Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating
Abstract
:1. Introduction
2. Results and Discussion
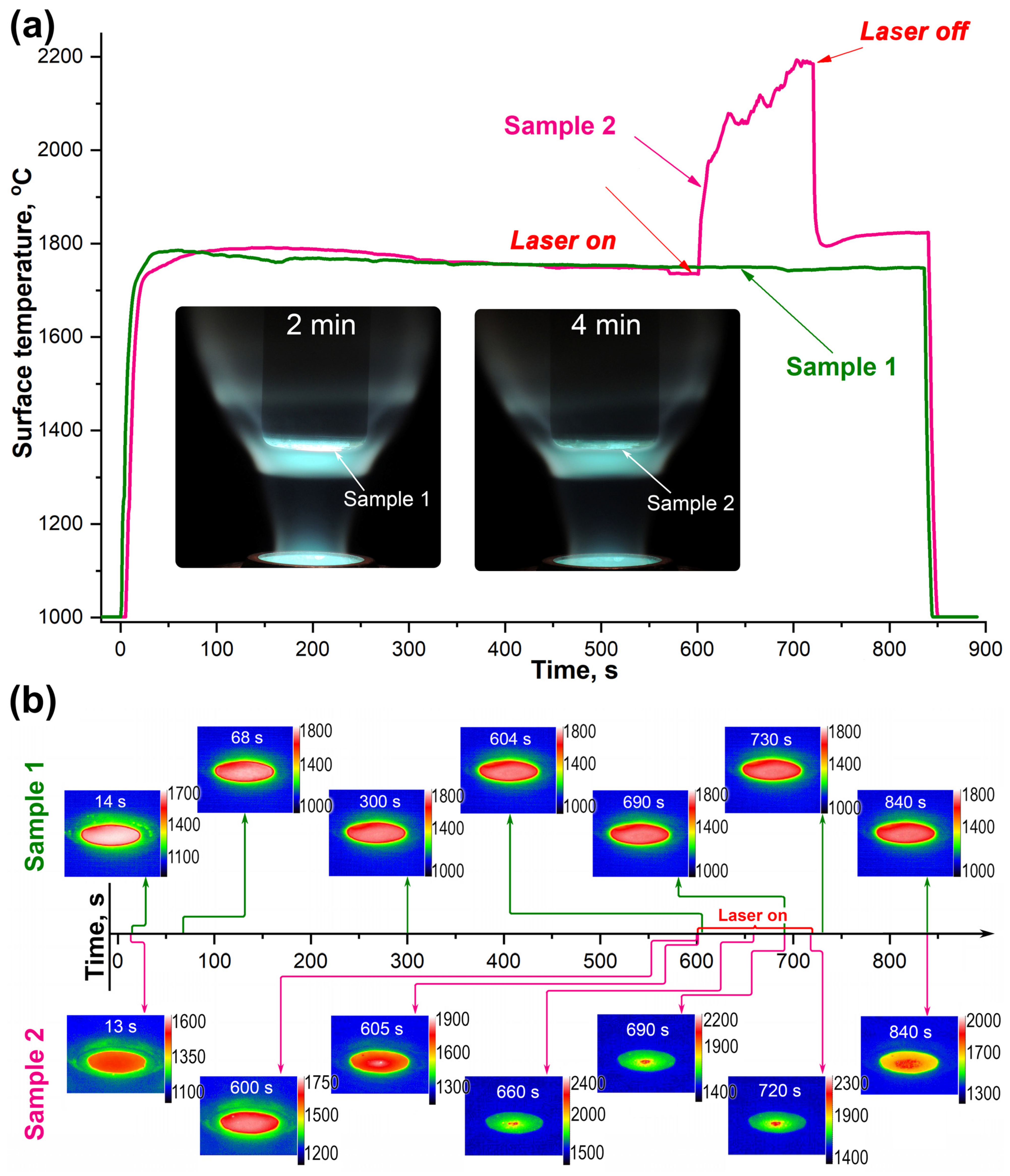
2.1. Thermochemical Effects of Supersonic Carbon Dioxide Flow and Combined Heating with Laser Irradiation on the Surface of HfB2-SiC-CG Samples
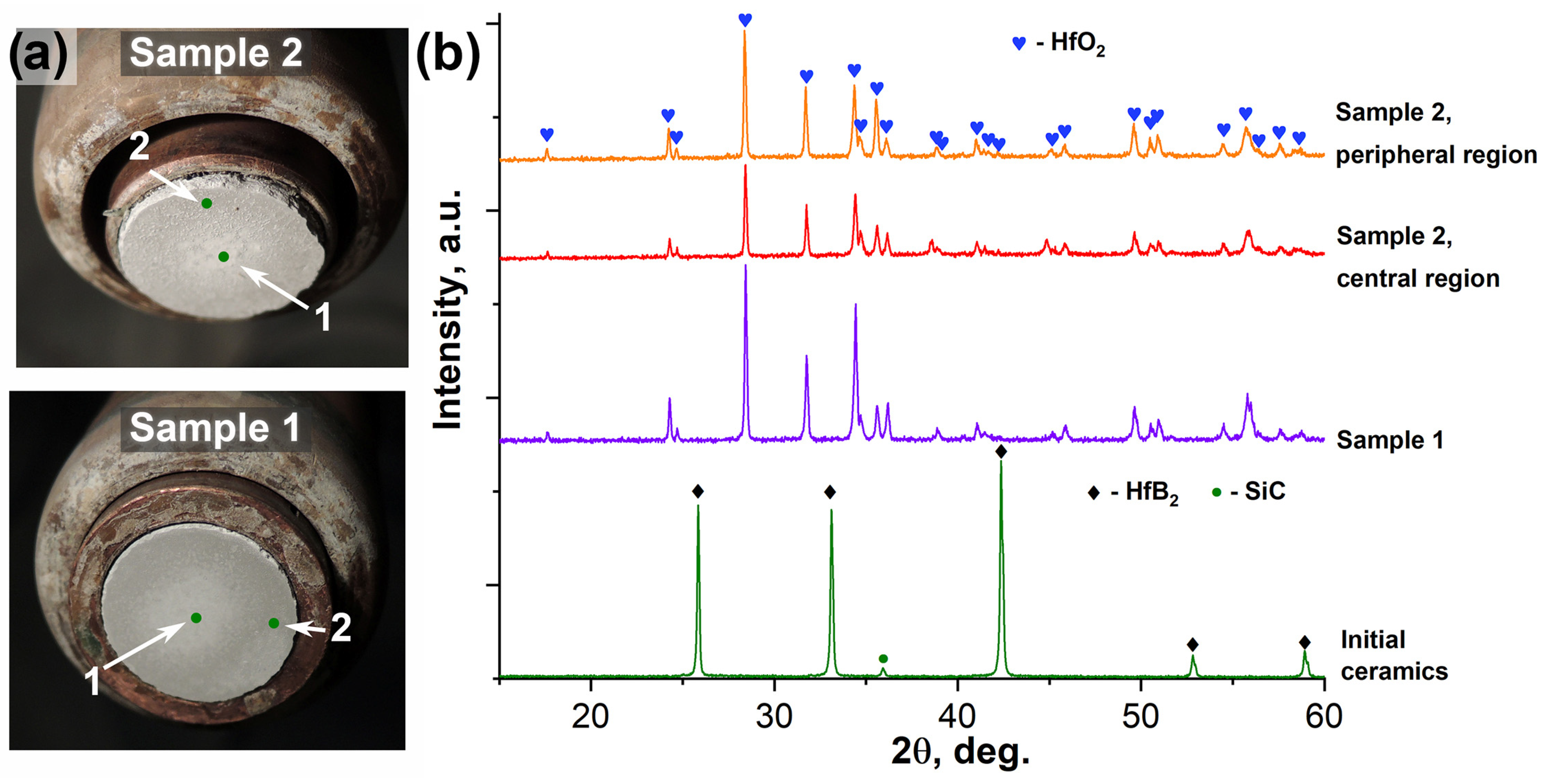
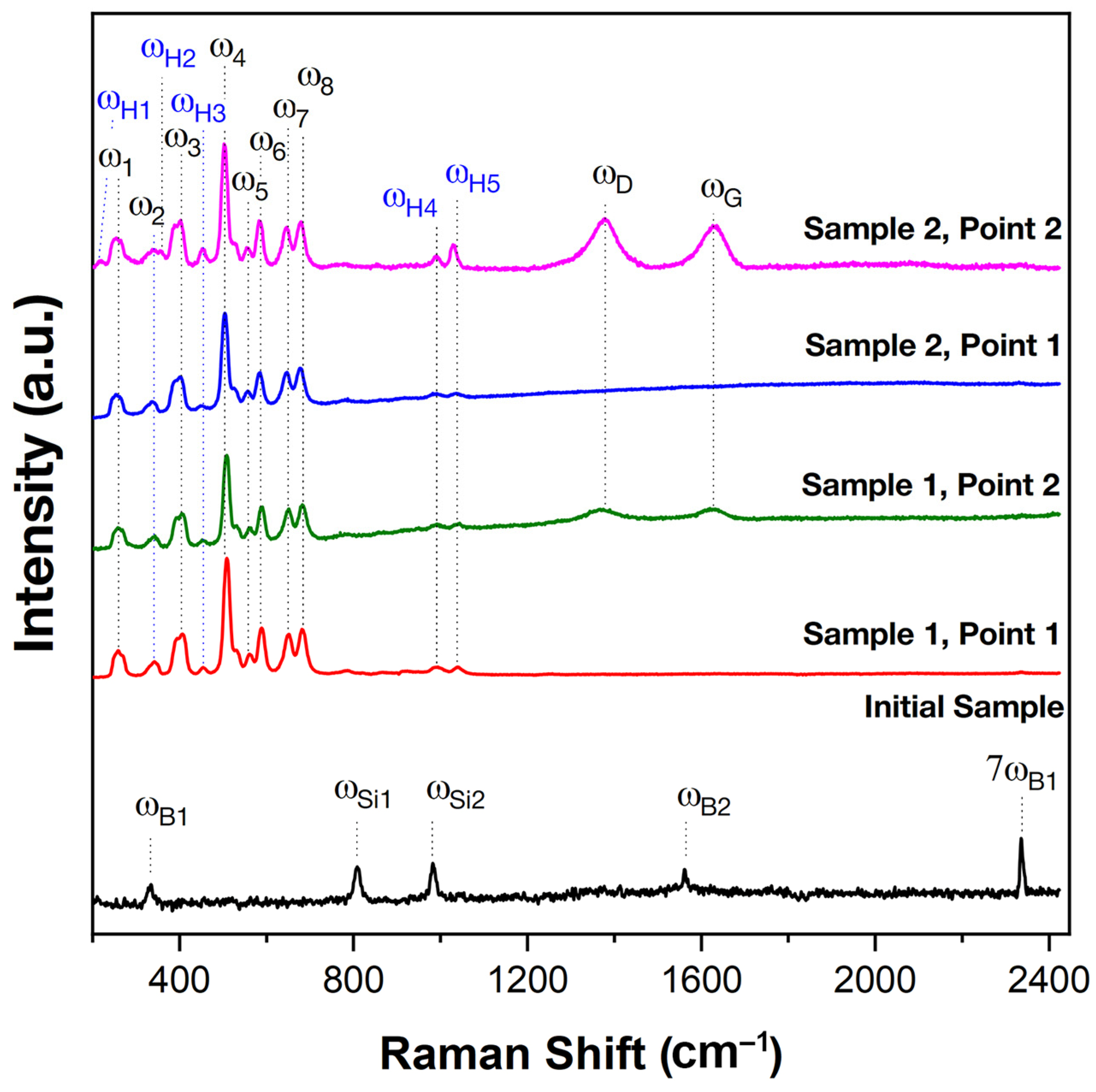
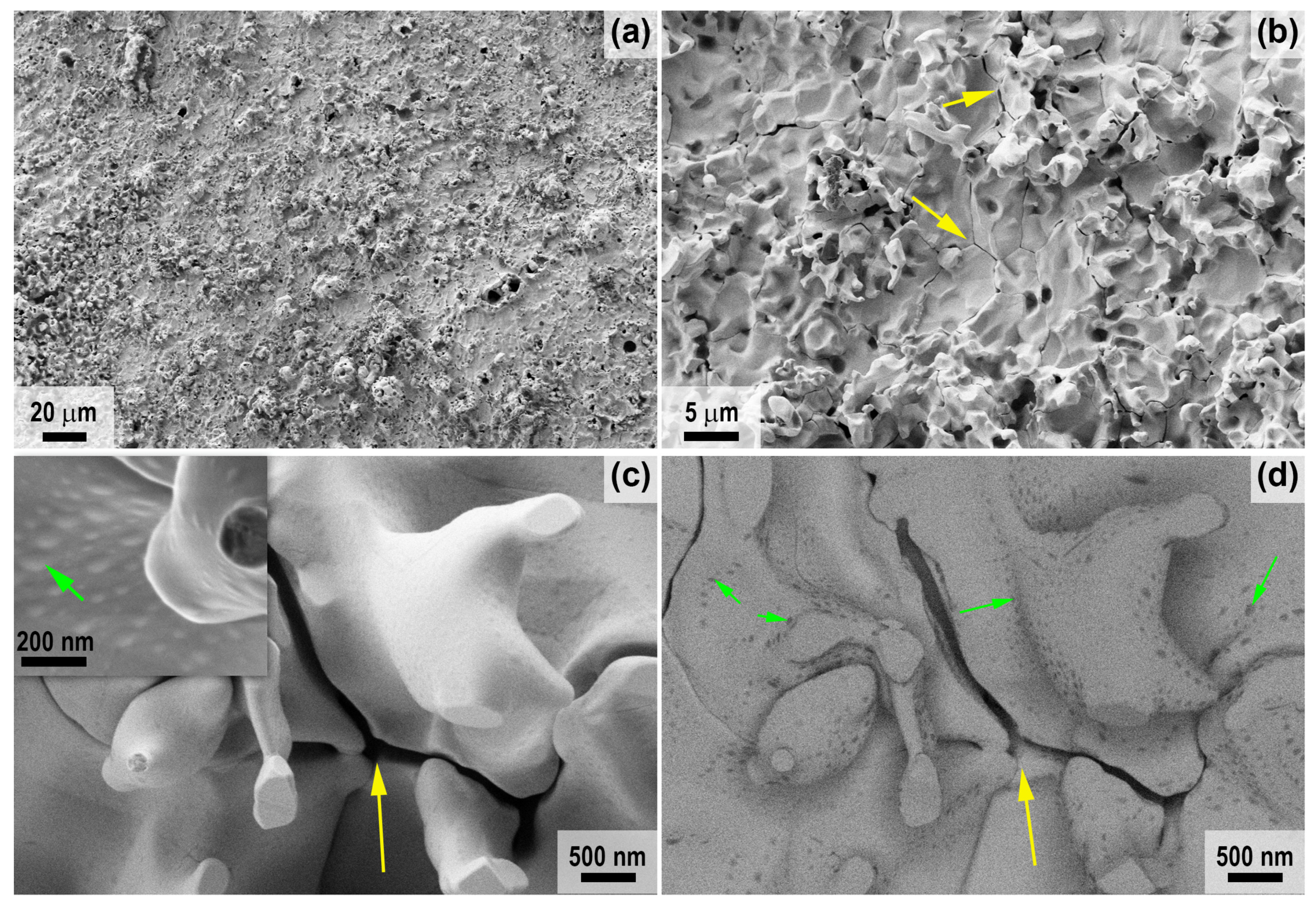
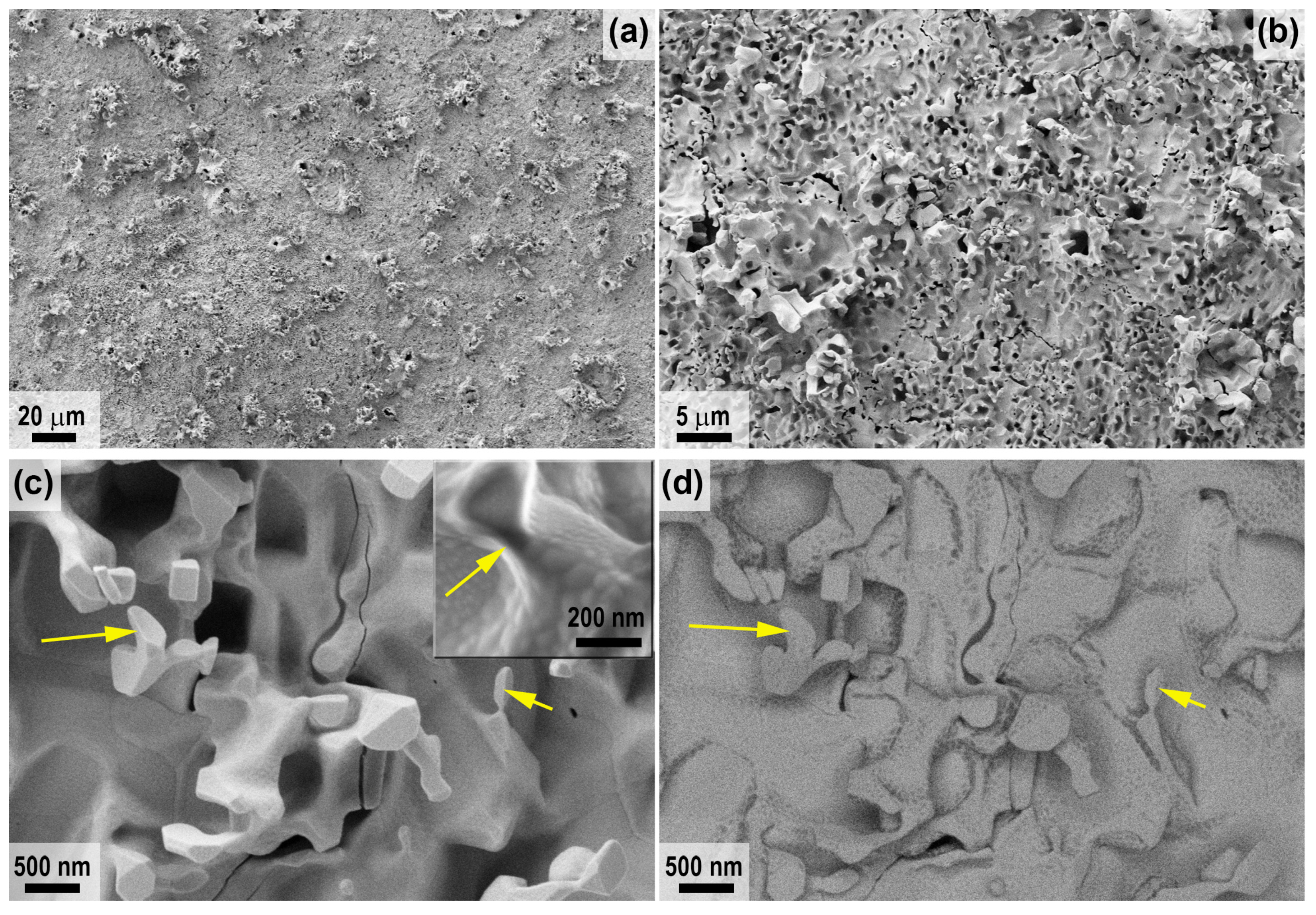
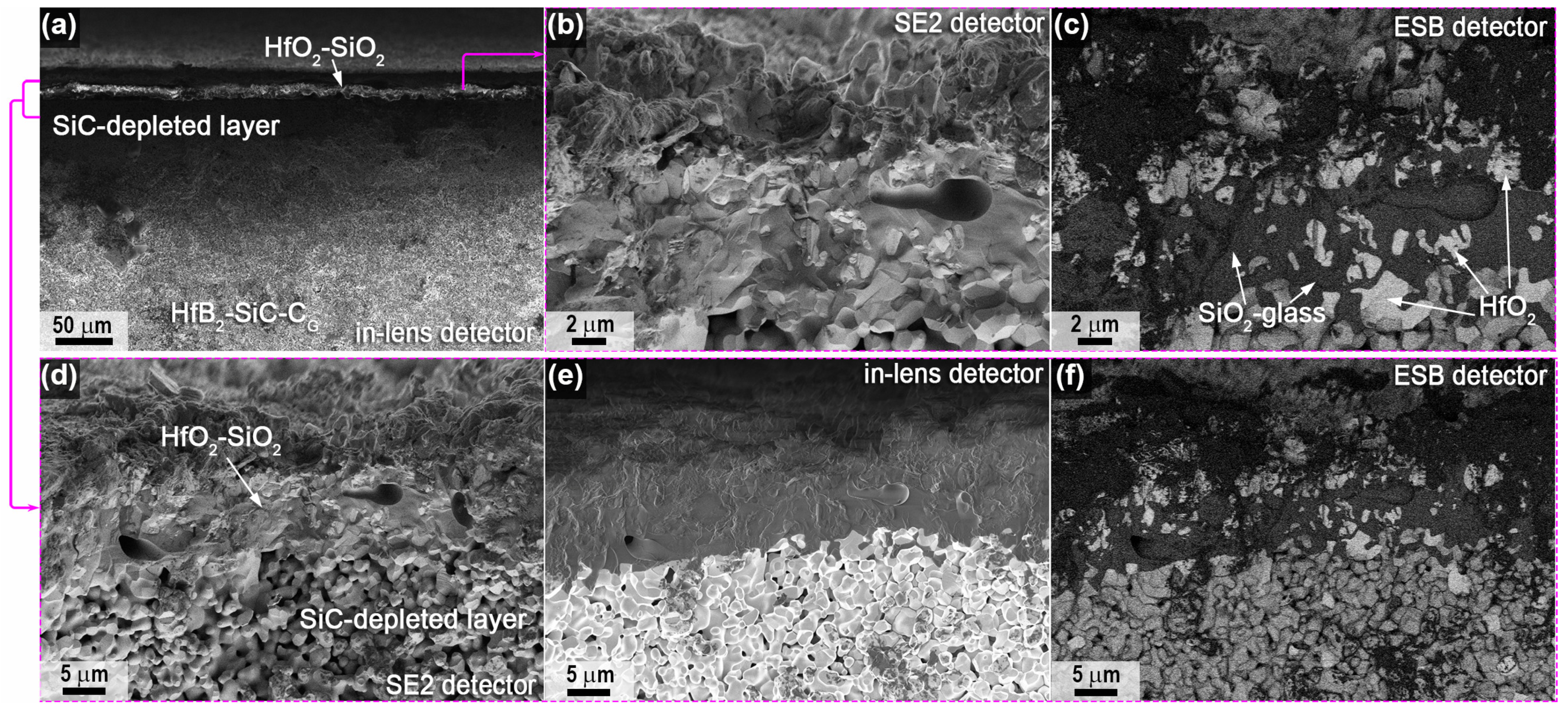
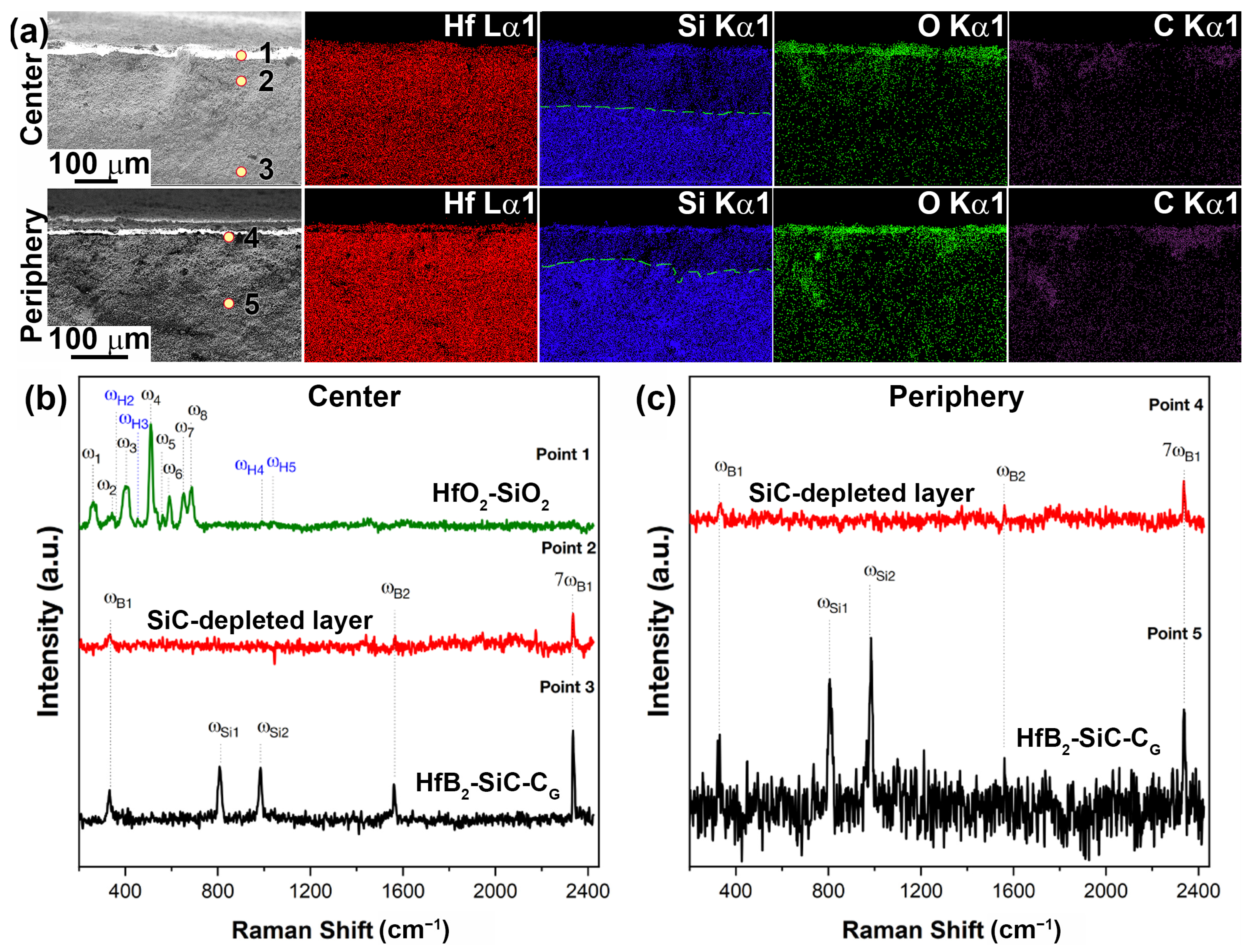
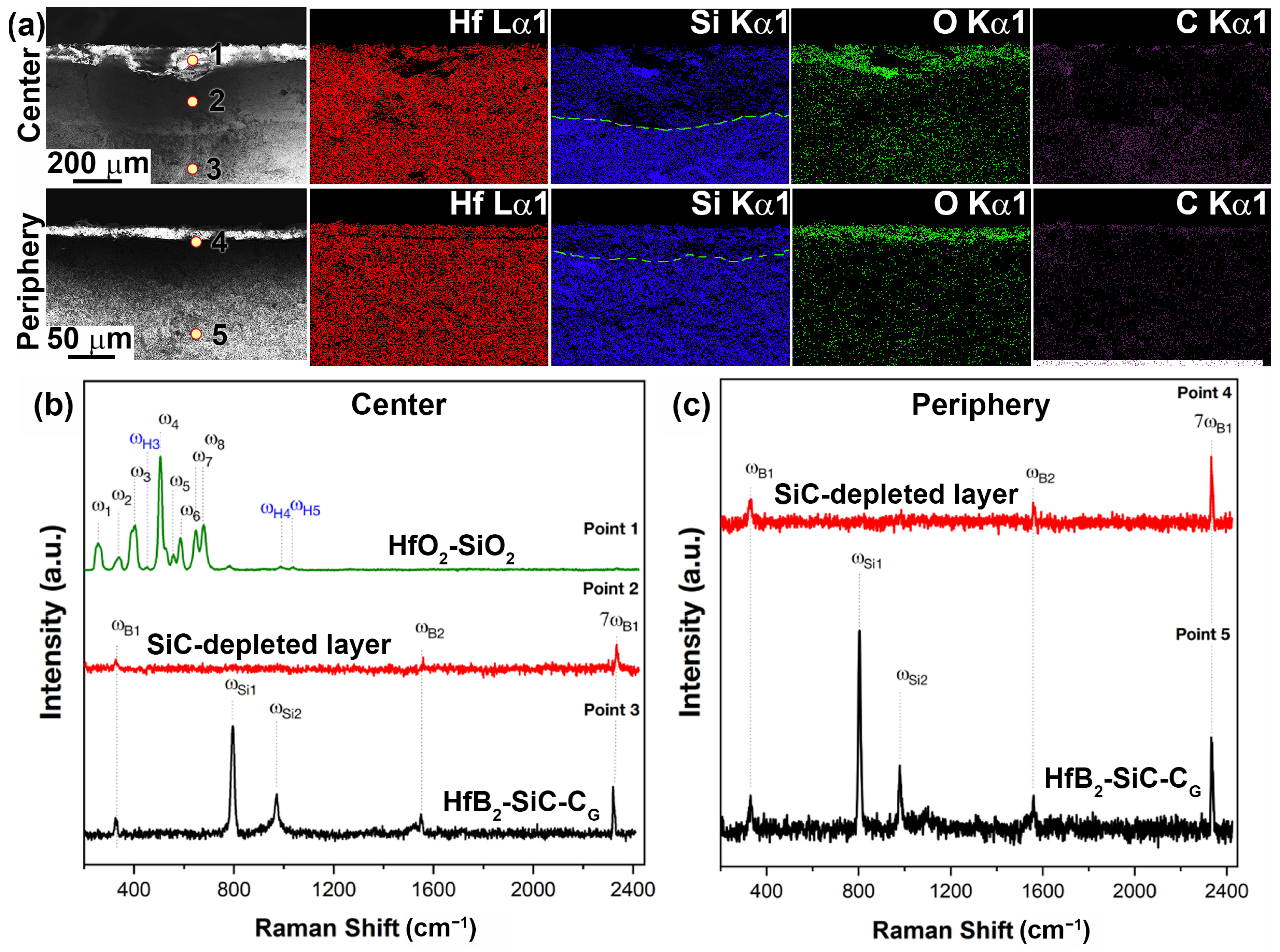
2.2. Characteristics of Oxidation of HfB2-SiC-CG Samples by Supersonic Flow of Carbon Dioxide and Additional Exposure to Laser Radiation
- (1)
- External oxide layer based on HfO2 with admixture of HfSiO4 and, probably, silicate melt;
- (2)
- SiC-depleted layer based on HfB2 with B4C admixture, which transitions to the unoxidized HfB2-SiC-CG material.
3. Materials and Methods
3.1. Synthesis and Sample Preparation
3.2. Instrumentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nisar, A.; Hassan, R.; Agarwal, A.; Balani, K. Ultra-High Temperature Ceramics: Aspiration to Overcome Challenges in Thermal Protection Systems. Ceram. Int. 2022, 48, 8852–8881. [Google Scholar] [CrossRef]
- Savino, R.; Criscuolo, L.; Di Martino, G.D.; Mungiguerra, S. Aero-Thermo-Chemical Characterization of Ultra-High-Temperature Ceramics for Aerospace Applications. J. Eur. Ceram. Soc. 2018, 38, 2937–2953. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Sevast’yanov, D.V.; Simonenko, N.P.; Sevast’yanov, V.G.; Kuznetsov, N.T. Promising Ultra-High-Temperature Ceramic Materials for Aerospace Applications. Russ. J. Inorg. Chem. 2013, 58, 1669–1693. [Google Scholar] [CrossRef]
- Aguirre, T.G.; Lamm, B.W.; Cramer, C.L.; Mitchell, D.J. Zirconium-Diboride Silicon-Carbide Composites: A Review. Ceram. Int. 2022, 48, 7344–7361. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.-X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in Ultra-High Temperature Ceramics, Composites, and Coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Liu, G.; Yan, C.; Jin, H. Colloidal Processing of Complex-Shaped ZrB2-Based Ultra-High-Temperature Ceramics: Progress and Prospects. Materials 2022, 15, 2886. [Google Scholar] [CrossRef]
- Sonber, J.K.; Murthy, T.S.R.C.; Majumdar, S.; Kain, V. Processing of ZrB2- and HfB2-Based Ultra-High Temperature Ceramic Materials: A Review. Mater. Perform. Charact. 2021, 10, 20200133. [Google Scholar] [CrossRef]
- Dan, A.; Basu, B. Understanding Spectrally Selective Properties of Solar Absorbers. In Energy Saving Coating Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–151. [Google Scholar]
- Squire, T.H.; Marschall, J. Material Property Requirements for Analysis and Design of UHTC Components in Hypersonic Applications. J. Eur. Ceram. Soc. 2010, 30, 2239–2251. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Oxidation of Ultra-High Temperature Transition Metal Diboride Ceramics. Int. Mater. Rev. 2012, 57, 61–72. [Google Scholar] [CrossRef]
- He, R.J.; Zhang, X.H.; Hu, P. Ablation Property of ZrB2-SiC Composite Sharp Leading Edges with Varying Radiuses of Curvature under Oxy-Acetylene Torch. Key Eng. Mater. 2012, 512–515, 710–714. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Papynov, E.K.; Shichalin, O.O.; Kolesnikov, A.F.; Avramenko, V.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Study of the Thermal Behavior of Wedge-Shaped Samples of HfB2–45 Vol % SiC Ultra-High-Temperature Composite in a High-Enthalpy Air Flow. Russ. J. Inorg. Chem. 2018, 63, 421–432. [Google Scholar] [CrossRef]
- Monteverde, F.; Savino, R. ZrB2-SiC Sharp Leading Edges in High Enthalpy Supersonic Flows. J. Am. Ceram. Soc. 2012, 95, 2282–2289. [Google Scholar] [CrossRef]
- Jin, X.; He, R.; Zhang, X.; Hu, P. Ablation Behavior of ZrB2–SiC Sharp Leading Edges. J. Alloys Compd. 2013, 566, 125–130. [Google Scholar] [CrossRef]
- Sciti, D.; Savino, R.; Silvestroni, L. Aerothermal Behaviour of a SiC Fibre-Reinforced ZrB2 Sharp Component in Supersonic Regime. J. Eur. Ceram. Soc. 2012, 32, 1837–1845. [Google Scholar] [CrossRef]
- Bianco, G.; Nisar, A.; Zhang, C.; Boesl, B.; Agarwal, A. Predicting Oxidation Damage of Ultra High-Temperature Carbide Ceramics in Extreme Environments Using Machine Learning. Ceram. Int. 2023, 49, 19974–19981. [Google Scholar] [CrossRef]
- Samsonov, G.V.; Bolgar, A.S.; Guseva, E.A.; Klochkov, L.A.; Kovenskaya, B.A.; Serebryakova, T.I.; Timofeeva, I.I.; Turchanin, A.G.; Fesenko, V.V. Thermophysical Properties of Transition Metal Carbides and Diborides. High Temp.-High Press. 1973, 5, 29–33. [Google Scholar]
- Kinoshita, H.; Otani, S.; Kamiyama, S.; Amano, H.; Akasaki, I.; Suda, J.; Matsunami, H. Zirconium Diboride (0001) as an Electrically Conductive Lattice-Matched Substrate for Gallium Nitride. Jpn. J. Appl. Phys. 2001, 40, L1280. [Google Scholar] [CrossRef]
- Zhang, L.; Pejaković, D.A.; Marschall, J.; Gasch, M. Thermal and Electrical Transport Properties of Spark Plasma-Sintered HfB2 and ZrB2 Ceramics. J. Am. Ceram. Soc. 2011, 94, 2562–2570. [Google Scholar] [CrossRef]
- Guria, J.F.; Bansal, A.; Kumar, V. Effect of Additives on the Thermal Conductivity of Zirconium Diboride Based Composites—A Review. J. Eur. Ceram. Soc. 2021, 41, 1–23. [Google Scholar] [CrossRef]
- Opeka, M.M.; Talmy, I.G.; Wuchina, E.J.; Zaykoski, J.A.; Causey, S.J. Mechanical, Thermal, and Oxidation Properties of Refractory Hafnium and Zirconium Compounds. J. Eur. Ceram. Soc. 1999, 19, 2405–2414. [Google Scholar] [CrossRef]
- Weng, L.; Han, W.; Li, X.; Hong, C. High Temperature Thermo-Physical Properties and Thermal Shock Behavior of Metal–Diborides-Based Composites. Int. J. Refract. Met. Hard Mater. 2010, 28, 459–465. [Google Scholar] [CrossRef]
- Povolny, S.J.; Seidel, G.D.; Tallon, C. Numerical Investigation of Thermomechanical Response of Multiscale Porous Ultra-High Temperature Ceramics. Ceram. Int. 2022, 48, 11502–11517. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Astapov, A.N.; Pogozhev, Y.S.; Rupasov, S.I.; Shvyndina, N.V.; Klechkovskaya, V.V.; Levashov, E.A.; Timofeev, I.A.; Timofeev, A.N. Oxidation of HfB2–SiC Ceramics under Static and Dynamic Conditions. J. Eur. Ceram. Soc. 2021, 41, 34–47. [Google Scholar] [CrossRef]
- Sevastyanov, V.G.; Simonenko, E.P.; Gordeev, A.N.; Simonenko, N.P.; Kolesnikov, A.F.; Papynov, E.K.; Shichalin, O.O.; Avramenko, V.A.; Kuznetsov, N.T. Behavior of a Sample of the Ceramic Material HfB2-SiC (45 Vol %) in the Flow of Dissociated Air and the Analysis of the Emission Spectrum of the Boundary Layer above Its Surface. Russ. J. Inorg. Chem. 2015, 60, 1360–1373. [Google Scholar] [CrossRef]
- Li, N.; Hu, P.; Zhang, X.; Liu, Y.; Han, W. Effects of Oxygen Partial Pressure and Atomic Oxygen on the Microstructure of Oxide Scale of ZrB2–SiC Composites at 1500 °C. Corros. Sci. 2013, 73, 44–53. [Google Scholar] [CrossRef]
- Scatteia, L.; Borrelli, R.; Cosentino, G.; Beche, E.; Sans, J.-L.; Balat-Pichelin, M. Catalytic and Radiative Behaviors of ZrB2-SiC Ultrahigh Temperature Ceramic Composites. J. Spacecr. Rockets 2006, 43, 1004–1012. [Google Scholar] [CrossRef]
- Marschall, J.; Pejakovic, D.; Fahrenholtz, W.G.; Hilmas, G.E.; Panerai, F.; Chazot, O. Temperature Jump Phenomenon during Plasmatron Testing of ZrB2-SiC Ultrahigh-Temperature Ceramics. J. Thermophys. Heat Transf. 2012, 26, 559–572. [Google Scholar] [CrossRef]
- Nisar, A.; Zhang, C.; Boesl, B.; Agarwal, A. A Perspective on Challenges and Opportunities in Developing High Entropy-Ultra High Temperature Ceramics. Ceram. Int. 2020, 46, 25845–25853. [Google Scholar] [CrossRef]
- Bianco, G.; Nisar, A.; Zhang, C.; Boesl, B.; Agarwal, A. A Critical Analysis of the Parameters Affecting the Oxidation Behavior of Ultra-high-temperature Diboride Ceramics. J. Am. Ceram. Soc. 2022, 105, 1939–1953. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, C.; Jin, X.; Li, P.; Wang, Z.; Fan, X. Oxidation Behaviors of ZrB2–SiC Ceramics with Different Porosity. Adv. Eng. Mater. 2023, 25, 2201313. [Google Scholar] [CrossRef]
- Sengupta, P.; Basu, S.; Manna, I. Effect of TiC Reinforcement on Densification, Structural Evolution and High-Temperature Oxidation Behaviour of ZrB2-20 Vol Pct SiC Composite. Metall. Mater. Trans. A 2023, 54, 1252–1270. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Kolesnikov, A.F.; Papynov, E.K.; Shichalin, O.O.; Tal’skikh, K.Y.; Gridasova, E.A.; Avramenko, V.A.; Sevastyanov, V.G.; et al. Impact of a Supersonic Dissociated Air Flow on the Surface of HfB2–30 Vol % SiC UHTC Produced by the Sol–Gel Method. Russ. J. Inorg. Chem. 2018, 63, 1484–1493. [Google Scholar] [CrossRef]
- Shahedi Asl, M.; Zamharir, M.J.; Ahmadi, Z.; Parvizi, S. Effects of Nano-Graphite Content on the Characteristics of Spark Plasma Sintered ZrB2–SiC Composites. Mater. Sci. Eng. A 2018, 716, 99–106. [Google Scholar] [CrossRef]
- Shahedi Asl, M.; Ghassemi Kakroudi, M. Characterization of Hot-Pressed Graphene Reinforced ZrB2–SiC Composite. Mater. Sci. Eng. A 2015, 625, 385–392. [Google Scholar] [CrossRef]
- An, Y.; Xu, X.; Gui, K. Effect of SiC Whiskers and Graphene Nanosheets on the Mechanical Properties of ZrB2-SiCw-Graphene Ceramic Composites. Ceram. Int. 2016, 42, 14066–14070. [Google Scholar] [CrossRef]
- Nisar, A.; Balani, K. Phase and Microstructural Correlation of Spark Plasma Sintered HfB2-ZrB2 Based Ultra-High Temperature Ceramic Composites. Coatings 2017, 7, 110. [Google Scholar] [CrossRef]
- Gui, K.; Hu, P.; Hong, W.; Zhang, X.; Dong, S. Microstructure, Mechanical Properties and Thermal Shock Resistance of ZrB2-SiC-C f Composite with Inhibited Degradation of Carbon Fibers. J. Alloys Compd. 2017, 706, 16–23. [Google Scholar] [CrossRef]
- Arai, Y.; Inoue, R.; Goto, K.; Kogo, Y. Carbon Fiber Reinforced Ultra-High Temperature Ceramic Matrix Composites: A Review. Ceram. Int. 2019, 45, 14481–14489. [Google Scholar] [CrossRef]
- Chen, Y. The Effects of Sintering Temperature on the Mechanical Properties and Toughening Mechanisms of Carbon Fibre-Reinforced HfB2-SiC Composites. Mater. Res. Express 2021, 8, 055602. [Google Scholar] [CrossRef]
- Dubey, S.; Ariharan, S.; Nisar, A.; Saini, S.; Jana, S.S.; Wangaskar, B.; Das, A.; Khandekar, S.; Maiti, T.; Omar, S.; et al. Domination of Phononic Scattering in Solid Solutioning and Interfaces of HfB2–ZrB2–SiC-Carbon Nanotube Based Ultra High Temperature Composites. Scr. Mater. 2022, 218, 114776. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Sakharov, V.I.; Lysenkov, A.S.; Nagornov, I.A.; Kuznetsov, N.T. Effect of 2 Vol % Graphene Additive on Heat Transfer of Ceramic Material in Underexpanded Jets of Dissociated Air. Russ. J. Inorg. Chem. 2022, 67, 2050–2061. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Modification of HfB2–30% SiC UHTC with Graphene (1 Vol %) and Its Influence on the Behavior in a Supersonic Air Jet. Russ. J. Inorg. Chem. 2021, 66, 1405–1415. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Simonenko, T.L.; Gubin, S.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Oxidation of Graphene-Modified HfB2-SiC Ceramics by Supersonic Dissociated Air Flow. J. Eur. Ceram. Soc. 2022, 42, 30–42. [Google Scholar] [CrossRef]
- Anderson, J.D.; Lewis, M.J.; Kothari, A.P.; Corda, S. Hypersonic Waveriders for Planetary Atmospheres. J. Spacecr. Rockets 1991, 28, 401–410. [Google Scholar] [CrossRef]
- Rodi, P.E. Evaluation of the Capsule/Waverider Concept for Mars Entry, Descent, and Landing. In Proceedings of the AIAA Aviation 2021 Forum, Online, 2–6 August 2021; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2021. [Google Scholar]
- Monteverde, F.; Savino, R. Stability of Ultra-High-Temperature ZrB2–SiC Ceramics under Simulated Atmospheric Re-Entry Conditions. J. Eur. Ceram. Soc. 2007, 27, 4797–4805. [Google Scholar] [CrossRef]
- Savino, R.; De Stefano Fumo, M.; Silvestroni, L.; Sciti, D. Arc-Jet Testing on HfB2 and HfC-Based Ultra-High Temperature Ceramic Materials. J. Eur. Ceram. Soc. 2008, 28, 1899–1907. [Google Scholar] [CrossRef]
- Kolesnikov, A.F.; Kuznetsov, N.T.; Murav’eva, T.I.; Nagornov, I.A.; Sakharov, V.I.; Sevastyanov, V.G.; Simonenko, E.P.; Simonenko, N.P.; Chaplygin, A.V.; Shcherbakova, O.O. Investigation of Heat Transfer to HfB2-SiC-Based Ceramics in Underexpanded Dissociated-Nitrogen Jets and Analysis of the Surface. Fluid Dyn. 2022, 57, 513–523. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; Kuznetsov, N.T. Investigation of the Effect of Supersonic Flow of Dissociated Nitrogen on ZrB2–HfB2–SiC Ceramics Doped with 10 Vol.% Carbon Nanotubes. Materials 2022, 15, 8507. [Google Scholar] [CrossRef]
- Alosime, E.M.; Alsuhybani, M.S.; Almeataq, M.S. The Oxidation Behavior of ZrB2-SiC Ceramic Composites Fabricated by Plasma Spray Process. Materials 2021, 14, 392. [Google Scholar] [CrossRef]
- Moroz, V.I. Chemical Composition of the Atmosphere of Mars. Adv. Sp. Res. 1998, 22, 449–457. [Google Scholar] [CrossRef]
- Oyama, V.I.; Carle, G.C.; Woeller, F.; Pollack, J.B.; Reynolds, R.T.; Craig, R.A. Pioneer Venus Gas Chromatography of the Lower Atmosphere of Venus. J. Geophys. Res. 1980, 85, 7891. [Google Scholar] [CrossRef]
- Marcq, E.; Mills, F.P.; Parkinson, C.D.; Vandaele, A.C. Composition and Chemistry of the Neutral Atmosphere of Venus. Space Sci. Rev. 2018, 214, 10. [Google Scholar] [CrossRef]
- Chaplygin, A.; Kotov, M.; Yakimov, M.; Lukomskii, I.; Galkin, S.; Kolesnikov, A.; Shemyakin, A.; Solovyov, N. Combined Surface Heating by Laser Beam and Subsonic Nitrogen Plasma Jet. Fluids 2022, 8, 11. [Google Scholar] [CrossRef]
- Cushman, G.; Alunni, A.; Balboni, J.; Zell, P.; Hartman, J.; Empey, D. The Laser Enhanced Arc-Jet Facility (LEAF-Lite): Simulating Convective and Radiative Heating with Arc-Jets and Multiple 50-KW CW Lasers. In Proceedings of the 2018 Joint Thermophysics and Heat Transfer Conference, Atlanta, GA, USA, 25–29 June 2018; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2018. [Google Scholar]
- Gokcen, T.; Alunni, A. CFD Simulations of the IHF Arc-Jet Flow: 9-Inch Nozzle, Flow Surveys, LEAF Wedge Calibration Data. In Proceedings of the AIAA Aviation 2019 Forum, Dallas, TX, USA, 17–21 June 2019; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2019. [Google Scholar]
- Momozawa, A.; Yokote, N.; Terutsuki, D.; Komurasaki, K. Dynamic Oxidation of SiC with Arc-Heated Plasma Wind Tunnel and Laser Heating. Vacuum 2021, 185, 109899. [Google Scholar] [CrossRef]
- Mungiguerra, S.; Cecere, A.; Savino, R.; Saraga, F.; Monteverde, F.; Sciti, D. Improved Aero-Thermal Resistance Capabilities of ZrB2-Based Ceramics in Hypersonic Environment for Increasing SiC Content. Corros. Sci. 2021, 178, 109067. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Rapp, R.A.; Opeka, M.; Cinibulk, M.K. Modeling Oxidation Kinetics of SiC-Containing Refractory Diborides. J. Am. Ceram. Soc. 2012, 95, 338–349. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Gordeev, A.N.; Simonenko, N.P.; Vasilevskii, S.A.; Kolesnikov, A.F.; Papynov, E.K.; Shichalin, O.O.; Avramenko, V.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Behavior of HfB2-SiC (10, 15, and 20 Vol %) Ceramic Materials in High-Enthalpy Air Flows. Russ. J. Inorg. Chem. 2016, 61, 1203–1218. [Google Scholar] [CrossRef]
- Shin, D.; Arróyave, R.; Liu, Z.-K. Thermodynamic Modeling of the Hf–Si–O System. Calphad 2006, 30, 375–386. [Google Scholar] [CrossRef]
- Holleck, H. Legierungsverhalten von HfB2 Mit Uran- Und Übergangsmetalldiboriden. J. Nucl. Mater. 1967, 21, 14–20. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Second Edition. Interscience Publishers, New York, New Yorkrocksalt Structure. Cryst. Struct. 1963, 1, 85–237. [Google Scholar]
- Whittle, K.R.; Lumpkin, G.R.; Ashbrook, S.E. Neutron Diffraction and MAS NMR of Cesium Tungstate Defect Pyrochlores. J. Solid State Chem. 2006, 179, 512–521. [Google Scholar] [CrossRef]
- Ruh, R.; Corfield, P.W.R. Crystal Structure of Monoclinic Hafnia and Comparison with Monoclinic Zirconia. J. Am. Ceram. Soc. 1970, 53, 126–129. [Google Scholar] [CrossRef]
- Werheit, H.; Leithe-Jasper, A.; Tanaka, T.; Rotter, H.W.; Schwetz, K.A. Some Properties of Single-Crystal Boron Carbide. J. Solid State Chem. 2004, 177, 575–579. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.; Fujita, T.; Goto, T.; Chen, M. Pressure-Induced Depolarization and Resonance in Raman Scattering of Single-Crystalline Boron Carbide. Phys. Rev. B 2010, 81, 060102. [Google Scholar] [CrossRef]
- Nakashima, S.; Harima, H. Raman Investigation of SiC Polytypes. Phys. Status Solidi 1997, 162, 39–64. [Google Scholar] [CrossRef]
- Ghosh, D.; Subhash, G.; Orlovskaya, N. Measurement of Scratch-Induced Residual Stress within SiC Grains in ZrB2–SiC Composite Using Micro-Raman Spectroscopy. Acta Mater. 2008, 56, 5345–5354. [Google Scholar] [CrossRef]
- Shafiq, M.; Subhash, G. A Novel Technique for the Determination of Surface Biaxial Stress under External Confinement Using Raman Spectroscopy. Exp. Mech. 2014, 54, 763–774. [Google Scholar] [CrossRef]
- Stadelmann, R.; Hughes, B.; Orlovskaya, N.; Grasso, S.; Reece, M.J. 2D Raman Mapping and Thermal Residual Stresses in SiC Grains of ZrB2–SiC Ceramic Composites. Ceram. Int. 2015, 41, 13630–13637. [Google Scholar] [CrossRef]
- Tkachev, S.N.; Manghnani, M.H.; Niilisk, A.; Aarik, J.; Mändar, H. Raman and Brillouin Scattering Spectroscopy Studies of Atomic Layer-Deposited ZrO2 and HfO2 Thin Films. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2434–2438. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, B.; Li, Q.; Jiang, Z.; Wang, W.; Ma, W.; Zhang, X. Elastic and Vibrational Properties of Monoclinic HfO2 from First-Principles Study. J. Phys. D Appl. Phys. 2012, 45, 125304. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, H.; Zhang, X.D.; Su, Q.; Jiang, Z.Y. The Simulated Vibrational Spectra of HfO2 Polymorphs. J. Phys. D. Appl. Phys. 2014, 47, 115502. [Google Scholar] [CrossRef]
- Manoun, B.; Downs, R.T.; Saxena, S.K. A High-Pressure Raman Spectroscopic Study of Hafnon, HfSiO4. Am. Mineral. 2006, 91, 1888–1892. [Google Scholar] [CrossRef]
- Grüneberger, A.M.; Schmidt, C.; Jahn, S.; Rhede, D.; Loges, A.; Wilke, M. Interpretation of Raman Spectra of the Zircon–Hafnon Solid Solution. Eur. J. Mineral. 2016, 28, 721–733. [Google Scholar] [CrossRef]
- Li, J.; Lenosky, T.J.; Först, C.J.; Yip, S. Thermochemical and Mechanical Stabilities of the Oxide Scale of ZrB2+SiC and Oxygen Transport Mechanisms. J. Am. Ceram. Soc. 2008, 91, 1475–1480. [Google Scholar] [CrossRef]
- Han, J.; Hu, P.; Zhang, X.; Meng, S.; Han, W. Oxidation-Resistant ZrB2–SiC Composites at 2200 °C. Compos. Sci. Technol. 2008, 68, 799–806. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Simonenko, E.P.; Simonenko, N.P.; Zavjalov, A.P.; Belov, A.A.; Portnyagin, A.S.; Gerasimenko, A.V.; et al. Spark Plasma Sintering-Reactive Synthesis of SiC and SiC–HfB2 Ceramics Based on Natural Renewable Raw Materials. Russ. J. Inorg. Chem. 2021, 66, 629–637. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Nagornov, I.A.; Gorobtsov, P.Y.; Kuznetsov, N.T. Effect of Nanocrystalline SiC Addition on Reactive SPS and Oxidation Resistance of Ta4HfC5 Ceramics. Ceram. Int. 2022, 49, 9691–9701. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Papynov, E.K.; Gridasova, E.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Production of HfB2–SiC (10–65 Vol % SiC) Ultra-High-Temperature Ceramics by Hot Pressing of HfB2–(SiO2–C) Composite Powder Synthesized by the Sol–Gel Method. Russ. J. Inorg. Chem. 2018, 63, 1–15. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Kolesnikov, A.F.; Sevastyanov, V.G.; Kuznetsov, N.T. Behavior of HfB2–30 Vol% SiC UHTC Obtained by Sol–Gel Approach in the Supersonic Airflow. J. Sol-Gel Sci. Technol. 2019, 92, 386–397. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Oxidation of HfB2-SiC-Ta4HfC5 Ceramic Material by a Supersonic Flow of Dissociated Air. J. Eur. Ceram. Soc. 2021, 41, 1088–1098. [Google Scholar] [CrossRef]
- Papynov, E.K.; Portnyagin, A.S.; Modin, E.B.; Mayorov, V.Y.; Shichalin, O.O.; Golikov, A.P.; Pechnikov, V.S.; Gridasova, E.A.; Tananaev, I.G.; Avramenko, V.A. A Complex Approach to Assessing Porous Structure of Structured Ceramics Obtained by SPS Technique. Mater. Charact. 2018, 145, 294–302. [Google Scholar] [CrossRef]
- Gordeev, A. Overview of Characteristics and Experiments in IPM Plasmatrons. VKI, RTO AVT/VKI Special Course on Measurement Techniques for High Enthalpy Plasma Flows. Available online: https://apps.dtic.mil/sti/citations/ADP010736 (accessed on 25 October 1999).
- Chaplygin, A.V.; Vasil’evskii, S.A.; Galkin, S.S.; Kolesnikov, A.F. Thermal State of Uncooled Quartz Discharge Channel of Powerful High-Frequency Induction Plasmatron. Phys. Kinet. Gas Dyn. 2022, 23, 38–56. [Google Scholar] [CrossRef]
- Kolesnikov, A.F.; Lukomskii, I.V.; Sakharov, V.I.; Chaplygin, A.V. Experimental and Numerical Modeling of Heat Transfer to Graphite Surface in Underexpanded Dissociated-Nitrogen Jets. Fluid Dyn. 2021, 56, 897–905. [Google Scholar] [CrossRef]
- Lukomskii, I.V.; Chaplygin, A.V.; Kolesnikov, A.F. A Device for Measuring the Heat Flux to the Surface of a Material Heated in a Jet of High-Enthalpy Gas to a High Temperature. Patent RU 205572, 21 July 2021. [Google Scholar]





| Sample 1 | Sample 2 | |||
|---|---|---|---|---|
| Central Region | Peripheral Region | Central Region | Peripheral Region | |
| tmax, °C | 1765–1785 | 1665–1720 | 2200–2300 | 1750–1800 |
| lox, µm | 40 ± 20 * | 12 ± 4 | 140 ± 25 | 20 ± 5 |
| lSiC-depl, µm | 120 ± 20 | 90 ± 10 ** | 230 ± 20 | 70 ± 10 ** |
| lΣ, µm | 165 ± 10 | 100 ± 10 ** | 380 ± 2 | 90 ± 10 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonenko, E.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Kotov, M.A.; Yakimov, M.Y.; Lukomskii, I.V.; Galkin, S.S.; Shemyakin, A.N.; Solovyov, N.G.; Lysenkov, A.S.; et al. Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating. Int. J. Mol. Sci. 2023, 24, 13634. https://doi.org/10.3390/ijms241713634
Simonenko EP, Kolesnikov AF, Chaplygin AV, Kotov MA, Yakimov MY, Lukomskii IV, Galkin SS, Shemyakin AN, Solovyov NG, Lysenkov AS, et al. Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating. International Journal of Molecular Sciences. 2023; 24(17):13634. https://doi.org/10.3390/ijms241713634
Chicago/Turabian StyleSimonenko, Elizaveta P., Anatoly F. Kolesnikov, Aleksey V. Chaplygin, Mikhail A. Kotov, Mikhail Yu. Yakimov, Ilya V. Lukomskii, Semen S. Galkin, Andrey N. Shemyakin, Nikolay G. Solovyov, Anton S. Lysenkov, and et al. 2023. "Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating" International Journal of Molecular Sciences 24, no. 17: 13634. https://doi.org/10.3390/ijms241713634
APA StyleSimonenko, E. P., Kolesnikov, A. F., Chaplygin, A. V., Kotov, M. A., Yakimov, M. Y., Lukomskii, I. V., Galkin, S. S., Shemyakin, A. N., Solovyov, N. G., Lysenkov, A. S., Nagornov, I. A., Mokrushin, A. S., Simonenko, N. P., & Kuznetsov, N. T. (2023). Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating. International Journal of Molecular Sciences, 24(17), 13634. https://doi.org/10.3390/ijms241713634









