Plasticity of Plantago lanceolata L. in Adaptation to Extreme Environmental Conditions
Abstract
1. Introduction
2. Results
2.1. Leaf Morphology and Anatomy
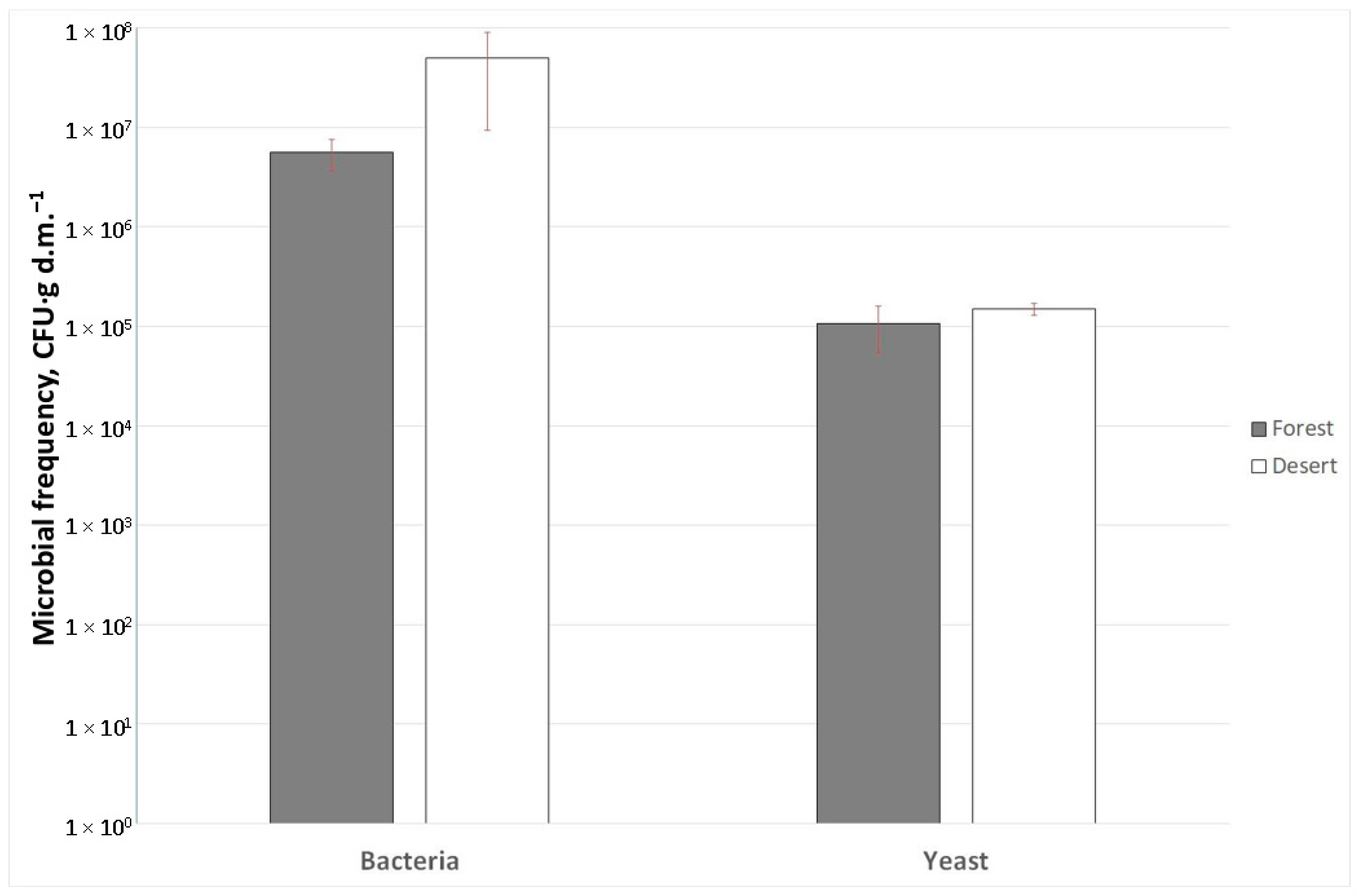
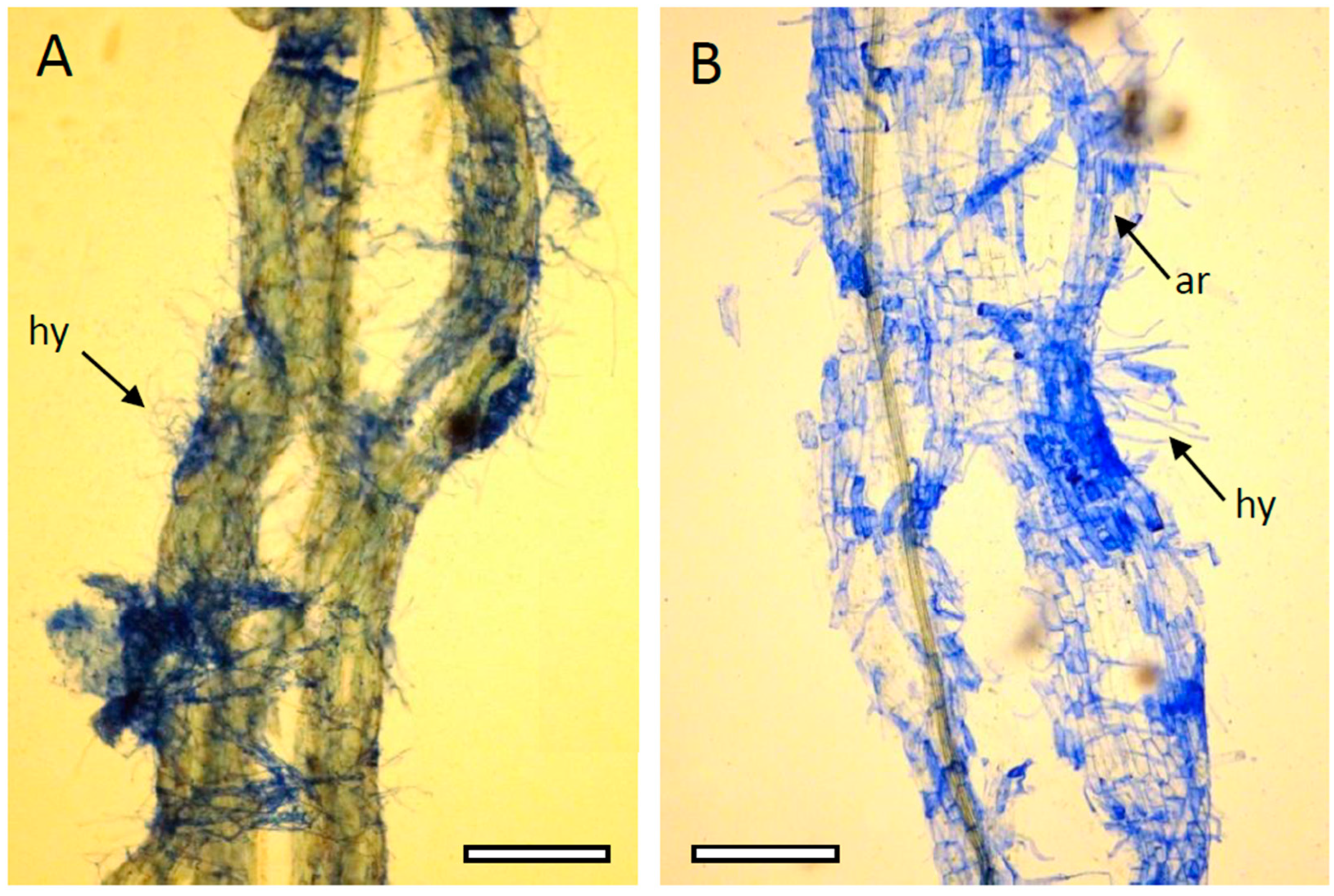
2.2. Microbial Colonization and Mycorrhiza
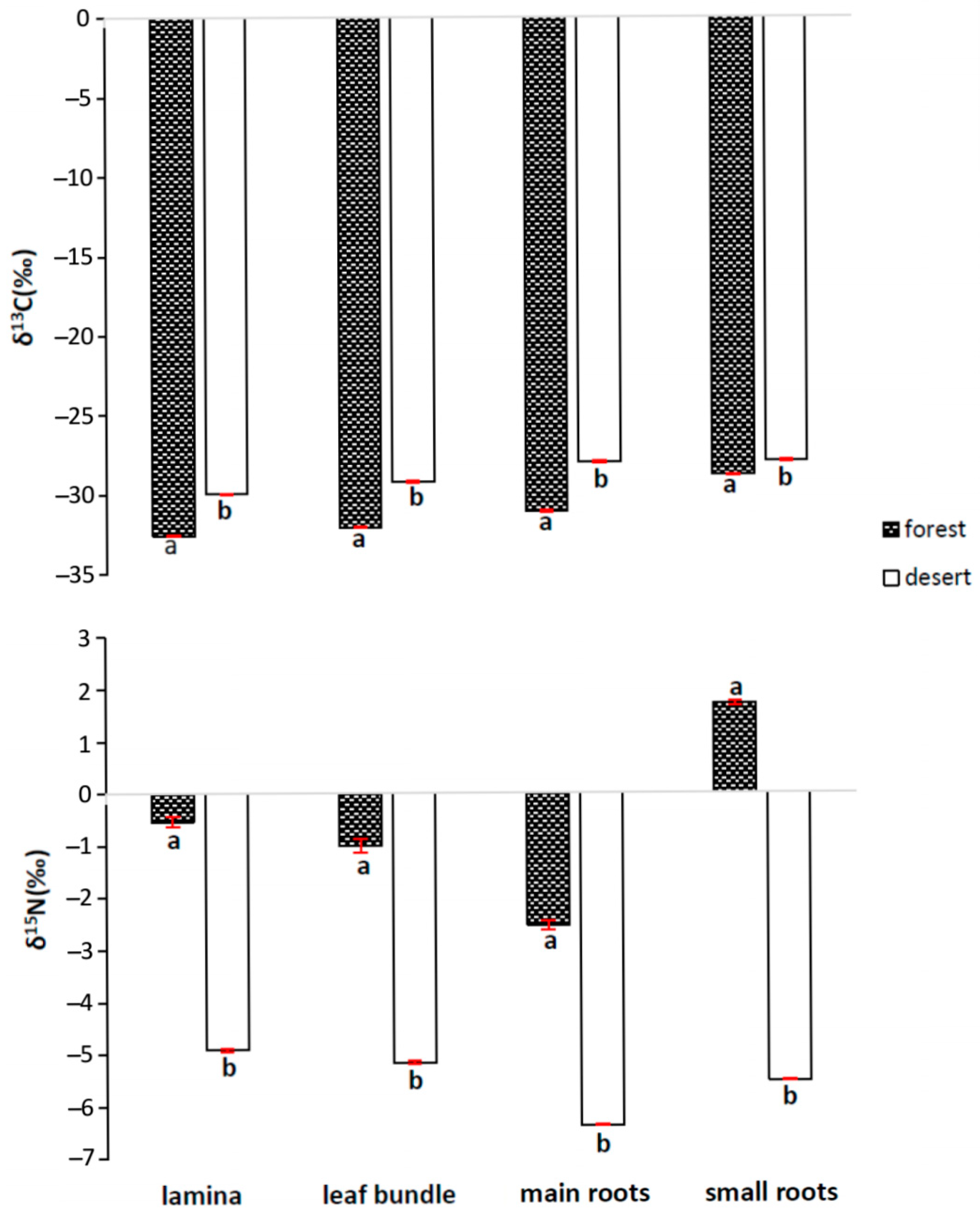
2.3. The Leaf Signature: 13C and 15N Discrimination
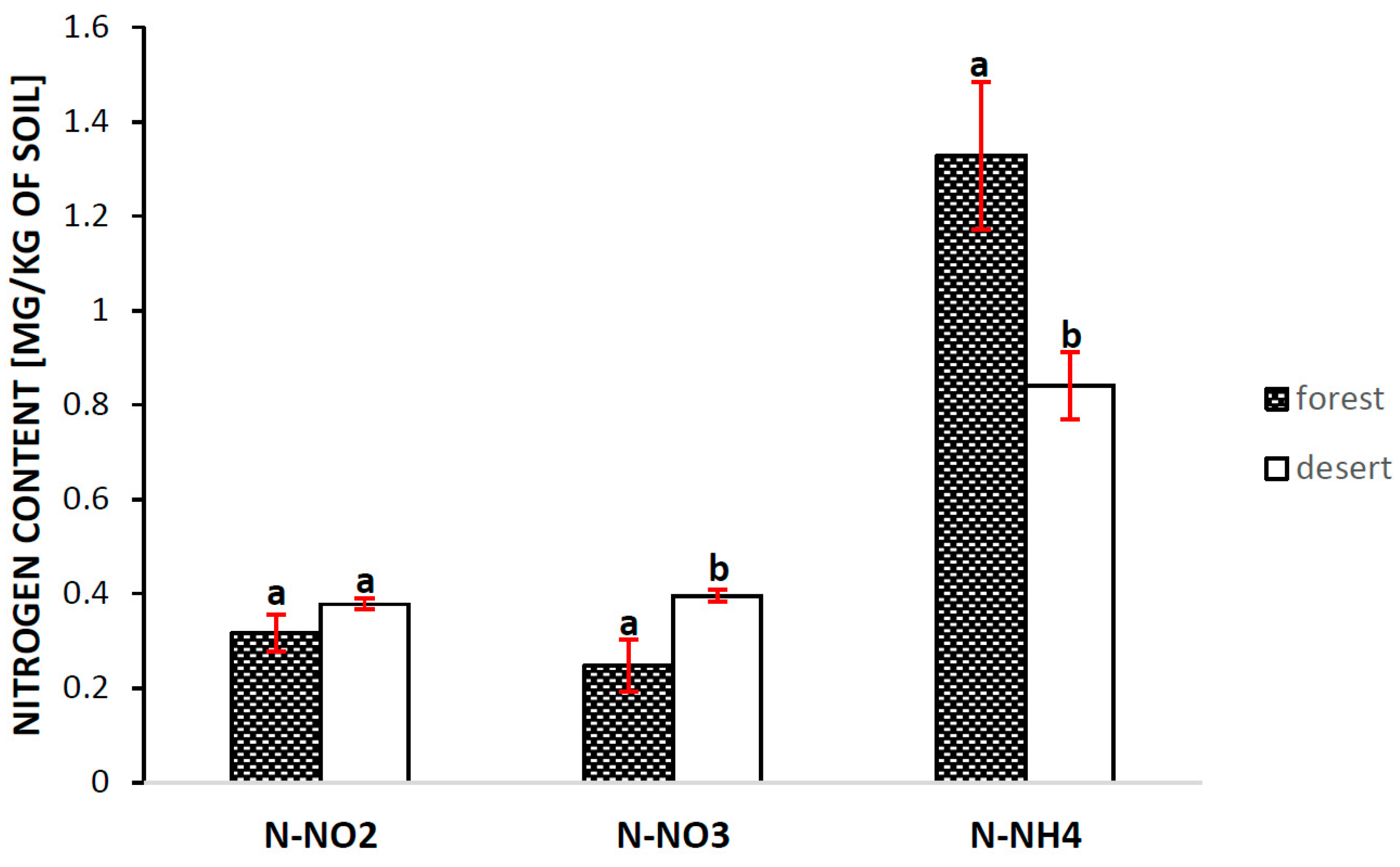
2.4. Analysis of the Content of Various Forms of Nitrogen in the Soil
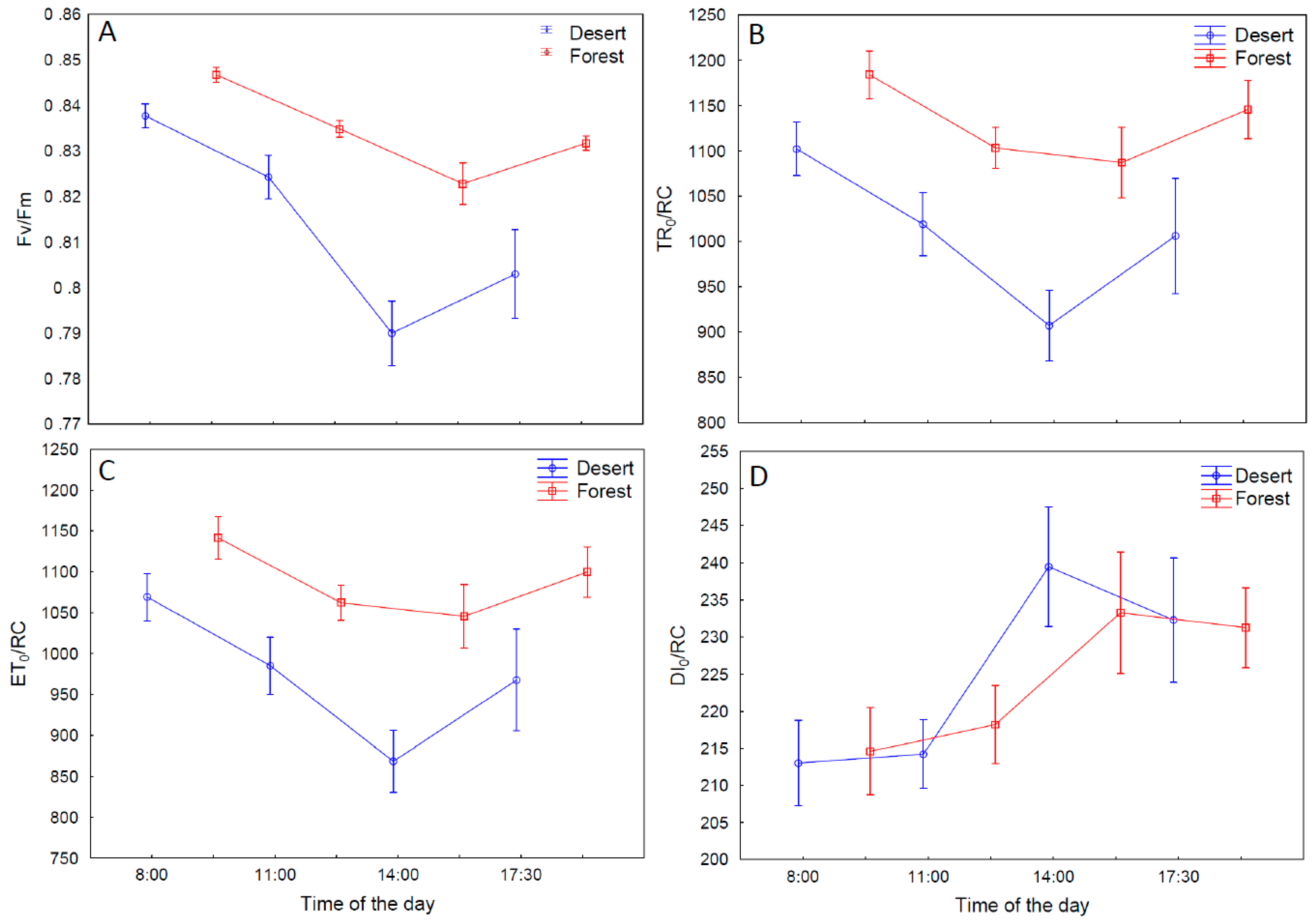
2.5. Analyses of Chlorophyll a Fluorescence
3. Discussion
4. Materials and Methods
4.1. Study Sites and Plant Material
4.2. Leaf Morphology Parameters
4.3. Bacterial Frequency Determination and Mycorrhizal Colonization Assessment
- Frequency of mycorrhiza in the root system
- Intensity of the mycorrhizal colonization in the root system
- Intensity of the mycorrhizal colonization in the root fragments
- Arbuscule abundance in the mycorrhizal parts of the root fragments
- Arbuscule abundance in the root system
4.4. The Content of Carbon 13C and Nitrogen 15N Isotopes
4.5. The Content of Various Forms of Nitrogen in the Soil
4.6. Chl a Fluorescence Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lüttge, U. Clusia: Holy Grail and enigma. J. Exp. Bot. 2008, 59, 1503–1514. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Nosek, M.; Rozpądek, P.; Kornas, A.; Kuźniak, E.; Schmitt, A.; Miszalski, Z. Veinal-mesophyll interaction under biotic stress. J. Plant Physiol. 2015, 185, 52–56. [Google Scholar] [CrossRef]
- Kocurek, M.; Kornas, A.; Wierzchnicki, R.; Lüttge, U.; Miszalski, Z. Importance of stem photosynthesis in plant carbon allocation of Clusia minor. Tress—Struct. Funct. 2020, 34, 1009–1020. [Google Scholar] [CrossRef]
- Miszalski, Z.; Kornaś, A.; Kuźniak, E. Photosynthesis-Related Functions of Vasculature-Associated Chlorenchymatous Cells. In Progress in Botany; Cánovas, F., Lüttge, U., Matyssek, R., Eds.; Springer: Cham, Switzerland, 2017; Volume 79, pp. 173–196. [Google Scholar] [CrossRef]
- Miszalski, Z.; Skoczowski, A.; Silina, E.; Dymova, O.; Golovko, T.; Kornas, A.; Strzalka, K. Photosynthetic activity of vascular bundles in Plantago media leaves. J. Plant Physiol. 2016, 204, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Walters, R.G.; Jansson, S.; Horton, P. Acclimation of Arabidopsis thaliana to the light environment: The existence of separate low light and high light responses. Planta 2001, 213, 794–801. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Quick, W.P. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 2002, 415, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Ślesak, I.; Libik, M.; Miszalski, Z. The foliar concentration of hydrogen peroxide during salt-induced C3-CAM transition in Mesembryanthemum crystallinum L. Plant Sci. 2008, 174, 221–226. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Aschan, G.; Pfanz, H. Non-foliar photosynthesis—A strategy of additional carbon acquisition. Flora 2003, 198, 81–97. [Google Scholar] [CrossRef]
- Kocurek, M.; Kornas, A.; Pilarski, J.; Tokarz, K.; Lüttge, U.; Miszalski, Z. Photosynthetic activity of stems in two Clusia species. Tress—Struct. Funct. 2015, 29, 1029–1040. [Google Scholar] [CrossRef]
- Kuźniak, E.; Kornas, A.; Kaźmierczak, A.; Rozpądek, P.; Nosek, M.; Zellnig, G.; Müller, M.; Miszalski, Z. Photosynthesis-related characteristics of the midrib and the interveinal lamina in leaves of the C3–CAM intermediate plant Mesembryanthemum crystallinum. Ann. Bot. 2016, 117, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Pfanz, H.; Aschan, G.; Langenfeld-Heyser, R.; Wittmann, C.; Loose, M. Ecology and ecophysiology of tree stems: Corticular and wood photosynthesis. Naturwissenschaften 2002, 89, 147–162. [Google Scholar] [CrossRef]
- Edwards, E.J. Evolutionary trajectories, accessibility and other metaphors: The case of C4 and CAM photosynthesis. New Phytol. 2019, 223, 1742–1755. [Google Scholar] [CrossRef]
- Nimmo, H.G. The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci. 2000, 5, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Feria, A.B.; Ruíz-Ballesta, I.; Baena, G.; Ruíz-López, N.; Echevarría, C.; Vidal, J. Phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxylase kinase isoenzymes play an important role in the filling and quality of Arabidopsis thaliana seed. Plant Physiol. Biochem. 2022, 190, 70–80. [Google Scholar] [CrossRef]
- Shu, J.P.; Yan, Y.H.; Wang, R.J. Convergent molecular evolution of phosphoenolpyruvate carboxylase gene family in C4 and crassulacean acid metabolism plants. PeerJ 2022, 10, e12828. Available online: https://peerj.com/articles/12828/ (accessed on 15 July 2023). [CrossRef]
- Feria, A.B.; Bosch, N.; Sánchez, A.; Nieto-Ingelmo, A.; de la Osa, C.; Echevarría, C.; García-Mauriño, S.; Monreal, J.A. Phosphoenolpyruvate carboxylase (PPC) and PPC-kinase (PPC-k) isoenzymes in Arabidopsis thaliana: Role in control and abiotic stress conditions. Planta 2016, 244, 901–913. [Google Scholar] [CrossRef]
- Brown, N.J.; Palmer, B.G.; Stanley, S.; Hajaji, H.; Janacek, S.H.; Astley, H.M.; Parsley, K.; Kajala, K.; Quick, W.P.; Trenkamp, S.; et al. C acid decarboxylases required for C photosynthesis are active in the mid-vein of the C species Arabidopsis thaliana, and are important in sugar and amino acid metabolism. Plant J. 2010, 61, 122–133. [Google Scholar] [CrossRef]
- Shi, J.; Yi, K.; Liu, Y.; Xie, L.; Zhou, Z.; Chen, Y.; Hu, Z.; Zheng, T.; Liu, R.; Chen, Y.; et al. Phosphoenolpyruvate carboxylase in Arabidopsis leaves plays a crucial role in Carbon and Nitrogen metabolism. Plant Physiol. 2015, 167, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Skrynetska, I.; Karcz, J.; Barczyk, G.; Kandziora-Ciupa, M.; Ryszard Ciepał, R.; Nadgórska-Socha, A. Using Plantago major and Plantago lanceolata in environmental pollution research in an urban area of Southern Poland. Environ. Sci. Pollut. Res. 2019, 26, 23359–23371. [Google Scholar] [CrossRef]
- Bachar, A.; Soares, M.I.M.; Gillor, O. The effect of resource islands on abundance and diversity of bacteria in arid soils. Microb. Ecol. 2012, 63, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. Reclamation of arid and semi-arid soils: The role of plant growth-promoting archaea and bacteria. Curr. Plant Biol. 2021, 25, 100173. [Google Scholar] [CrossRef]
- Köberl, M.; Müller, H.; Ramadan, E.M.; Berg, G. Desert farming benefits from microbial potential in arid soils and promotes diversity and plant health. PLoS ONE 2011, 6, e24452. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and arid soils: Impact on bacteria, plants, and their interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef]
- Alkobaisy, J.S. Factors affecting mycorrhizal activity. In Arbuscular Mycorrhizal Fungi in Agriculture; de Sousa, R.N., Ed.; New Insights Edition; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Vasar, M.; Davison, J.; Sepp, S.-K.; Öpik, M.; Moora, M.; Koorem, K.; Meng, Y.; Oja, J.; Akhmetzhanova, A.A.; Al-Quraishy, S.; et al. Arbuscular mycorrhizal fungal communities in the soils of desert habitats. Microorganisms 2021, 9, 229. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Livingston, N.J.; Guy, R.D.; Sun, Z.J.; Ethier, G.J. The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell Environ. 1999, 22, 281–289. [Google Scholar] [CrossRef]
- Scheidegger, Y.; Saurer, M.; Bahn, M.; Siegwolf, R. Linking stable oxygen and carbon isotopes with stomatal conductance and photosynthetic capacity: A conceptual model. Oecologia 2000, 125, 350–357. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Tcherkez, G.; Keitel, C.; Cornwell, W.K.; Santiago, L.S.; Knohl, A.; Barbour, M.M.; Williams, D.G.; Reich, P.B.; Ellsworth, D.S.; et al. Why are non-photosynthetic tissues generally 13C enriched comparedwith leaves in C3 plants? Review and synthesis of current hypotheses. Funct. Plant Biol. 2009, 36, 199–213. [Google Scholar] [CrossRef]
- Kornas, A.; Fischer-Schliebs, E.; Lüttge, U.; Miszalski, Z. Adaptation of the obligate CAM plant Clusia alata to light stress: Metabolic responses. J. Plant Physiol. 2009, 166, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Handley, L.L.; Raven, J.A. The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ. 1992, 15, 965–985. [Google Scholar] [CrossRef]
- Kalcsits, L.; Buschhaus, H.; Guy, R. Nitrogen isotope discrimination as an integrated measure of nitrogen fluxes, assimilation and allocation in plants. Physiol. Plant. 2014, 151, 293–304. [Google Scholar] [CrossRef]
- Śliwa, M.; Kaszycki, P.; Supel, P.; Kornaś, A.; Kaproń, A.; Lüttge, U.; Miszalski, Z. Selected physiological parameters of creeping willow [Salix repens subsp. arenaria (L.) Hiit.]: A shrubby plant inhabiting degraded industrial areas. Tress—Struct. Funct. 2019, 33, 1447–1457. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee; Bosa, K.; Kościelniak, J.; Żuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Ordinance of the Regional Director for Environmental Protection, Kraków, Katowice, 2014, (in Polish). Zarządzenie Regionalnego Dyrektora Ochrony Środowiska w Krakowie i Regionalnego Dyrektora Ochrony Środowiska w Katowicach z dnia 31 lipca 2014 r. w Sprawie Ustanowienia Planu zadań Ochronnych dla Obszaru Natura 2000 Pustynia Błędowska PLH120014, Dz. Urz. Województwa Małopolskiego z 04.08.2014 r. Poz. 4258, Dz. Urz. Województwa Śląskiego z 04.08.2014 r. Poz. 4210. Available online: https://www.infor.pl/dzienniki-urzedowe/wojewodztwa-slaskiego/r2014/nr136 (accessed on 15 July 2023).
- Szostak, M.; Wężyk, P.; Hawryło, P.; Puchała, M. Monitoring the secondary forest succession and land cover/use changes of the Błędów Desert (Poland) using geospatial analyses. Quaest. Geogr. 2016, 35, 5–13. [Google Scholar] [CrossRef]
- Rahmonov, O.; Oleś, W. Vegetation succession over an area of a medieval ecological disaster. The case of the Błędów Desert, Poland. Erdkunde 2010, 64, 241–255. [Google Scholar] [CrossRef]
- Gus, M.; Drewniak, M. Evolution of sandy soils within deflation hollows in shifting areas of sand—A case study from the Błędów Desert (Poland). Soil Water Res. 2017, 12, 161–169. [Google Scholar] [CrossRef]
- Rahmonov, O.; Szczypek, T.; Wach, J. The Błędów Desert (Pustynia Błędowska)—A unique phenomenon of the Polish landscape. Ann. Geogr. 2006, 39, 34–41. [Google Scholar]
- Kaszycki, P.; Supel, P.; Petryszak, P. Bacterial population dynamics in waste oily emulsions from the metal-processing industry. J. Ecol. Eng. 2014, 15, 14–22. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158. [Google Scholar] [CrossRef]
- Ważny, R.; Rozpądek, P.; Jędrzejczyk, R.J.; Śliwa, M.; Stojakowska, A.; Anielska, T.; Turnau, K. Does co-inoculation of Lactuca serriola with endophytic and arbuscular mycorrhizal fungi improve plant growth in a polluted environment? Mycorrhiza 2018, 28, 235–246. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesere du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Mycorhizes: Physiologie Et Génétique; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Edition: Paris, France, 1986; pp. 217–220. [Google Scholar]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Gröning, M.; Meijer, H.A.J.; Toman, B.; Verkouteren, R.M. New guidelines for 13C measurements. Anal. Chem. 2006, 78, 2439–2441. [Google Scholar] [CrossRef]


| Habitat | ||
|---|---|---|
| Forest | Desert | |
| F% | 100 ± 0.0 | 100 ± 0.0 |
| M% | 57.9 ± 3.8 a | 66.1 ± 2.3 b |
| m% | 57.9 ± 3.8 a | 66.1 ± 2.3 b |
| A% | 8.8 ± 0.8 a | 52.6 ± 1.3 b |
| a% | 15.2 ± 0.5 a | 79.6 ± 2.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miszalski, Z.; Kaszycki, P.; Śliwa-Cebula, M.; Kaczmarczyk, A.; Gieniec, M.; Supel, P.; Kornaś, A. Plasticity of Plantago lanceolata L. in Adaptation to Extreme Environmental Conditions. Int. J. Mol. Sci. 2023, 24, 13605. https://doi.org/10.3390/ijms241713605
Miszalski Z, Kaszycki P, Śliwa-Cebula M, Kaczmarczyk A, Gieniec M, Supel P, Kornaś A. Plasticity of Plantago lanceolata L. in Adaptation to Extreme Environmental Conditions. International Journal of Molecular Sciences. 2023; 24(17):13605. https://doi.org/10.3390/ijms241713605
Chicago/Turabian StyleMiszalski, Zbigniew, Paweł Kaszycki, Marta Śliwa-Cebula, Adriana Kaczmarczyk, Miron Gieniec, Paulina Supel, and Andrzej Kornaś. 2023. "Plasticity of Plantago lanceolata L. in Adaptation to Extreme Environmental Conditions" International Journal of Molecular Sciences 24, no. 17: 13605. https://doi.org/10.3390/ijms241713605
APA StyleMiszalski, Z., Kaszycki, P., Śliwa-Cebula, M., Kaczmarczyk, A., Gieniec, M., Supel, P., & Kornaś, A. (2023). Plasticity of Plantago lanceolata L. in Adaptation to Extreme Environmental Conditions. International Journal of Molecular Sciences, 24(17), 13605. https://doi.org/10.3390/ijms241713605








