of type H vessel formation at the TBI caused changes in knee joint functions. The histological analysis indicated that the promoted type H vessel at the TBI favored bony ingrowth towards the tendon graft. Importantly, the promoted angiogenesis did not induce more fibrous scar tissue formation. The enhanced osseous ingrowth at the TBI was also accompanied with increased peri-tunnel bone mass and decreased tunnel size, indicating that the local injection of slit3 affected bone remodeling at both the TBI and the peri-tunnel tissue. The aim of ACL reconstruction is to restore the pre-injury activity level as much as possible in an accelerated pattern. The gait analysis was then recorded and compared. Consistent to the histological and radiographic findings, the slit3 treatment significantly improved the gait performance of mice relative to the HMP group. Although a series of cytokines exhibit angiogenesis or osteogenesis effects, their in situ injection may still not be recommended in ACL reconstruction due to their pro-inflammatory properties (e.g., VEGF, PDGF-BB, and calcitonin gene-related peptide, etc.) [
26], which would cause pain, impairing gait performance. Therefore, slit3 may be considered as a potential angiogenic factor suitable for in situ injection into bone tunnels without causing extra pain and inflammatory responses. As expected, increased bony ingrowth into the TBI contributed to improved biomechanical performance. Although only 6 weeks were selected to monitor the graft healing in mice, the biomechanical parameters of the slit3-treated mice, including knee joint laxity displacement, the maximum load to failure, and the graft stiffness, showed values very close to the reference range in the normal mice.
There are still some limitations in this study. First, we did not monitor the in vivo release behavior and tissue distribution of slit3. In the following study, we will label slit3 with a fluorescent probe for real-time imaging. Second, a murine model was used and the findings may not be directly applicable to clinical practice. A beagle model will be considered to test the translational potential of in situ injection of slit3 loaded hydrogels in our future work. In addition, an extended period will be designed to assess the complete healing process in the beagle dog model after ACL reconstruction to address the concern with the short observation time. Third, sex is an important factor influencing tendon or bone healing [
27,
28], so we just considered a single-sex group in this study to investigate if the type H vessel affects the graft osteointegration towards the bone. It will be interesting to conduct the experiments in female mice to verify the findings observed in the male mice in the future. Finally, a delivery system targeting CD31
hiEmcn
hi vascular endothelial cells for in situ release of slit3 may be a better strategy for the modulation of type H vessel formation at the TBI.