Acrolein Induces Retinal Abnormalities of Alzheimer’s Disease in Mice
Abstract
:1. Introduction
2. Results
2.1. Acrolein Induced Memory and Learning Impairment in Mice
2.2. Acrolein Induced Apparent Olfactory Impairment in Mice
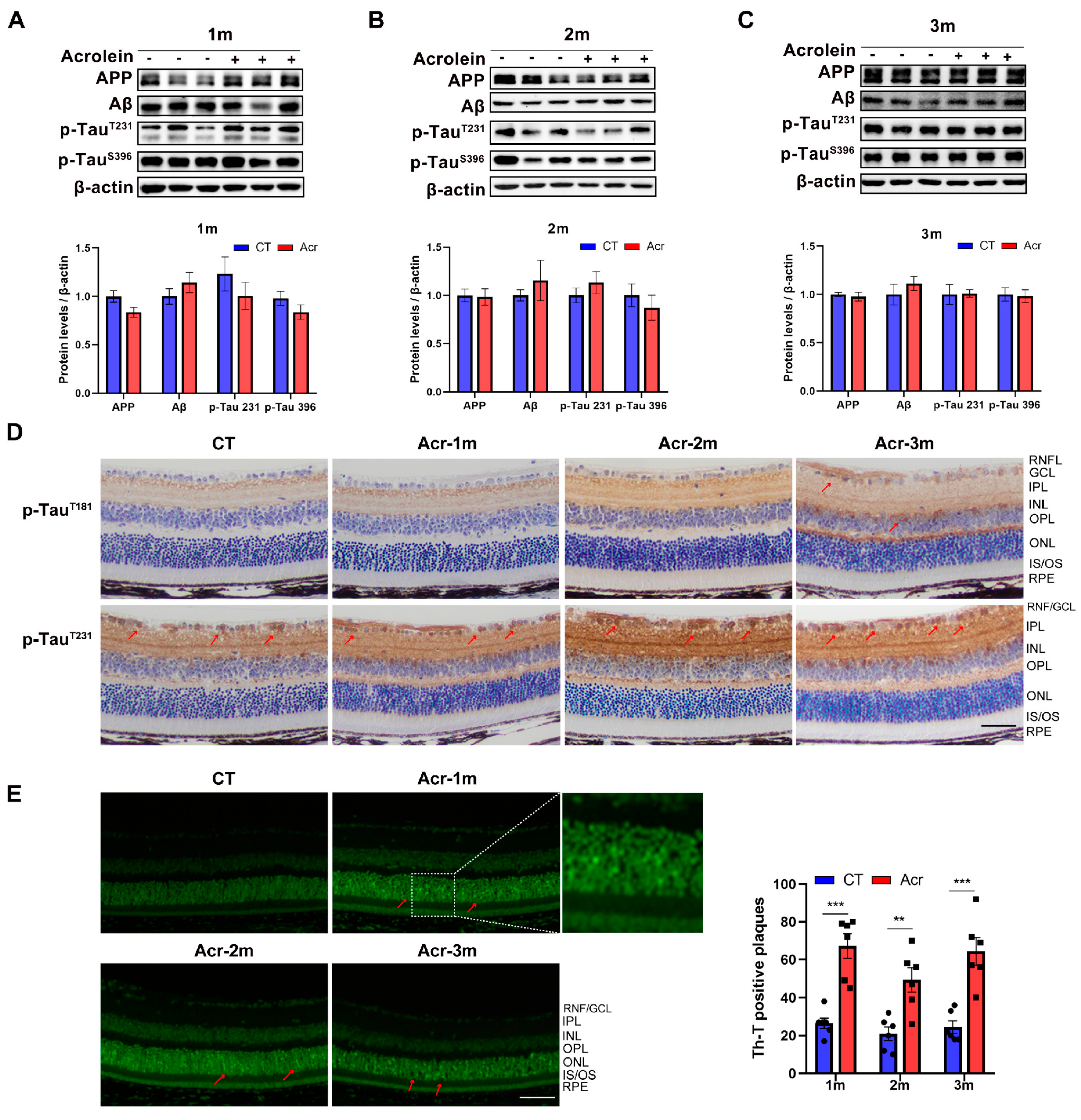
2.3. Acrolein Induced Tau Accumulation in Mice Retina
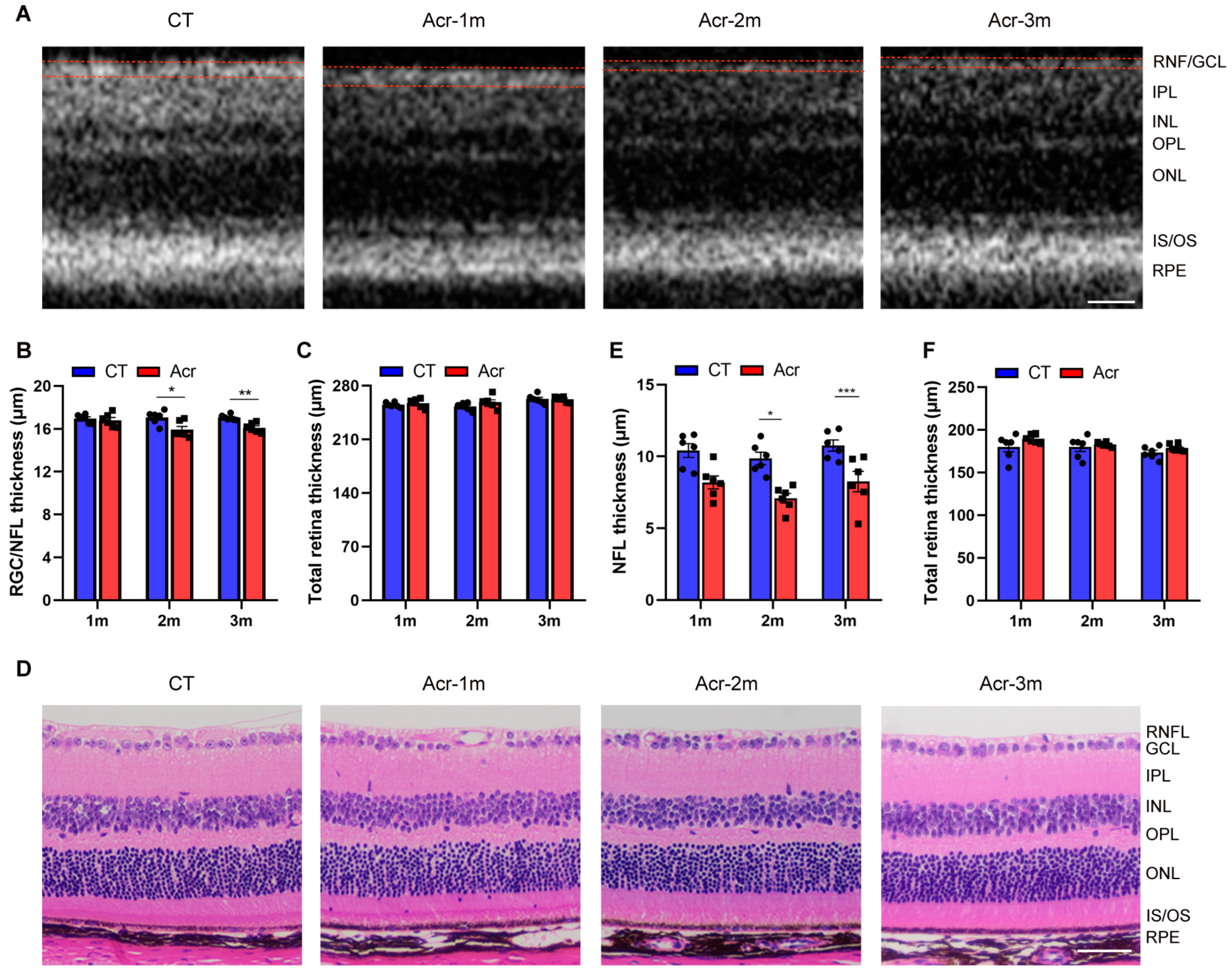
2.4. Acrolein Induced RNF/GCL Thickness Decrease in Mice
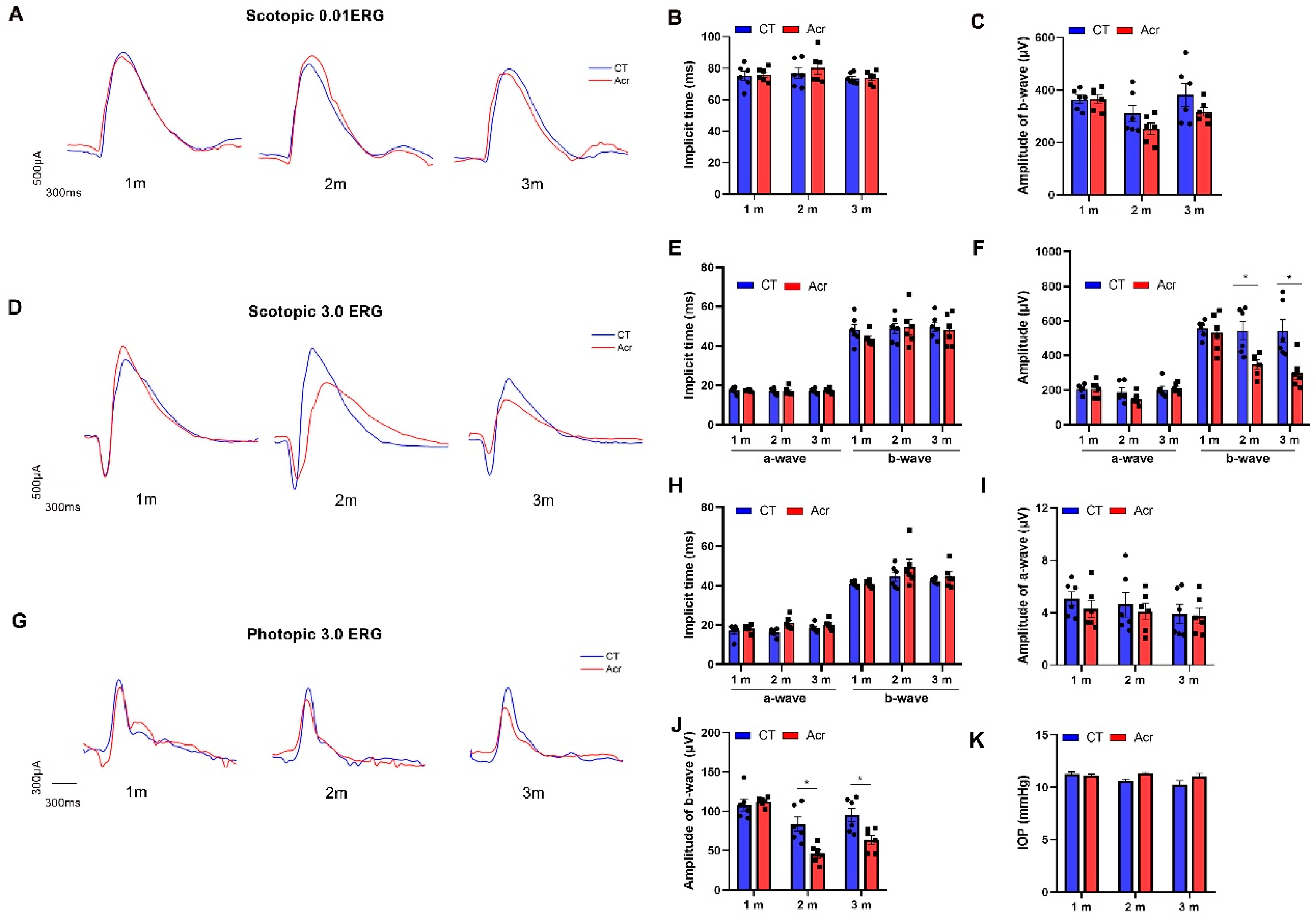
2.5. Acrolein Induced Abnormal Visual Function in Mice
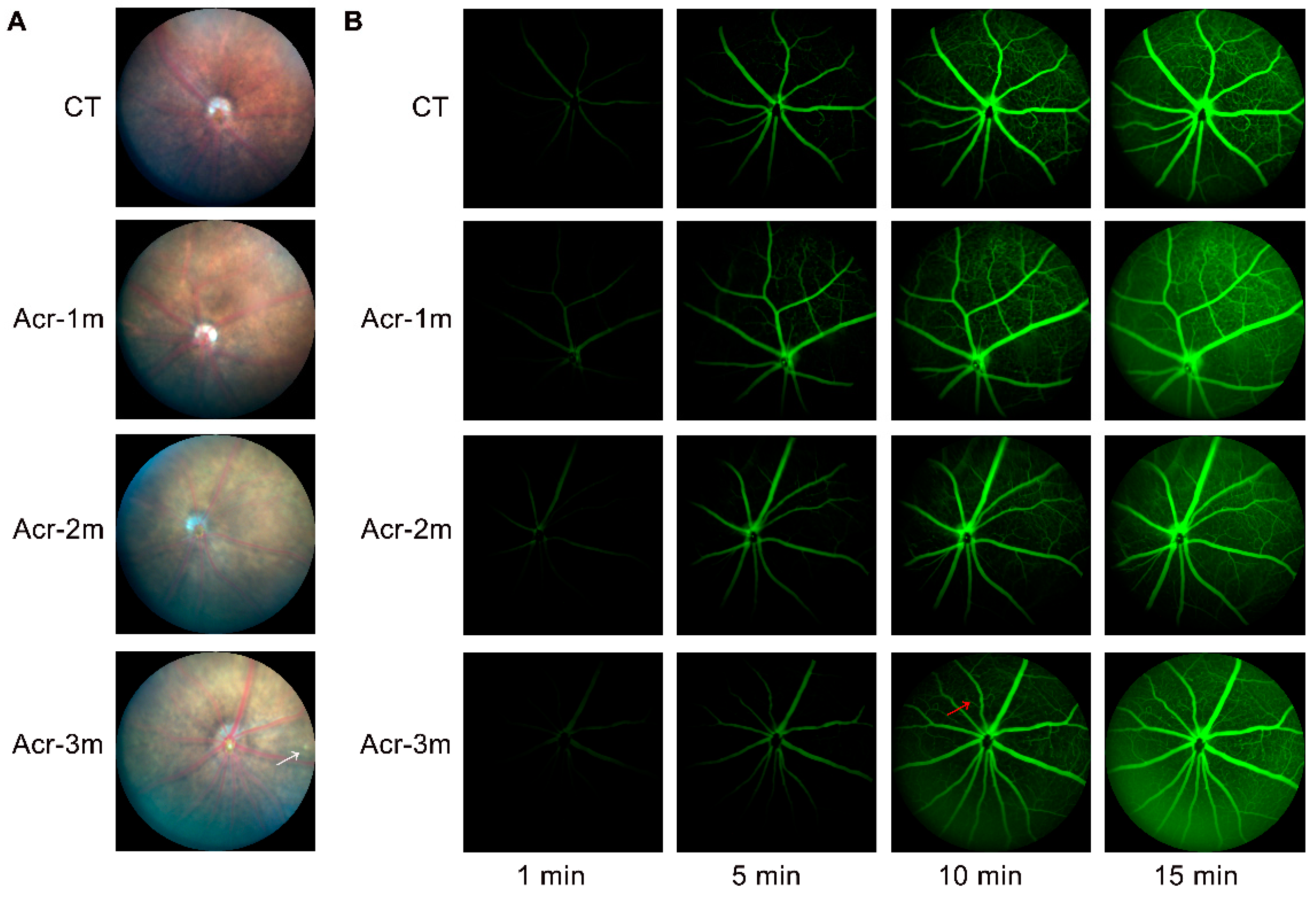
2.6. Acrolein Caused Mild Retinal Vascular Damage
3. Discussion
4. Materials and Methods
4.1. Experimental Model and Subject Details
4.2. STT
4.3. MWMT
4.4. BFPT
4.5. Western Blotting Analysis
4.6. Histological Examinations
4.7. IHC
4.8. OCT Imaging
4.9. IOP Measure
4.10. ERG Recording
4.11. FFA
4.12. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [CrossRef] [PubMed]
- Zhang, L.; Chen, C.; Mak, M.S.; Lu, J.; Wu, Z.; Chen, Q.; Han, Y.; Li, Y.; Pi, R. Advance of sporadic Alzheimer’s disease animal models. Med. Res. Rev. 2020, 40, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Matyas, N.; Keser Aschenberger, F.; Wagner, G.; Teufer, B.; Auer, S.; Gisinger, C.; Kil, M.; Klerings, I.; Gartlehner, G. Continuing education for the prevention of mild cognitive impairment and Alzheimer’s-type dementia: A systematic review and overview of systematic reviews. BMJ Open 2019, 9, e027719. [Google Scholar] [CrossRef] [PubMed]
- Dorszewska, J.; Prendecki, M.; Oczkowska, A.; Dezor, M.; Kozubski, W. Molecular basis of familial and sporadic Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 952–963. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Barbieri, A. Targeting the microbiota in pharmacology of psychiatric disorders. Pharmacol. Res. 2020, 157, 104856. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular neurodegenerative diseases: Interconnection between retina and cortical areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef]
- Frost, S.; Kanagasingam, Y.; Sohrabi, H.; Vignarajan, J.; Bourgeat, P.; Salvado, O.; Villemagne, V.; Rowe, C.C.; Macaulay, S.L.; Szoeke, C.; et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl. Psychiatry 2013, 3, e233. [Google Scholar] [CrossRef]
- Salamone, G.; Di Lorenzo, C.; Mosti, S.; Lupo, F.; Cravello, L.; Palmer, K.; Musicco, M.; Caltagirone, C. Color discrimination performance in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009, 27, 501–507. [Google Scholar] [CrossRef]
- Cormack, F.K.; Tovee, M.; Ballard, C. Contrast sensitivity and visual acuity in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2000, 15, 614–620. [Google Scholar] [CrossRef]
- Salobrar-García, E.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Rojas, P.; Vazirani, R.; Amarante, C.; Yubero, R.; Gil, P.; Pinazo-Durán, M.D.; et al. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PLoS ONE 2019, 14, e0220535. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.R.; Owsley, C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol. Clin. 2003, 21, 709–728. [Google Scholar] [CrossRef]
- Liao, C.; Xu, J.; Chen, Y.; Ip, N.Y. Retinal Dysfunction in Alzheimer’s Disease and Implications for Biomarkers. Biomolecules 2021, 11, 1215. [Google Scholar] [CrossRef]
- Lim, J.K.H.; Li, Q.X.; He, Z.; Vingrys, A.J.; Chinnery, H.R.; Mullen, J.; Bui, B.V.; Nguyen, C.T.O. Retinal functional and structural changes in the 5xFAD mouse model of Alzheimer’s disease. Front. Neurosci. 2020, 14, 862. [Google Scholar] [CrossRef]
- Chan, V.T.T.; Sun, Z.; Tang, S.; Chen, L.J.; Wong, A.; Tham, C.C.; Wong, T.Y.; Chen, C.; Ikram, M.K.; Whitson, H.E.; et al. Spectral-Domain OCT Measurements in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011, 54 (Suppl. S1), S204–S217. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2, e93621. [Google Scholar] [CrossRef]
- Schön, C.; Hoffmann, N.A.; Ochs, S.M.; Burgold, S.; Filser, S.; Steinbach, S.; Seeliger, M.W.; Arzberger, T.; Goedert, M.; Kretzschmar, H.A.; et al. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS ONE 2012, 7, e53547. [Google Scholar] [CrossRef]
- Chiasseu, M.; Alarcon-Martinez, L.; Belforte, N.; Quintero, H.; Dotigny, F.; Destroismaisons, L.; Vande Velde, C.; Panayi, F.; Louis, C.; Di Polo, A. Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 58. [Google Scholar] [CrossRef]
- Gasparini, L.; Crowther, R.A.; Martin, K.R.; Berg, N.; Coleman, M.; Goedert, M.; Spillantini, M.G. Tau inclusions in retinal ganglion cells of human P301S tau transgenic mice: Effects on axonal viability. Neurobiol. Aging 2011, 32, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Chiquita, S.; Rodrigues-Neves, A.C.; Baptista, F.I.; Carecho, R.; Moreira, P.I.; Castelo-Branco, M.; Ambrosio, A.F. The retina as a window or mirror of the brain changes detected in Alzheimer’s disease: Critical aspects to unravel. Mol. Neurobiol. 2019, 56, 5416–5435. [Google Scholar] [CrossRef]
- Chen, C.; Lu, J.; Peng, W.; Mak, M.S.; Yang, Y.; Zhu, Z.; Wang, S.; Hou, J.; Zhou, X.; Xin, W.; et al. Acrolein, an endogenous aldehyde induces Alzheimer’s disease-like pathologies in mice: A new sporadic AD animal model. Pharmacol. Res. 2021, 175, 106003. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xie, C.; Markesbery, W.R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 2001, 22, 187–194. [Google Scholar] [CrossRef]
- Williams, T.I.; Lynn, B.C.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Nardini, E.; Hogan, R.; Flamier, A.; Bernier, G. Alzheimer’s disease: A tale of two diseases? Neural Regen. Res. 2021, 16, 1958–1964. [Google Scholar] [PubMed]
- Athar, T.; Al Balushi, K.; Khan, S.A. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 5629–5645. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, W.; Wu, Q.; Zhou, Q. Acrolein: Formation, health hazards and its controlling by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2023, 1–14. [Google Scholar] [CrossRef]
- Perez, S.E.; Lumayag, S.; Kovacs, B.; Mufson, E.J.; Xu, S. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Investig. Ophthalmol. Vis. Sci. 2009, 50, 793–800. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, R.; Che, H.; Wang, J.; Yang, L.; Fang, W.; Xia, Y.; Li, N.; Ma, Q.; Wang, X. Hyperphosphorylation of tau protein by calpain regulation in retina of Alzheimer’s disease transgenic mouse. Neurosci. Lett. 2013, 551, 12–16. [Google Scholar] [CrossRef]
- Rodrigues-Neves, A.C.; Carecho, R.; Correia, S.C.; Carvalho, C.; Campos, E.J.; Baptista, F.I.; Moreira, P.I.; Ambrosio, A.F. Retina and brain display early and differential molecular and cellular changes in the 3xTG-AD mouse model of Alzheimer’s disease. Mol. Neurobiol. 2021, 58, 3043–3060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, F.; Ma, Y.; Xue, T.; Shen, Y. Identification of early-onset photoreceptor degeneration in transgenic mouse models of Alzheimer’s disease. iScience 2021, 24, 103327. [Google Scholar] [CrossRef]
- Lazarov, O.; Lee, M.; Peterson, D.A.; Sisodia, S.S. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J. Neurosci. 2002, 22, 9785–9793. [Google Scholar] [CrossRef] [PubMed]
- More, S.S.; Vince, R. Hyperspectral imaging signatures detect amyloidopathy in Alzheimer’s mouse retina well before onset of cognitive decline. ACS Chem. Neurosci. 2015, 6, 306–315. [Google Scholar] [CrossRef]
- Lim, J.K.H.; Li, Q.X.; Ryan, T.; Bedggood, P.; Metha, A.; Vingrys, A.J.; Bui, B.V.; Nguyen, C.T.O. Retinal hyperspectral imaging in the 5xFAD mouse model of Alzheimer’s disease. Sci. Rep. 2021, 11, 6387. [Google Scholar] [CrossRef]
- Kromer, R.; Serbecic, N.; Hausner, L.; Froelich, L.; Aboul-Enein, F.; Beutelspacher, S.C. Detection of retinal nerve fiber layer defects in Alzheimer’s disease using SD-OCT. Front. Psychiatry 2014, 5, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Buccarello, L.; Sclip, A.; Sacchi, M.; Castaldo, A.M.; Bertani, I.; ReCecconi, A.; Maestroni, S.; Zerbini, G.; Nucci, P.; Borsello, T. The c-jun N-terminal kinase plays a key role in ocular degenerative changes in a mouse model of Alzheimer disease suggesting a correlation between ocular and brain pathologies. Oncotarget 2017, 8, 83038–83051. [Google Scholar] [CrossRef]
- Parisi, V.; Restuccia, R.; Fattapposta, F.; Mina, C.; Bucci, M.G.; Pierelli, F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin. Neurophysiol. 2001, 112, 1860–1867. [Google Scholar] [CrossRef]
- Li, Z.B.; Lin, Z.J.; Li, N.; Yu, H.; Wu, Y.L.; Shen, X. Evaluation of retinal and choroidal changes in patients with Alzheimer’s type dementia using optical coherence tomography angiography. Int. J. Ophthalmol. 2021, 14, 860–868. [Google Scholar] [CrossRef] [PubMed]
- McAnany, J.J.; Matei, N.; Chen, Y.F.; Liu, K.; Park, J.C.; Shahidi, M. Rod pathway and cone pathway retinal dysfunction in the 5xFAD mouse model of Alzheimer’s disease. Sci. Rep. 2021, 11, 4824. [Google Scholar] [CrossRef]
- Vandenabeele, M.; Veys, L.; Lemmens, S.; Hadoux, X.; Gelders, G.; Masin, L.; Serneels, L.; Theunis, J.; Saito, T.; Saido, T.C.; et al. The App(NL-G-F) mouse retina is a site for preclinical Alzheimer’s disease diagnosis and research. Acta Neuropathol. Commun. 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Regis, G.C.; Sheyn, J.; Fuchs, D.-T.; Kramerov, A.A.; Ljubimov, A.V.; Dumitrascu, O.M.; Rodriguez, A.R.; et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020, 139, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.A.; Dantas, M.A. Retinal venous beading associated with recurrent branch vein occlusion. Can. J. Ophthalmol. 2002, 37, 182–183. [Google Scholar] [CrossRef]
- Abdel-Hay, A.F.; Raman, V. Unilateral retinal venous beading. BMJ Case Rep. 2018, 11, bcr2018226116. [Google Scholar] [CrossRef]
- Van Dam, D.; D’Hooge, R.; Staufenbiel, M.; Van Ginneken, C.; Van Meir, F.; De Deyn, P.P. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur. J. Neurosci. 2003, 17, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Rao, X.; Chai, T.; Zeng, B.; Zhang, X.; Yu, Y.; Zhou, C.; Pu, J.; Zhou, W.; et al. Commensal microbiota regulation of metabolic networks during olfactory dysfunction in mice. Neuropsychiatr. Dis. Treat. 2020, 16, 761–769. [Google Scholar] [CrossRef]
- Chidlow, G.; Wood, J.P.; Manavis, J.; Finnie, J.; Casson, R.J. Investigations into Retinal Pathology in the Early Stages of a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 56, 655–675. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, J.H.; Mook-Jung, I.; Kim, K.W.; Park, W.J.; Park, K.H.; Kim, J.H. Intracellular amyloid beta alters the tight junction of retinal pigment epithelium in 5XFAD mice. Neurobiol. Aging 2014, 35, 2013–2020. [Google Scholar] [CrossRef]
- Gabriele, M.L.; Ishikawa, H.; Schuman, J.S.; Ling, Y.; Bilonick, R.A.; Kim, J.S.; Kagemann, L.; Wollstein, G. Optic nerve crush mice followed longitudinally with spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2250–2254. [Google Scholar] [CrossRef]
- Georgiou, A.L.; Guo, L.; Francesca Cordeiro, M.; Salt, T.E. Electroretinogram and visual-evoked potential assessment of retinal and central visual function in a rat ocular hypertension model of glaucoma. Curr. Eye Res. 2014, 39, 472–486. [Google Scholar] [CrossRef]
- Robson, A.G.; Nilsson, J.; Li, S.; Jalali, S.; Fulton, A.B.; Tormene, A.P.; Holder, G.E.; Brodie, S.E. ISCEV guide to visual electrodiagnostic procedures. Doc. Ophthalmol. 2018, 136, 1–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jiang, X.; Peng, W.; Yang, S.; Pi, R.; Zhou, S. Acrolein Induces Retinal Abnormalities of Alzheimer’s Disease in Mice. Int. J. Mol. Sci. 2023, 24, 13576. https://doi.org/10.3390/ijms241713576
Wang S, Jiang X, Peng W, Yang S, Pi R, Zhou S. Acrolein Induces Retinal Abnormalities of Alzheimer’s Disease in Mice. International Journal of Molecular Sciences. 2023; 24(17):13576. https://doi.org/10.3390/ijms241713576
Chicago/Turabian StyleWang, Shuyi, Xiuying Jiang, Weijia Peng, Shuangjian Yang, Rongbiao Pi, and Shiyou Zhou. 2023. "Acrolein Induces Retinal Abnormalities of Alzheimer’s Disease in Mice" International Journal of Molecular Sciences 24, no. 17: 13576. https://doi.org/10.3390/ijms241713576
APA StyleWang, S., Jiang, X., Peng, W., Yang, S., Pi, R., & Zhou, S. (2023). Acrolein Induces Retinal Abnormalities of Alzheimer’s Disease in Mice. International Journal of Molecular Sciences, 24(17), 13576. https://doi.org/10.3390/ijms241713576



