Preliminary Comparison of Molecular Antioxidant and Inflammatory Mechanisms Determined in the Peripheral Blood Granulocytes of COVID-19 Patients
Abstract
:1. Introduction
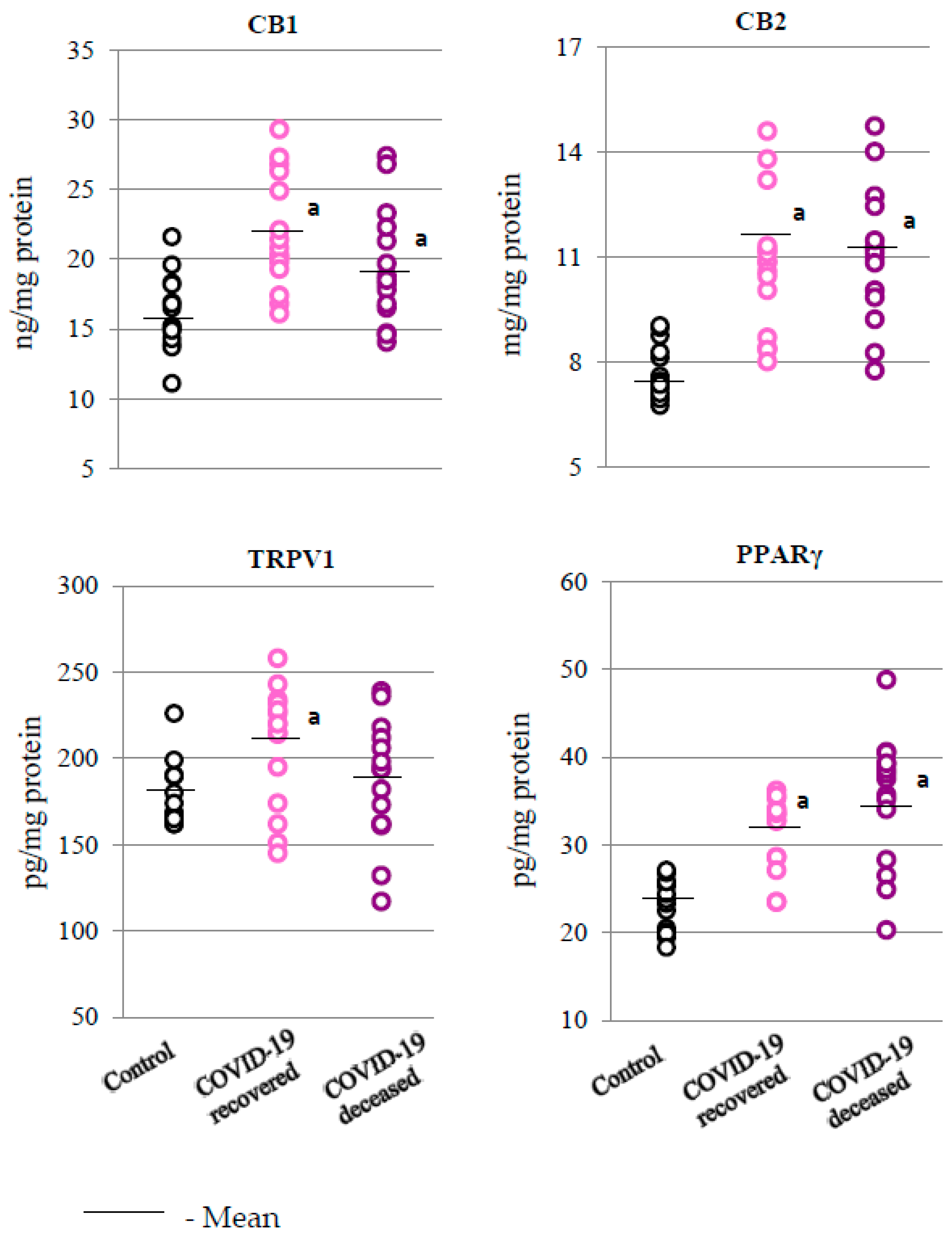
2. Results
2.1. Comparison of Laboratory Parameters of the Two Groups of COVID-19 Patients with Normal Values
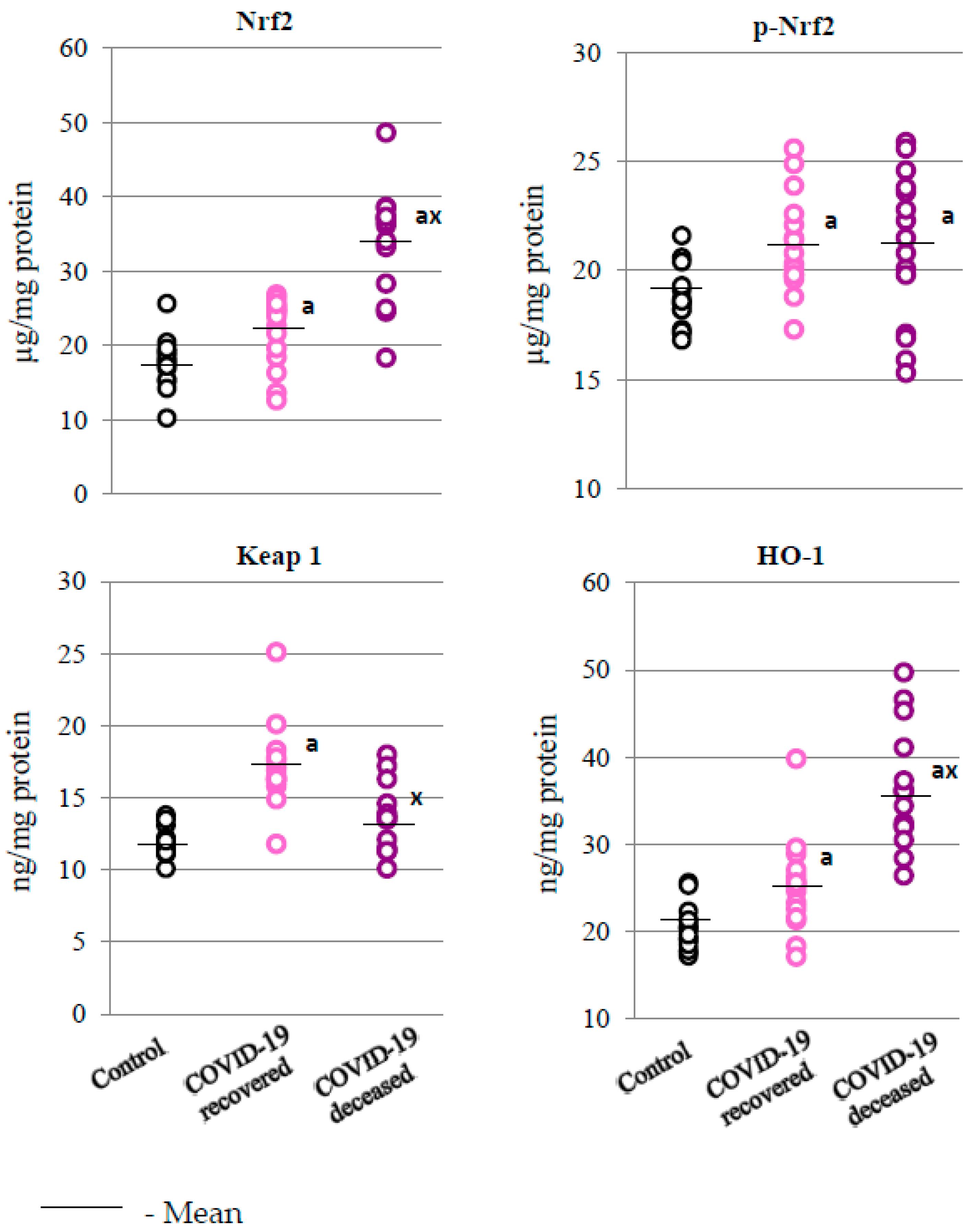
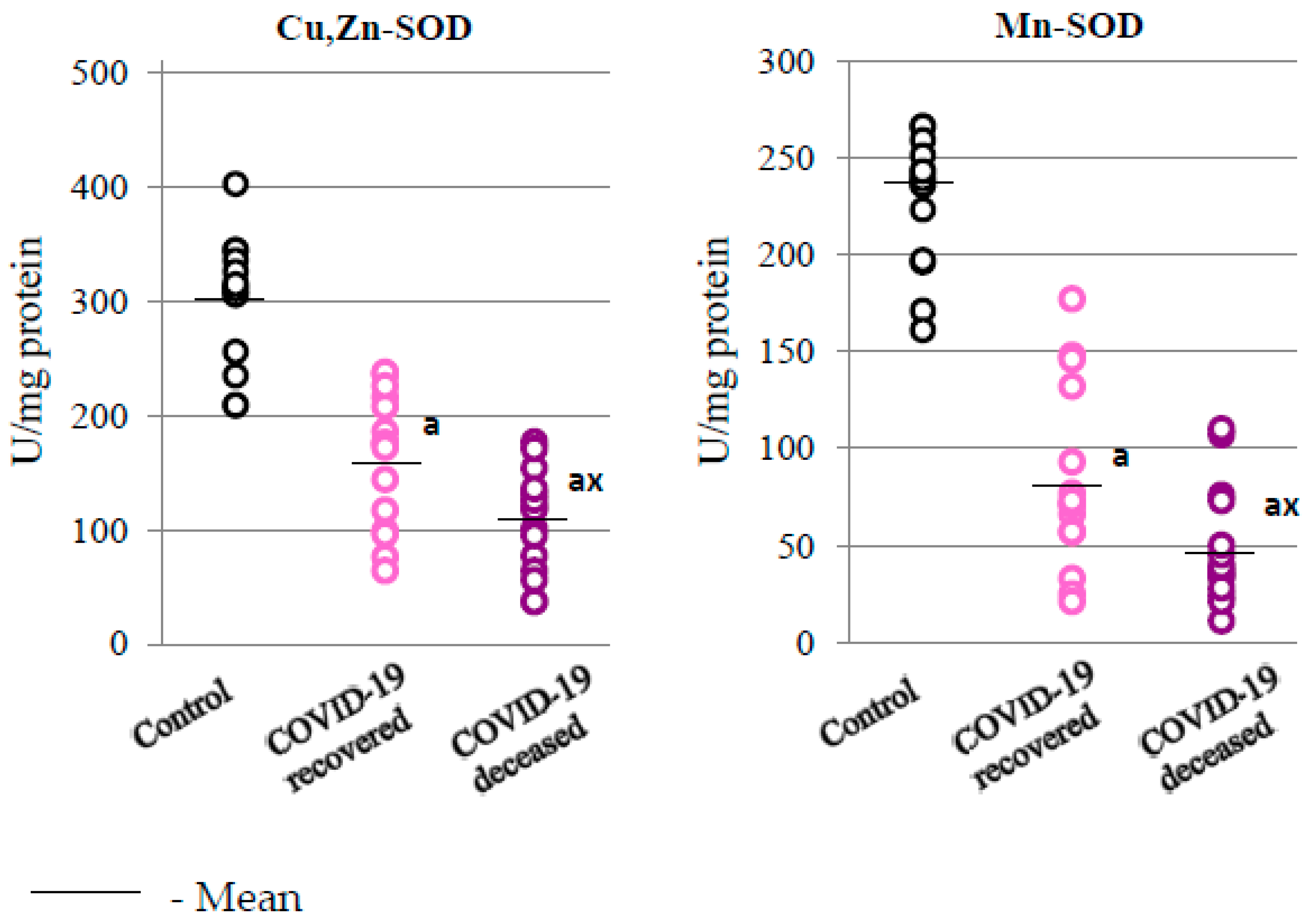
2.2. SARS-CoV-2 and the Antioxidant Response
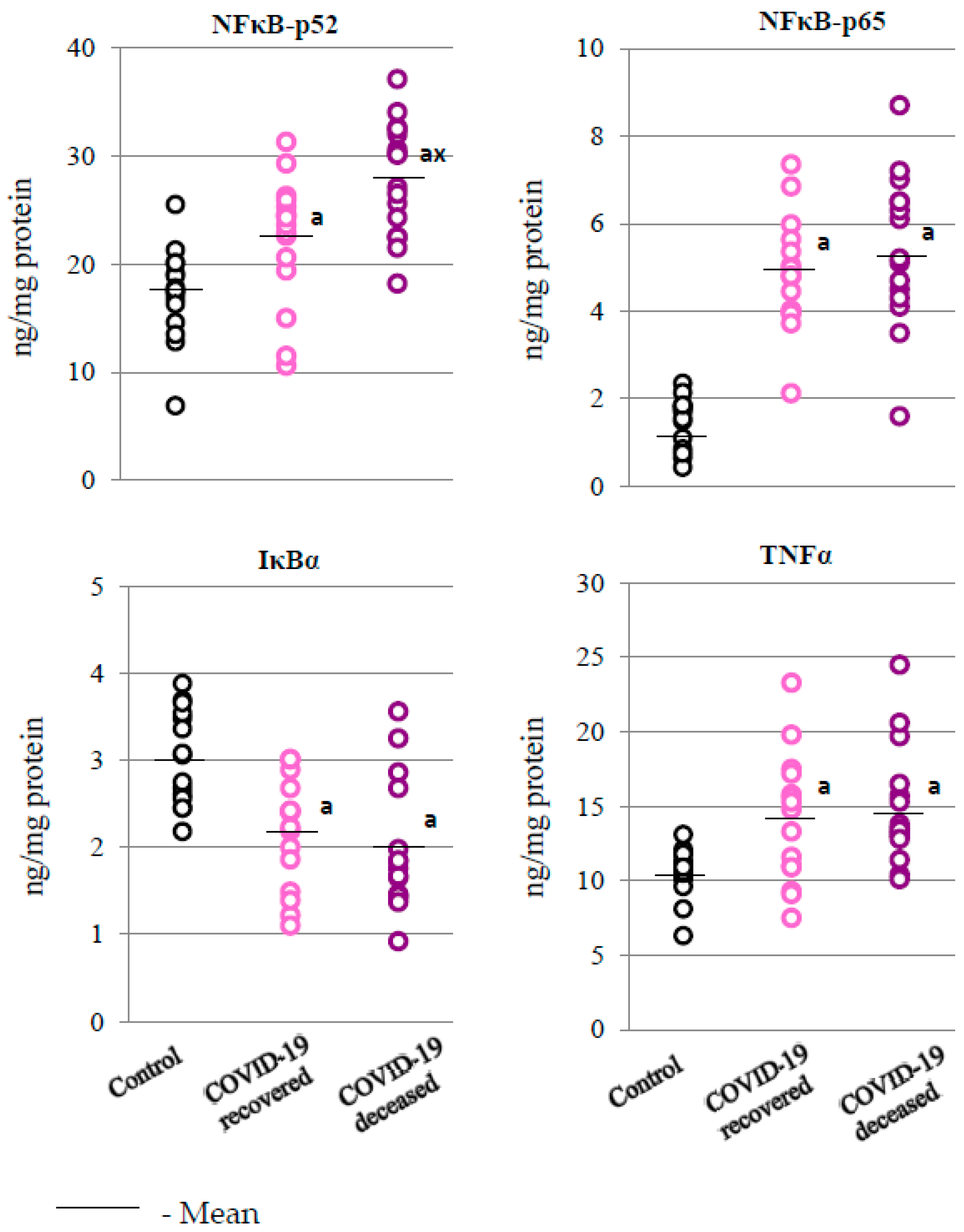
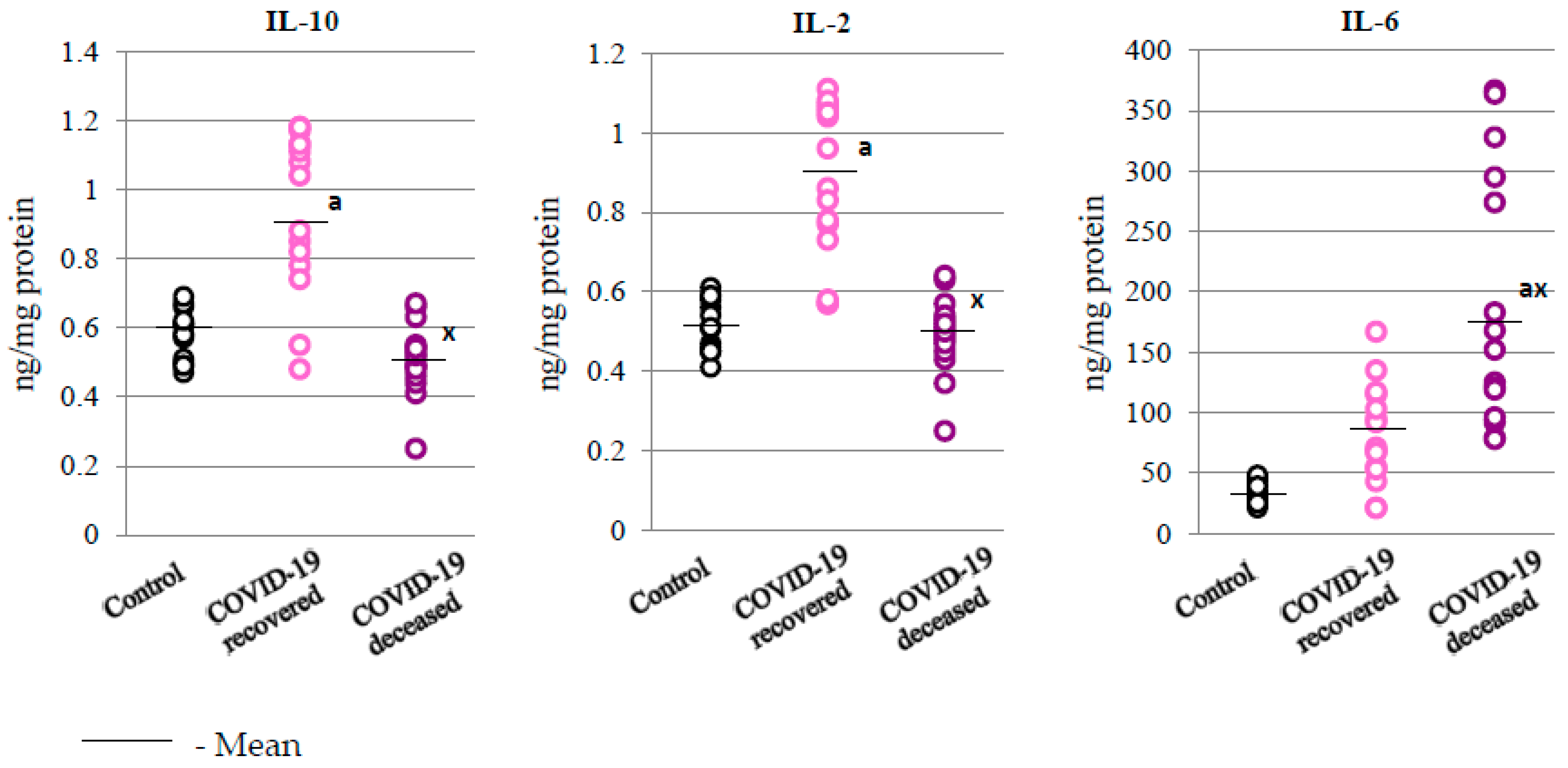
2.3. SARS-CoV-2 and the Body’s Response to Inflammation
3. Discussion
4. Materials and Methods
4.1. Samples Collection
4.2. Determination of Superoxide Dismutases Activity
4.3. Determination of Expression of the Analyzed Proteins
4.4. Determination of the Level of Eicosanoids
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzi, M.; D’Onghia, D.; Tonello, S.; Minisini, R.; Colangelo, D.; Bellan, M.; Castello, L.M.; Gavelli, F.; Avanzi, G.C.; Pirisi, M.; et al. COVID-19 Biomarkers at the Crossroad between Patient Stratification and Targeted Therapy: The Role of Validated and Proposed Parameters. Int. J. Mol. Sci. 2023, 24, 7099. [Google Scholar] [CrossRef] [PubMed]
- Passariello, M.; Esposito, S.; Manna, L.; Rapuano Lembo, R.; Zollo, I.; Sasso, E.; Amato, F.; De Lorenzo, C. Comparative Analysis of a Human Neutralizing MAb Specific for SARS-CoV-2 Spike-RBD with Cilgavimab and Tixagevimab for the Efficacy on the Omicron Variant in Neutralizing and Detection Assays. Int. J. Mol. Sci. 2023, 24, 10053. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Reusch, N.; De Domenico, E.; Bonaguro, L.; Schulte-Schrepping, J.; Baßler, K.; Schultze, J.L.; Aschenbrenner, A.C. Neutrophils in COVID-19. Front. Immunol. 2021, 12, 652470. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.; Turk, M.; Jenne, C.N.; Kim, S.-J. Neutrophils in Viral Infection. Cell Tissue Res. 2018, 371, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, E.M.; Ivanova, E.; Grechko, A.V.; Wu, W.-K.; Starodubova, A.V.; Orekhov, A.N. Involvement of Oxidative Stress and the Innate Immune System in SARS-CoV-2 Infection. Diseases 2021, 9, 17. [Google Scholar] [CrossRef]
- Fernandes, I.G.; de Brito, C.A.; Dos Reis, V.M.S.; Sato, M.N.; Pereira, N.Z. SARS-CoV-2 and Other Respiratory Viruses: What Does Oxidative Stress Have to Do with It? Oxid. Med. Cell. Longev. 2020, 2020, 8844280. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The Signal Pathways and Treatment of Cytokine Storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 Cytokine Storm: The Interplay between Inflammation and Coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Manik, M.; Singh, R.K. Role of Toll-like Receptors in Modulation of Cytokine Storm Signaling in SARS-CoV-2-Induced COVID-19. J. Med. Virol. 2021, 94, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of Control in Inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ripon, M.A.R.; Bhowmik, D.R.; Amin, M.T.; Hossain, M.S. Role of Arachidonic Cascade in COVID-19 Infection: A Review. Prostaglandins Other Lipid Mediat. 2021, 154, 106539. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Lawler, N.G.; Zeng, A.X.; Ryan, M.; Bong, S.H.; Boughton, B.A.; Bizkarguenaga, M.; Bruzzone, C.; Embade, N.; Wist, J.; et al. Diagnostic Potential of the Plasma Lipidome in Infectious Disease: Application to Acute SARS-CoV-2 Infection. Metabolites 2021, 11, 467. [Google Scholar] [CrossRef]
- Esser-von Bieren, J. Immune-Regulation and -Functions of Eicosanoid Lipid Mediators. Biol. Chem. 2017, 398, 1177–1191. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Huang, S.; Gartung, A.; Cortés-Puch, I.; Sime, P.J.; Phipps, R.P.; Serhan, C.N.; Hammock, B.D. Inflammation Resolution: A Dual-Pronged Approach to Averting Cytokine Storms in COVID-19? Cancer Metastasis Rev. 2020, 39, 337–340. [Google Scholar] [CrossRef]
- Parackova, Z.; Zentsova, I.; Bloomfield, M.; Vrabcova, P.; Smetanova, J.; Klocperk, A.; Mesežnikov, G.; Casas Mendez, L.F.; Vymazal, T.; Sediva, A. Disharmonic Inflammatory Signatures in COVID-19: Augmented Neutrophils’ but Impaired Monocytes’ and Dendritic Cells’ Responsiveness. Cells 2020, 9, 2206. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Cotta, L.A.; Brodt, J.K.; Hurst, J.W.; Ogle, C.K. Neutrophil Dysfunction in Sepsis. III. Degranulation as a Mechanism for Nonspecific Deactivation. J. Surg. Res. 1984, 36, 407–412. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Berg, D.J.; Zhang, J.; Lauricella, D.M.; Moore, S.A. Il-10 Is a Central Regulator of Cyclooxygenase-2 Expression and Prostaglandin Production. J. Immunol. 2001, 166, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Navarro, E.; Arribas Gómez, I.; Bernabeu-Andreu, F.A. IL-6 and Other Biomarkers Associated with Poor Prognosis in a Cohort of Hospitalized Patients with COVID-19 in Madrid. Biomark. Insights 2021, 16, 11772719211013364. [Google Scholar] [CrossRef] [PubMed]
- Ankerhold, J.; Giese, S.; Kolb, P.; Maul-Pavicic, A.; Voll, R.E.; Göppert, N.; Ciminski, K.; Kreutz, C.; Lother, A.; Salzer, U.; et al. Circulating Multimeric Immune Complexes Contribute to Immunopathology in COVID-19. Nat. Commun. 2022, 13, 5654. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Zarkovic, N.; Jakovcevic, A.; Mataic, A.; Jaganjac, M.; Vukovic, T.; Waeg, G.; Zarkovic, K. Post-Mortem Findings of Inflammatory Cells and the Association of 4-Hydroxynonenal with Systemic Vascular and Oxidative Stress in Lethal COVID-19. Cells 2022, 11, 444. [Google Scholar] [CrossRef]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Singh, D.; Wasan, H.; Reeta, K.H. Heme Oxygenase-1 Modulation: A Potential Therapeutic Target for COVID-19 and Associated Complications. Free Radic. Biol. Med. 2020, 161, 263–271. [Google Scholar] [CrossRef]
- Liu, G.-H.; Qu, J.; Shen, X. NF-KappaB/P65 Antagonizes Nrf2-ARE Pathway by Depriving CBP from Nrf2 and Facilitating Recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-Mediated Suppression of NRF2-Signaling Reveals Potent Antiviral and Anti-Inflammatory Activity of 4-Octyl-Itaconate and Dimethyl Fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.T.; Caster, D.J. The Potential of Nrf2 Activation as a Therapeutic Target in Systemic Lupus Erythematosus. Metabolites 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Hybertson, B.M.; Cota-Gomez, A.; Gao, B. Nrf2 Activator PB125® as a Potential Therapeutic Agent against COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 Signaling in Regulation of Antioxidants and Phase 2 Enzymes in Cardiac Fibroblasts: Protection against Reactive Oxygen and Nitrogen Species-Induced Cell Injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef]
- Žarković, N.; Jastrząb, A.; Jarocka-Karpowicz, I.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.; Łuczaj, W.; Skrzydlewska, E. The Impact of Severe COVID-19 on Plasma Antioxidants. Molecules 2022, 27, 5323. [Google Scholar] [CrossRef] [PubMed]
- Pyo, C.-W.; Shin, N.; Jung, K.I.; Choi, J.H.; Choi, S.-Y. Alteration of Copper-Zinc Superoxide Dismutase 1 Expression by Influenza A Virus Is Correlated with Virus Replication. Biochem. Biophys. Res. Commun. 2014, 450, 711–716. [Google Scholar] [CrossRef]
- Kettle, A.J.; Anderson, R.F.; Hampton, M.B.; Winterbourn, C.C. Reactions of Superoxide with Myeloperoxidase. Biochemistry 2007, 46, 4888–4897. [Google Scholar] [CrossRef]
- Bauer, G. HOCl and the Control of Oncogenesis. J. Inorg. Biochem. 2018, 179, 10–23. [Google Scholar] [CrossRef]
- Attiq, A.; Yao, L.; Afzal, S.; Khan, M. The Triumvirate of NF-ΚB, Inflammation and Cytokine Storm in COVID-19. Int. Immunopharmacol. 2021, 101, 108255. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Villeneuve, N.F.; Lau, A.; Zhang, D.D. Regulation of the Nrf2-Keap1 Antioxidant Response by the Ubiquitin Proteasome System: An Insight into Cullin-Ring Ubiquitin Ligases. Antioxid Redox Signal. 2010, 13, 1699–1712. [Google Scholar] [CrossRef]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in Redox Homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Žarković, N.; Orehovec, B.; Milković, L.; Baršić, B.; Tatzber, F.; Wonisch, W.; Tarle, M.; Kmet, M.; Mataić, A.; Jakovčević, A.; et al. Preliminary Findings on the Association of the Lipid Peroxidation Product 4-Hydroxynonenal with the Lethal Outcome of Aggressive COVID-19. Antioxidants 2021, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-ΚB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.G.; Fiorino, S.; Posabella, G.; Antonacci, D.; Tropeano, A.; Pausini, E.; Pausini, C.; Guarniero, T.; Hong, W.; Giampieri, E.; et al. The Function of Specialized Pro-Resolving Endogenous Lipid Mediators, Vitamins, and Other Micronutrients in the Control of the Inflammatory Processes: Possible Role in Patients with SARS-CoV-2 Related Infection. Prostaglandins Other Lipid Mediat. 2022, 159, 106619. [Google Scholar] [CrossRef]
- Pradelli, L.; Mayer, K.; Klek, S.; Omar Alsaleh, A.J.; Clark, R.A.C.; Rosenthal, M.D.; Heller, A.R.; Muscaritoli, M. ω-3 Fatty-Acid Enriched Parenteral Nutrition in Hospitalized Patients: Systematic Review With Meta-Analysis and Trial Sequential Analysis. J. Parenter. Enter. Nutr. 2020, 44, 44–57. [Google Scholar] [CrossRef]
- Archambault, A.-S.; Younes, Z.; Rakotoarivelo, V.; Doré, E.; Dubuc, I.; Martin, C.; Amar, Y.; Cheikh, A.; Fares, H.; Hassani, A.; et al. Lipid Storm within the Lungs of Severe COVID-19 Patients: Extensive Levels of Cyclooxygenase and Lipoxygenase-Derived Inflammatory Metabolites. medRxiv 2020. [Google Scholar] [CrossRef]
- Smeitink, J.; Jiang, X.; Pecheritsyna, S.; Renkema, H.; van Maanen, R.; Beyrath, J. Hypothesis: MPGES-1-Derived Prostaglandin E2, a So Far Missing Link in COVID-19 Pathophysiology? Preprints 2020, 2020040180. [Google Scholar] [CrossRef]
- Gross, S.; Tilly, P.; Hentsch, D.; Vonesch, J.-L.; Fabre, J.-E. Vascular Wall-Produced Prostaglandin E2 Exacerbates Arterial Thrombosis and Atherothrombosis through Platelet EP3 Receptors. J. Exp. Med. 2007, 204, 311–320. [Google Scholar] [CrossRef]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, Endothelial Injury and Complement-Induced Coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, C.; Wu, J. 15-Deoxy-∆-12,14-Prostaglandin J2 (15d-PGJ2), an Endogenous Ligand of PPAR-γ: Function and Mechanism. PPAR Res. 2019, 2019, 7242030. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-H.; Kim, S.-J.; Na, H.-K.; Han, W.; Kim, N.-J.; Suh, Y.-G.; Surh, Y.-J. 15-Deoxy-Δ12,14-Prostaglandin J2 Upregulates VEGF Expression via NRF2 and Heme Oxygenase-1 in Human Breast Cancer Cells. Cells 2021, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- Hedi, H.; Norbert, G. 5-Lipoxygenase Pathway, Dendritic Cells, and Adaptive Immunity. J. Biomed. Biotechnol. 2004, 2004, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Sharma, C.; Goyal, S.N.; Kumar, S.; Ojha, S. CB2 Receptor-Selective Agonists as Candidates for Targeting Infection, Inflammation, and Immunity in SARS-CoV-2 Infections. Drug Dev. Res. 2021, 82, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Stasiulewicz, A.; Znajdek, K.; Grudzień, M.; Pawiński, T.; Sulkowska, A.J.I. A Guide to Targeting the Endocannabinoid System in Drug Design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef]
- Žarković, N.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.; Tatzber, F.; Wonisch, W.; Skrzydlewska, E.; Łuczaj, W. Lipidomics Revealed Plasma Phospholipid Profile Differences between Deceased and Recovered COVID-19 Patients. Biomolecules 2022, 12, 1488. [Google Scholar] [CrossRef]
- Leung, C. Risk factors for predicting mortality in elderly patients with COVID-19: A review of clinical data in China. Mech. Ageing Dev. 2020, 188, 111255. [Google Scholar] [CrossRef]
- Ruiz-Huerta, C.; Canto, M.V.; Ruiz, C.; González, I.; Lozano-Montoya, I.; Quezada-Feijoo, M.; Gómez-Pavón, F.J. COVID-19 Mortality in Patients Aged 80 and over Residing in Nursing Homes-Six Pandemic Waves: OCTA-COVID Study. Int. J. Environ. Res. Public Health 2022, 19, 12019. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Sykes, J.A.; McCormack, F.X.; O’Brien, T.J. A Preliminary Study of the Superoxide Dismutase Content of Some Human Tumors. Cancer Res. 1978, 38, 2759–2762. [Google Scholar] [PubMed]
- Geller, B.L.; Winge, D.R. A Method for Distinguishing Cu,Zn- and Mn-Containing Superoxide Dismutases. Anal. Biochem. 1983, 128, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, R.; Lin, A.; McGarvey, J.A.; Stanker, L.H. A Rapid Method to Improve Protein Detection by Indirect ELISA. Biochem. Biophys. Res. Commun. 2011, 410, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Kim, J.; Kenny, A.; Pedersen, T.L.; Pappan, K.L.; Newman, J.W. Circulating Levels of Endocannabinoids and Oxylipins Altered by Dietary Lipids in Older Women Are Likely Associated with Previously Identified Gene Targets. Biochim. Biophys. Acta 2016, 1861, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Healthy People Normal Range n = 15 (7F + 8M) | COVID-19 Recovered n = 15 (7F + 8M) | COVID-19 Deceased n = 15 (7F + 8M) | |
|---|---|---|---|
| Average age | 55 (47–58) | 57 (49–71) | 66 (60–73) |
| Neutrophils (%) | 40.0–72.0 | 78.52 ± 5.34 | 88.12 ± 4.87 * |
| Platelets (103/μL) | 150–400 | 287.34 ± 101.21 | 224.17 ± 59.46 |
| Blood oxygen saturation (%) | >95% | 91.32 ± 5.38 | 90.12 ± 5.42 |
| Ferritin (μg/L) | 11–336 | 901 ± 448 | 953 ± 451 |
| PCT (ng/mL) | <0.1 | 0.39 ± 0.32 | 1.10 ± 0.71 * |
| LDH (U/L) | 140–280 | 429 ± 127 | 347 ± 132 |
| CRP (mg/L) | 0.00–5.00 | 135.27 ± 71.24 | 181.75 ± 56.24 |
| IL-6 (pg/mL) | 0–43.5 | 84 ± 41 | 189 ± 96 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzydlewska, E.; Łuczaj, W.; Biernacki, M.; Wójcik, P.; Jarocka-Karpowicz, I.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.; et al. Preliminary Comparison of Molecular Antioxidant and Inflammatory Mechanisms Determined in the Peripheral Blood Granulocytes of COVID-19 Patients. Int. J. Mol. Sci. 2023, 24, 13574. https://doi.org/10.3390/ijms241713574
Skrzydlewska E, Łuczaj W, Biernacki M, Wójcik P, Jarocka-Karpowicz I, Orehovec B, Baršić B, Tarle M, Kmet M, Lukšić I, et al. Preliminary Comparison of Molecular Antioxidant and Inflammatory Mechanisms Determined in the Peripheral Blood Granulocytes of COVID-19 Patients. International Journal of Molecular Sciences. 2023; 24(17):13574. https://doi.org/10.3390/ijms241713574
Chicago/Turabian StyleSkrzydlewska, Elżbieta, Wojciech Łuczaj, Michał Biernacki, Piotr Wójcik, Iwona Jarocka-Karpowicz, Biserka Orehovec, Bruno Baršić, Marko Tarle, Marta Kmet, Ivica Lukšić, and et al. 2023. "Preliminary Comparison of Molecular Antioxidant and Inflammatory Mechanisms Determined in the Peripheral Blood Granulocytes of COVID-19 Patients" International Journal of Molecular Sciences 24, no. 17: 13574. https://doi.org/10.3390/ijms241713574
APA StyleSkrzydlewska, E., Łuczaj, W., Biernacki, M., Wójcik, P., Jarocka-Karpowicz, I., Orehovec, B., Baršić, B., Tarle, M., Kmet, M., Lukšić, I., Marušić, Z., Bauer, G., & Žarković, N. (2023). Preliminary Comparison of Molecular Antioxidant and Inflammatory Mechanisms Determined in the Peripheral Blood Granulocytes of COVID-19 Patients. International Journal of Molecular Sciences, 24(17), 13574. https://doi.org/10.3390/ijms241713574









