Effect of Targeted Cytokine Inhibition on Progression of Post-Traumatic Osteoarthritis Following Intra-Articular Fracture
Abstract
:1. Introduction
2. Results
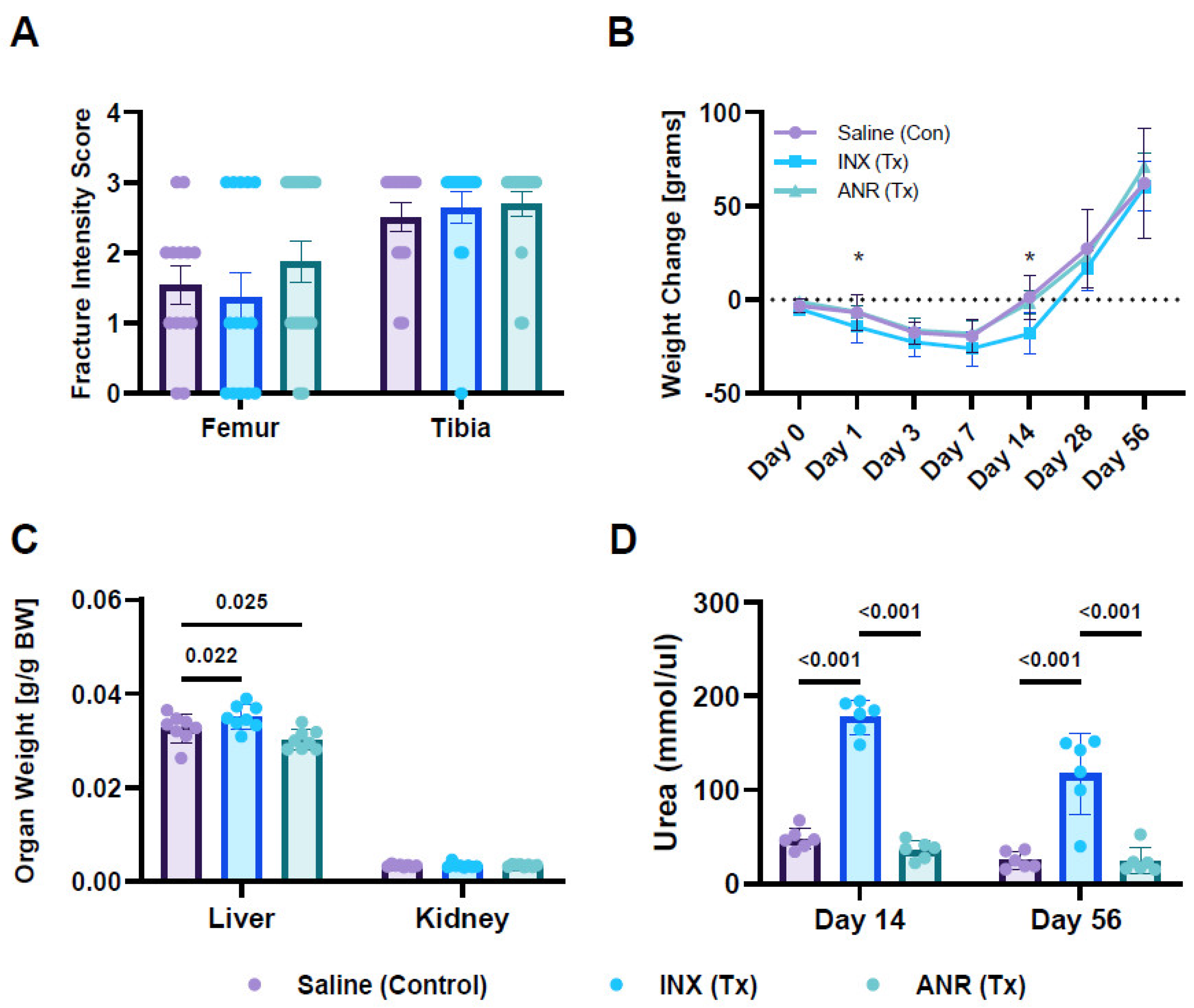
2.1. Fracture Characteristics
2.2. Systemic Changes Following Treatment with INX and ANR
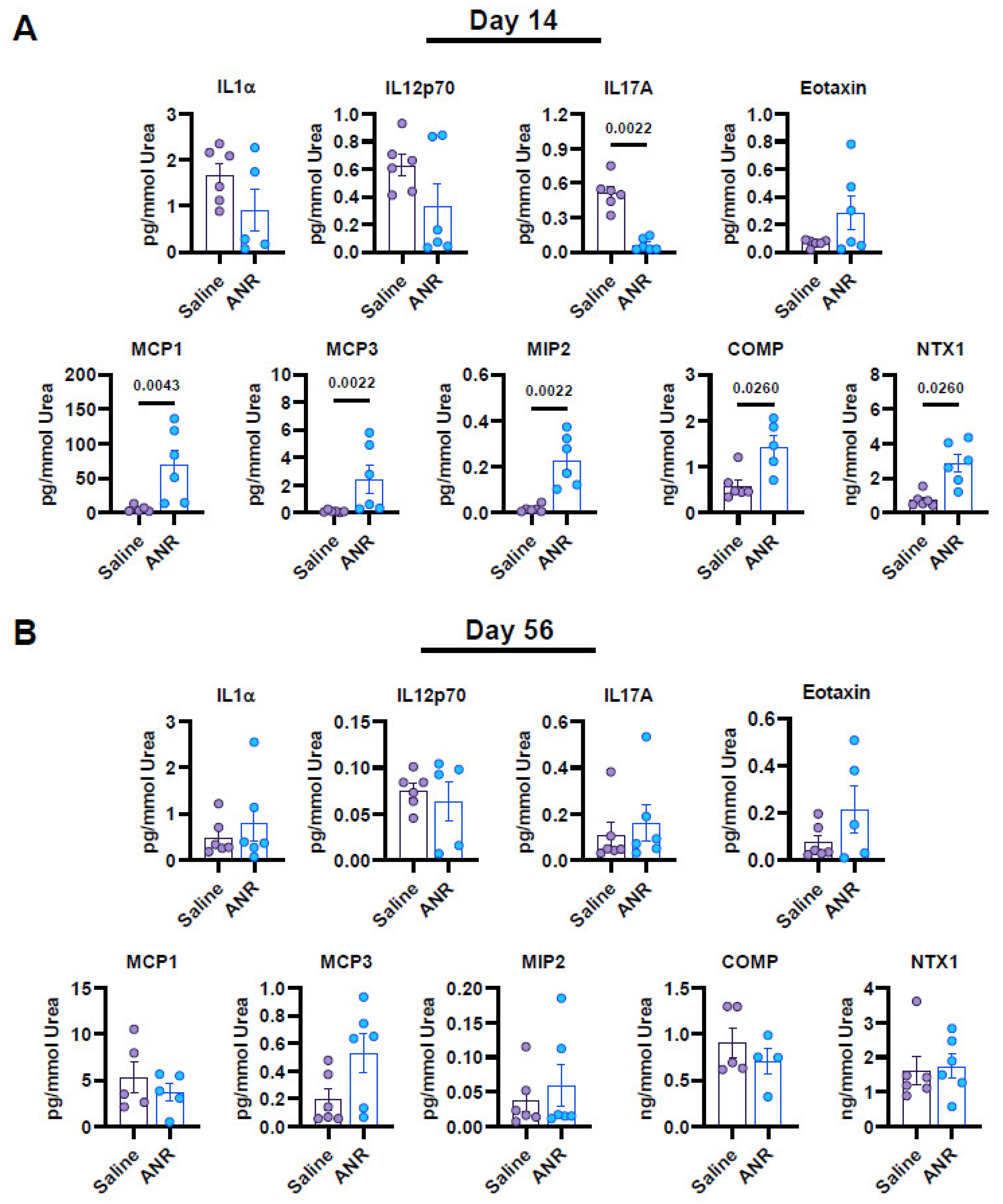
2.3. Effect of IL-1 Inhibition on Systemic Soluble Factor Expression Following IAF
2.4. Effect of IL-1 Inhibition on Systemic Expression of Osteochondral Markers Following IAF
2.5. Effect of IL-1 Inhibition on Local Synovial Fluid Expression of Inflammatory and Osteochondral Markers Following IAF
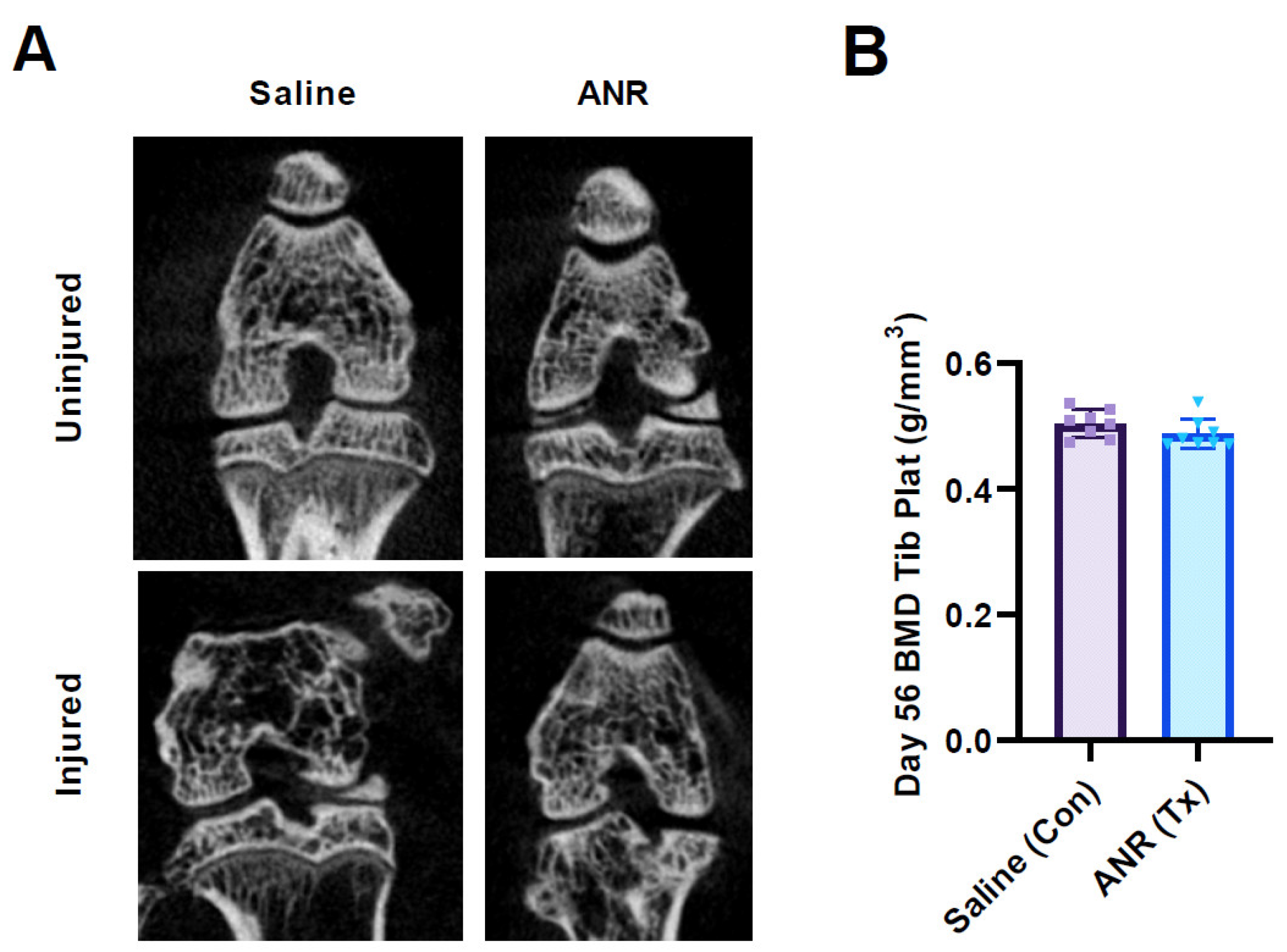
2.6. Effect of IL-1 Inhibition on Bone Mineral Density
2.7. Effect of IL-1 Inhibition on Bone Morphometry
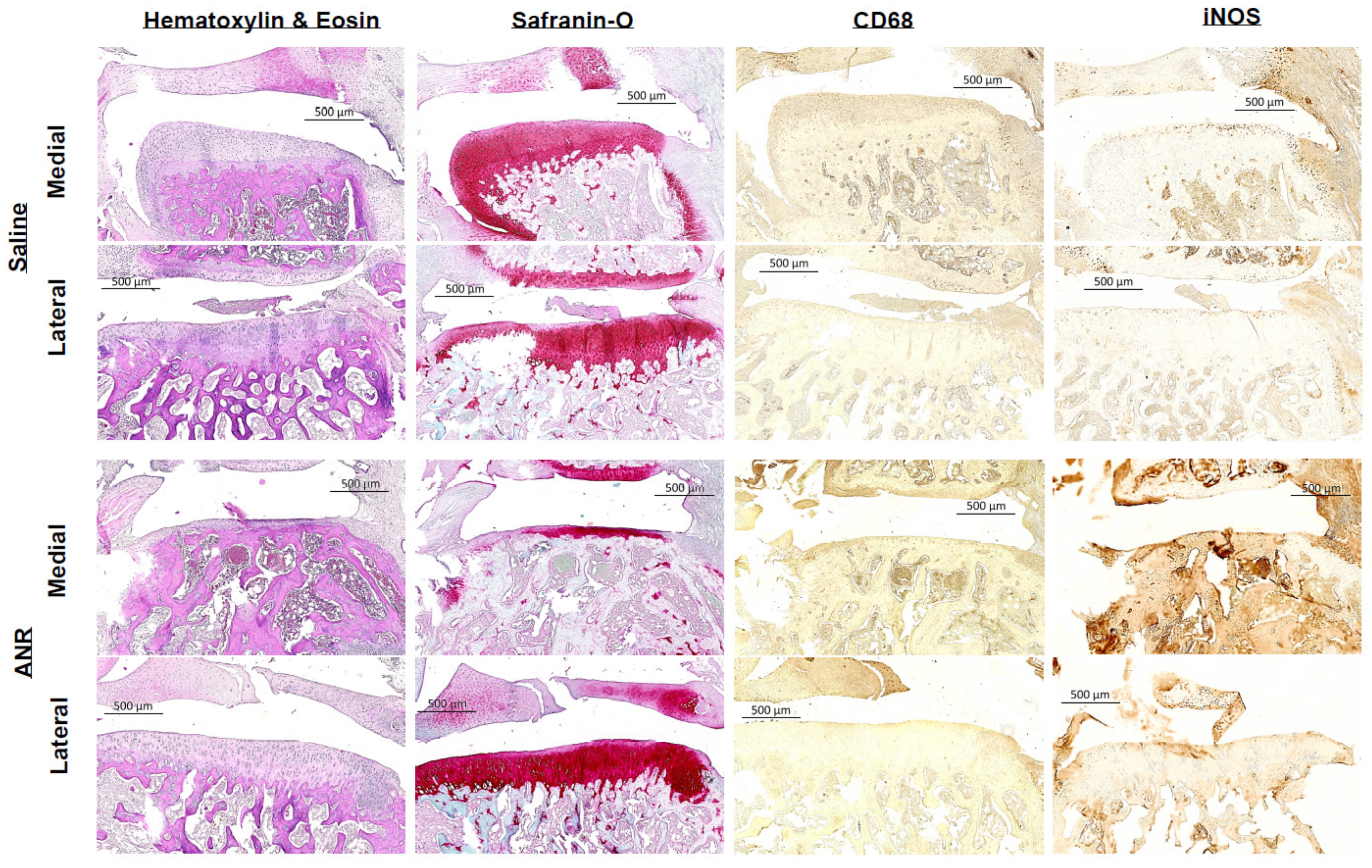
2.8. Effect of IL-1 Inhibition on Histopathological Presentation of Joint Tissues
2.9. Effect of IL-1 Inhibition on IAF Cartilage Content Measured Ex Vivo by CECT
3. Discussion
4. Materials and Methods
4.1. Preparation of Interventions
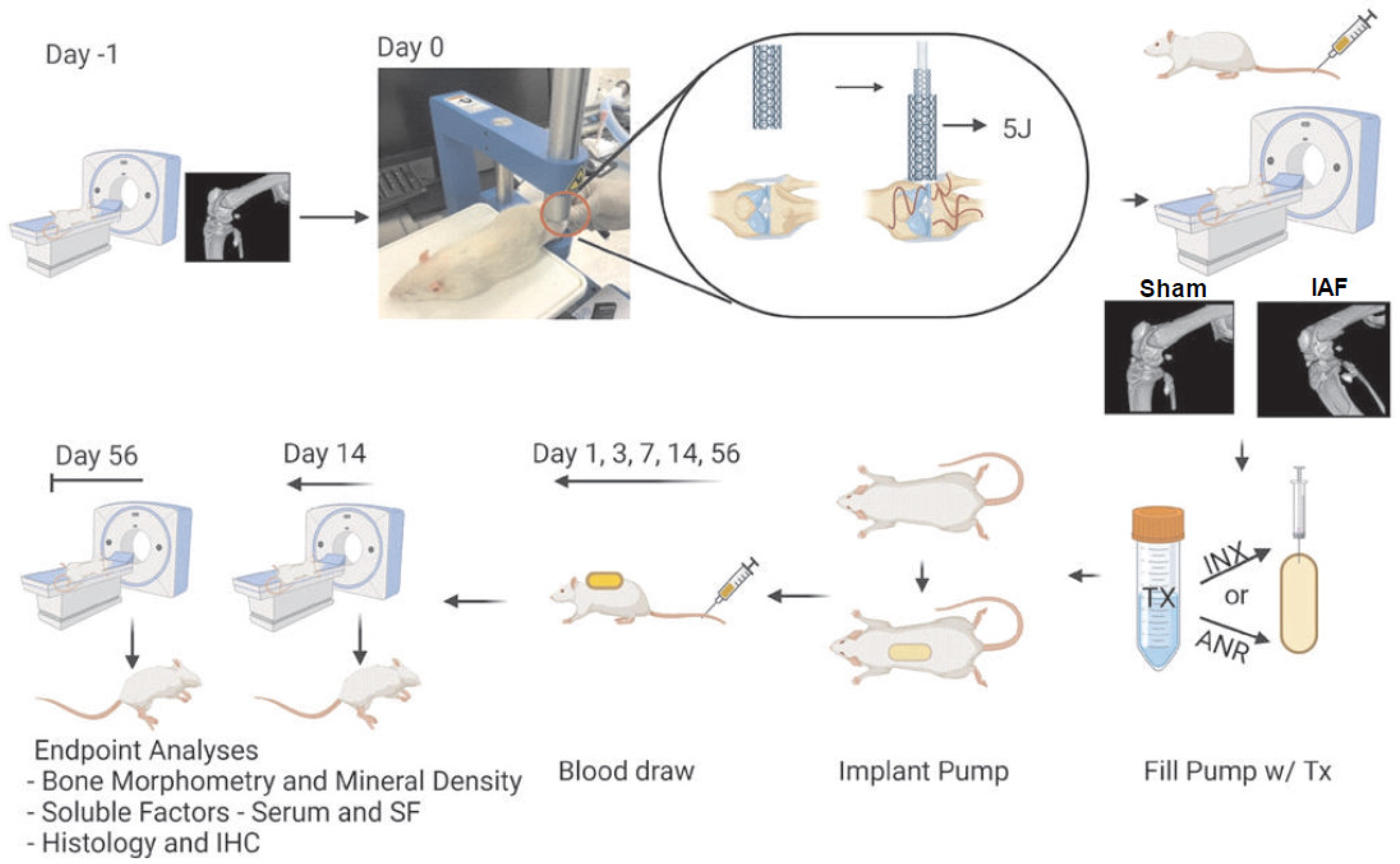
4.2. Intra-Articular Fracture Model and Drug Delivery
4.3. Tissue, Blood, and Synovial Fluid Harvest
4.4. Micro-Computed Tomography
4.5. Fracture Assessment
4.6. Synovial Fluid Protein Extraction
4.7. Protein Analysis
4.8. Contrasts Enhanced µCT and Analysis
4.9. Histological and Immunohistochemical Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statement
Disclaimer
References
- McKinley, T.O.; Borrelli, J., Jr.; D’Lima, D.D.; Furman, B.D.; Giannoudis, P.V. Basic science of intra-articular fractures and posttraumatic osteoarthritis. J. Orthop. Trauma 2010, 24, 567–570. [Google Scholar] [CrossRef]
- Schenker, M.L.; Mauck, R.L.; Ahn, J.; Mehta, S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J. Am. Acad. Orthop. Surg. 2014, 22, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gelber, A.C.; Hochberg, M.C.; Mead, L.A.; Wang, N.Y.; Wigley, F.M.; Klag, M.J. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med. 2000, 133, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Stanishewski, M.; Zimmermann, B. Osteoarthritis Treatment in the Veteran Population. Fed. Pract. 2015, 32, 21s–25s. [Google Scholar] [PubMed]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 2006, 20, 739–744. [Google Scholar] [CrossRef]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- Pham, T.M.; Frich, L.H.; Lambertsen, K.L.; Overgaard, S.; Schmal, H. Elevation of Inflammatory Cytokines and Proteins after Intra-Articular Ankle Fracture: A Cross-Sectional Study of 47 Ankle Fracture Patients. Mediat. Inflamm. 2021, 2021, 8897440. [Google Scholar] [CrossRef]
- Punzi, L.; Galozzi, P.; Luisetto, R.; Favero, M.; Ramonda, R.; Oliviero, F.; Scanu, A. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open 2016, 2, e000279. [Google Scholar] [CrossRef]
- Iversen, I.J.; Pham, T.M.; Schmal, H. Do acute inflammatory cytokines affect 3- and 12-month postoperative functional outcomes-a prospective cohort study of 12 patients with proximal tibia fractures. BMC Musculoskelet. Disord. 2021, 22, 342. [Google Scholar] [CrossRef]
- Garcia, K.S.; de Azevedo, M.F.C.; Carlos, A.S.; Barros, L.L.; Oba, J.; Sobrado Junior, C.W.; Sipahi, A.M.; Alves, O.D.C.; Navarro-Rodriguez, T.; Parra, R.S.; et al. Efficacy of Early Optimization of Infliximab Guided by Therapeutic Drug Monitoring during Induction-A Prospective Trial. Biomedicines 2023, 11, 1757. [Google Scholar] [CrossRef]
- Ku, E.J.; Kim, B.R.; Lee, J.I.; Lee, Y.K.; Oh, T.J.; Jang, H.C.; Choi, S.H. The Anti-Atherosclerosis Effect of Anakinra, a Recombinant Human Interleukin-1 Receptor Antagonist, in Apolipoprotein E Knockout Mice. Int. J. Mol. Sci. 2022, 23, 4906. [Google Scholar] [CrossRef]
- Lindsley, H.B.; Schue, J.; Tawfik, O.; Bolce, R.; Smith, D.D.; Hinson, G.; Wick, J.A. FRI0304 Treatment of knee osteoarthritis with intra-articular infliximab improves total womac score. High baseline levels of synovial cellularity predict improvement. Ann. Rheum. Dis. 2013, 71, 417. [Google Scholar] [CrossRef]
- Perdriger, A. Infliximab in the treatment of rheumatoid arthritis. Biologics 2009, 3, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, K.A.; Machan, J.T.; Waller, K.; Fleming, B.C.; Jay, G.D. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009, 60, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- van der Bijl, A.E.; Teng, Y.K.; van Oosterhout, M.; Breedveld, F.C.; Allaart, C.F.; Huizinga, T.W. Efficacy of intraarticular infliximab in patients with chronic or recurrent gonarthritis: A clinical randomized trial. Arthritis Rheum. 2009, 61, 974–978. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bersani-Amado, C.A.; Ishii-Iwamoto, E.L.; Bracht, L.; Caparroz-Assef, S.M. Protective effects of indomethacin and cyclophosphamide but not of infliximab on liver metabolic changes caused by adjuvant-induced arthritis. Inflammation 2011, 34, 519–530. [Google Scholar] [CrossRef]
- Mertens, M.; Singh, J.A. Anakinra for rheumatoid arthritis: A systematic review. J. Rheumatol. 2009, 36, 1118–1125. [Google Scholar] [CrossRef]
- Iqbal, I.; Fleischmann, R. Treatment of osteoarthritis with anakinra. Curr. Rheumatol. Rep. 2007, 9, 31–35. [Google Scholar] [CrossRef]
- Furman, B.D.; Mangiapani, D.S.; Zeitler, E.; Bailey, K.N.; Horne, P.H.; Huebner, J.L.; Kraus, V.B.; Guilak, F.; Olson, S.A. Targeting pro-inflammatory cytokines following joint injury: Acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res. Ther. 2014, 16, R134. [Google Scholar] [CrossRef]
- Kimmerling, K.A.; Furman, B.D.; Mangiapani, D.S.; Moverman, M.A.; Sinclair, S.M.; Huebner, J.L.; Chilkoti, A.; Kraus, V.B.; Setton, L.A.; Guilak, F.; et al. Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis. Eur. Cell Mater. 2015, 29, 124–139; discussion 139–140. [Google Scholar] [CrossRef]
- Nasi, S.; Ea, H.K.; So, A.; Busso, N. Revisiting the Role of Interleukin-1 Pathway in Osteoarthritis: Interleukin-1alpha and -1beta, and NLRP3 Inflammasome Are Not Involved in the Pathological Features of the Murine Menisectomy Model of Osteoarthritis. Front. Pharmacol. 2017, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Siu, W.-S.; Leung, P.-C. The Potential of MSC-Based Cell-Free Therapy in Wound Healing—A Thorough Literature Review. Int. J. Mol. Sci. 2023, 24, 9356. [Google Scholar] [PubMed]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- French, J.B.; Bonacini, M.; Ghabril, M.; Foureau, D.; Bonkovsky, H.L. Hepatotoxicity Associated with the Use of Anti-TNF-alpha Agents. Drug Saf. 2016, 39, 199–208. [Google Scholar] [CrossRef]
- Cayir, D.; Bozkurt, M.; Filik, M.; Gultekin, S.S. Infliximab-induced hot kidneys on bone scintigraphy. World J. Nucl. Med. 2019, 18, 437–439. [Google Scholar] [CrossRef]
- Bootun, R. Effects of immunosuppressive therapy on wound healing. Int. Wound J. 2013, 10, 98–104. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ito, H.; Kitaori, T.; Murata, K.; Shibuya, H.; Furu, M.; Yoshitomi, H.; Fujii, T.; Yamamoto, K.; Matsuda, S. MCP/CCR2 signaling is essential for recruitment of mesenchymal progenitor cells during the early phase of fracture healing. PLoS ONE 2014, 9, e104954. [Google Scholar] [CrossRef]
- Neunaber, C.; Oestern, S.; Andruszkow, H.; Zeckey, C.; Mommsen, P.; Kutter, D.; Stofen, M.; Krettek, C.; Hildebrand, F. Cytokine productive capacity of alveolar macrophages and Kupffer cells after femoral fracture and blunt chest trauma in a murine trauma model. Immunol. Lett. 2013, 152, 159–166. [Google Scholar] [CrossRef]
- Edderkaoui, B. Potential Role of Chemokines in Fracture Repair. Front. Endocrinol. 2017, 8, 39. [Google Scholar] [CrossRef]
- Bajrami, B.; Zhu, H.; Kwak, H.J.; Mondal, S.; Hou, Q.; Geng, G.; Karatepe, K.; Zhang, Y.C.; Nombela-Arrieta, C.; Park, S.Y.; et al. G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling. J. Exp. Med. 2016, 213, 1999–2018. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Iwasaki, T.; Kitano, S.; Satake, A.; Nomura, S.; Furukawa, T.; Matsui, K.; Sano, H. IL-2-Anti-IL-2 Monoclonal Antibody Immune Complexes Inhibit Collagen-Induced Arthritis by Augmenting Regulatory T Cell Functions. J. Immunol. 2018, 201, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Faust, H.J.; Zhang, H.; Han, J.; Wolf, M.T.; Jeon, O.H.; Sadtler, K.; Pena, A.N.; Chung, L.; Maestas, D.R., Jr.; Tam, A.J.; et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Investig. 2020, 130, 5493–5507. [Google Scholar] [CrossRef] [PubMed]
- Kellner, H. Targeting interleukin-17 in patients with active rheumatoid arthritis: Rationale and clinical potential. Ther. Adv. Musculoskelet. Dis. 2013, 5, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.A.; Furman, B.D.; Kraus, V.B.; Huebner, J.L.; Guilak, F. Therapeutic opportunities to prevent post-traumatic arthritis: Lessons from the natural history of arthritis after articular fracture. J. Orthop. Res. 2015, 33, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef]
- Huebner, J.L.; Hanes, M.A.; Beekman, B.; TeKoppele, J.M.; Kraus, V.B. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthr. Cartil. 2002, 10, 758–767. [Google Scholar] [CrossRef]
- Meinberg, E.G.; Agel, J.; Roberts, C.S.; Karam, M.D.; Kellam, J.F. Fracture and Dislocation Classification Compendium-2018. J. Orthop. Trauma 2018, 32, S1–S170. [Google Scholar] [CrossRef]
- Kraus, V.B.; Stabler, T.V.; Kong, S.Y.; Varju, G.; McDaniel, G. Measurement of synovial fluid volume using urea. Osteoarthr. Cartil. 2007, 15, 1217–1220. [Google Scholar] [CrossRef]
- Rieppo, L.; Janssen, L.; Rahunen, K.; Lehenkari, P.; Finnila, M.A.J.; Saarakkala, S. Histochemical quantification of collagen content in articular cartilage. PLoS ONE 2019, 14, e0224839. [Google Scholar] [CrossRef]



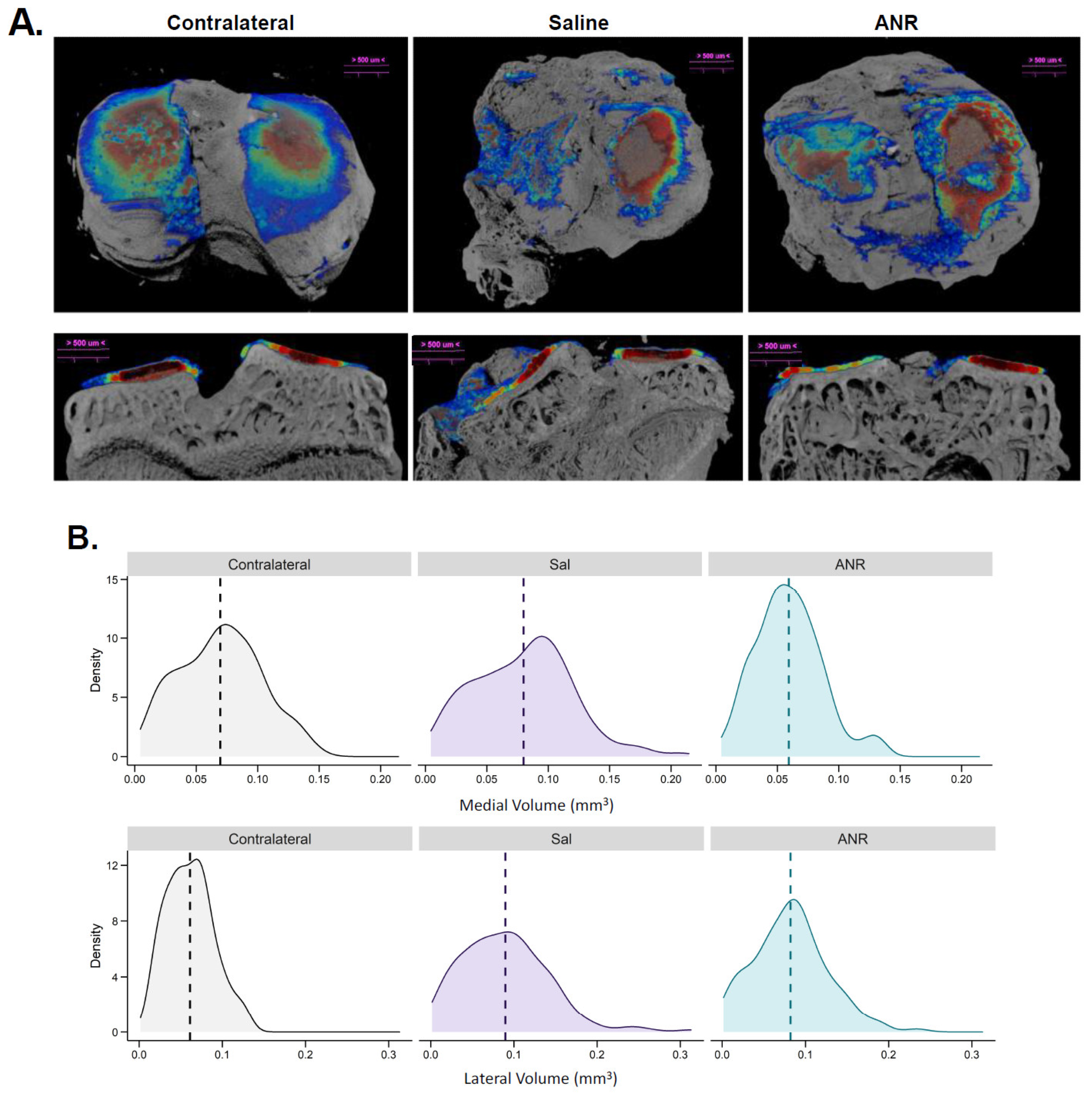
| Measurement | ID | Unit | Contralateral Mean ± SEM | Saline Mean ± SEM | ANR Mean ± SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| CTRL vs. Saline | CTRL vs. ARN | Saline vs. ARN | ||||||
| Tissue volume | TV | mm3 | 21.48 ± 0.72 | 17.00 ± 1.27 | 22.22 ± 2.44 | 0.060 | 0.696 | 0.058 |
| Bone volume | BV | mm3 | 5.54 ± 0.16 | 3.66 ± 0.47 | 4.57 ± 0.46 | <0.001 | 0.036 | 0.083 |
| Bone volume fraction | BV/TV | - | 25.84 ± 0.24 | 21.57 ± 2.29 | 20.82 ± 1.20 | 0.012 | 0.004 | 0.686 |
| Tissue surface | TS | mm2 | 91.15 ± 2.81 | 73.40 ± 5.08 | 94.04 ± 9.64 | 0.024 | 0.700 | 0.023 |
| Bone surface | BS | mm2 | 103.69 ± 1.77 | 65.41 ± 7.56 | 78.44 ± 7.38 | <0.001 | <0.001 | 0.104 |
| Intersection surface | i.S. | mm2 | 13.06 ± 0.56 | 9.08 ± 1.24 | 11.34 ± 1.28 | 0.004 | 0.189 | 0.138 |
| Specific bone surface | BS/BV | 1/mm | 18.80 ± 0.20 | 18.09 ± 0.34 | 17.31 ± 0.31 | 0.065 | <0.001 | 0.075 |
| Bone surface density | BS/TV | 1/mm | 4.86 ± 0.08 | 3.88 ± 0.39 | 3.59 ± 0.18 | 0.001 | <0.001 | 0.376 |
| Trabecular pattern factor | Tb.Pf | 1/mm | −3.41 ± 0.17 | 0.94 ± 0.93 | 1.95 ± 0.44 | <0.001 | <0.001 | 0.193 |
| Structure model index | SMI | - | 0.89 ± 0.02 | 1.39 ± 0.14 | 1.56 ± 0.06 | <0.001 | <0.001 | 0.149 |
| Trabecular thickness | Tb.Th | µm | 190.51 ± 1.62 | 213.19 ± 4.76 | 223.40 ± 3.56 | <0.001 | <0.001 | 0.040 |
| Trabecular number | Tb.N | 1/mm | 1.36 ± 0.02 | 1.02 ± 0.12 | 0.93 ± 0.05 | <0.001 | <0.001 | 0.337 |
| Trabecular separation | Tb.Sp | µm | 439.24 ± 4.07 | 457.57 ± 13.97 | 477.60 ± 8.08 | 0.105 | 0.002 | 0.125 |
| Degree of anisotropy | DA | - | 1.79 ± 0.03 | 2.43 ± 0.12 | 1.99 ± 0.07 | <0.001 | 0.036 | <0.001 |
| Fractal dimension | FD | - | 4.02 ± 0.03 | 3.60 ± 0.15 | 3.57 ± 0.07 | <0.001 | <0.001 | 0.803 |
| Number of objects | Obj.N | - | 2.44 ± 0.26 | 5.88 ± 2.26 | 9.00 ± 0.98 | 0.031 | <0.001 | 0.085 |
| Volume of open pore space | Po.V(op) | mm3 | 15.94 ± 0.57 | 13.34 ± 1.10 | 17.65 ± 2.10 | 0.112 | 0.292 | 0.026 |
| Open porosity (percent) | Po(op) | % | 74.16 ± 0.24 | 78.43 ± 2.29 | 79.18 ± 1.20 | 0.012 | 0.004 | 0.686 |
| Euler number | Eu.N | - | −279.60 ± 6.50 | −121.00 ± 20.06 | −110.00 ± 14.70 | <0.001 | <0.001 | 0.560 |
| Connectivity | Conn | - | 282.30 ± 6.39 | 126.90 ± 18.38 | 119.30 ± 14.68 | <0.001 | <0.001 | 0.687 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, M.S.; Edwards, J.B.; Dolan, C.P.; Motherwell, J.M.; Potter, B.K.; Dearth, C.L.; Goldman, S.M. Effect of Targeted Cytokine Inhibition on Progression of Post-Traumatic Osteoarthritis Following Intra-Articular Fracture. Int. J. Mol. Sci. 2023, 24, 13606. https://doi.org/10.3390/ijms241713606
Valerio MS, Edwards JB, Dolan CP, Motherwell JM, Potter BK, Dearth CL, Goldman SM. Effect of Targeted Cytokine Inhibition on Progression of Post-Traumatic Osteoarthritis Following Intra-Articular Fracture. International Journal of Molecular Sciences. 2023; 24(17):13606. https://doi.org/10.3390/ijms241713606
Chicago/Turabian StyleValerio, Michael S., Jorge B. Edwards, Connor P. Dolan, Jessica M. Motherwell, Benjamin K. Potter, Christopher L. Dearth, and Stephen M. Goldman. 2023. "Effect of Targeted Cytokine Inhibition on Progression of Post-Traumatic Osteoarthritis Following Intra-Articular Fracture" International Journal of Molecular Sciences 24, no. 17: 13606. https://doi.org/10.3390/ijms241713606
APA StyleValerio, M. S., Edwards, J. B., Dolan, C. P., Motherwell, J. M., Potter, B. K., Dearth, C. L., & Goldman, S. M. (2023). Effect of Targeted Cytokine Inhibition on Progression of Post-Traumatic Osteoarthritis Following Intra-Articular Fracture. International Journal of Molecular Sciences, 24(17), 13606. https://doi.org/10.3390/ijms241713606







