17 β-Estradiol Impedes Aortic Root Dilation and Rupture in Male Marfan Mice
Abstract
:1. Introduction
2. Results
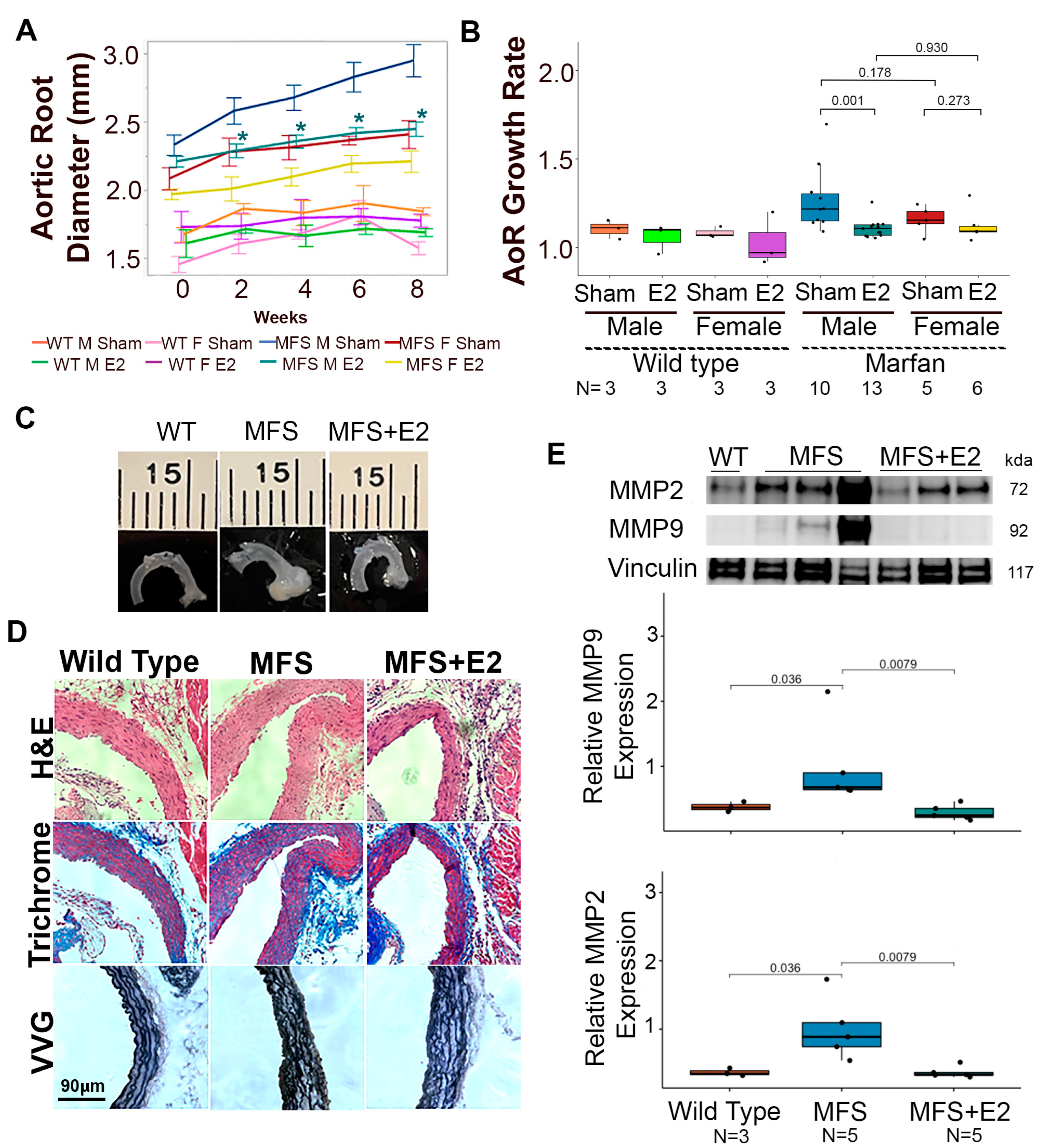
2.1. 17 β-Estradiol Attenuates Aortic Root Enlargement in Marfan Mice
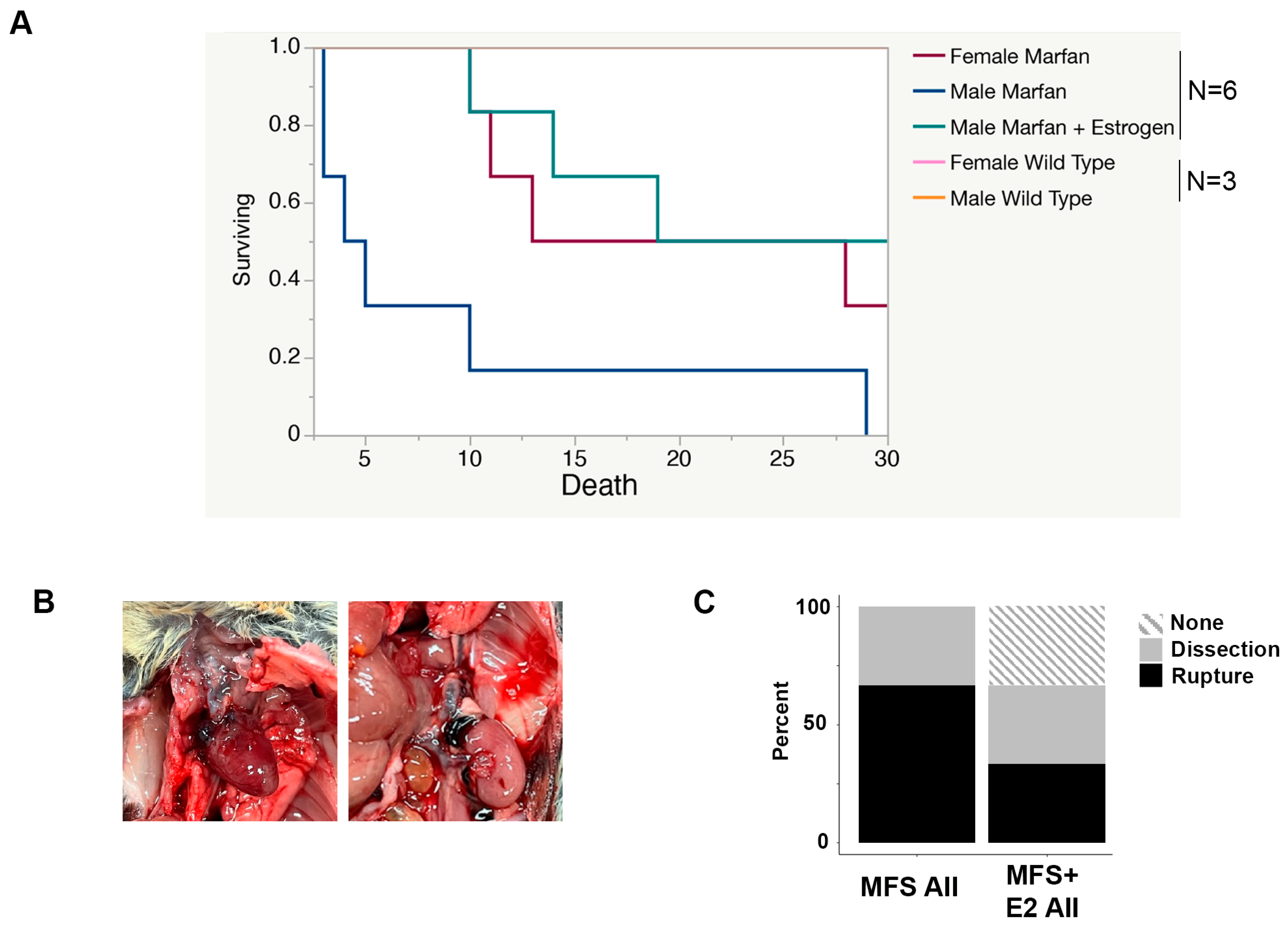
2.2. 17 β-Estradiol Prolongs Survival in an Aortic Rupture Mouse Model
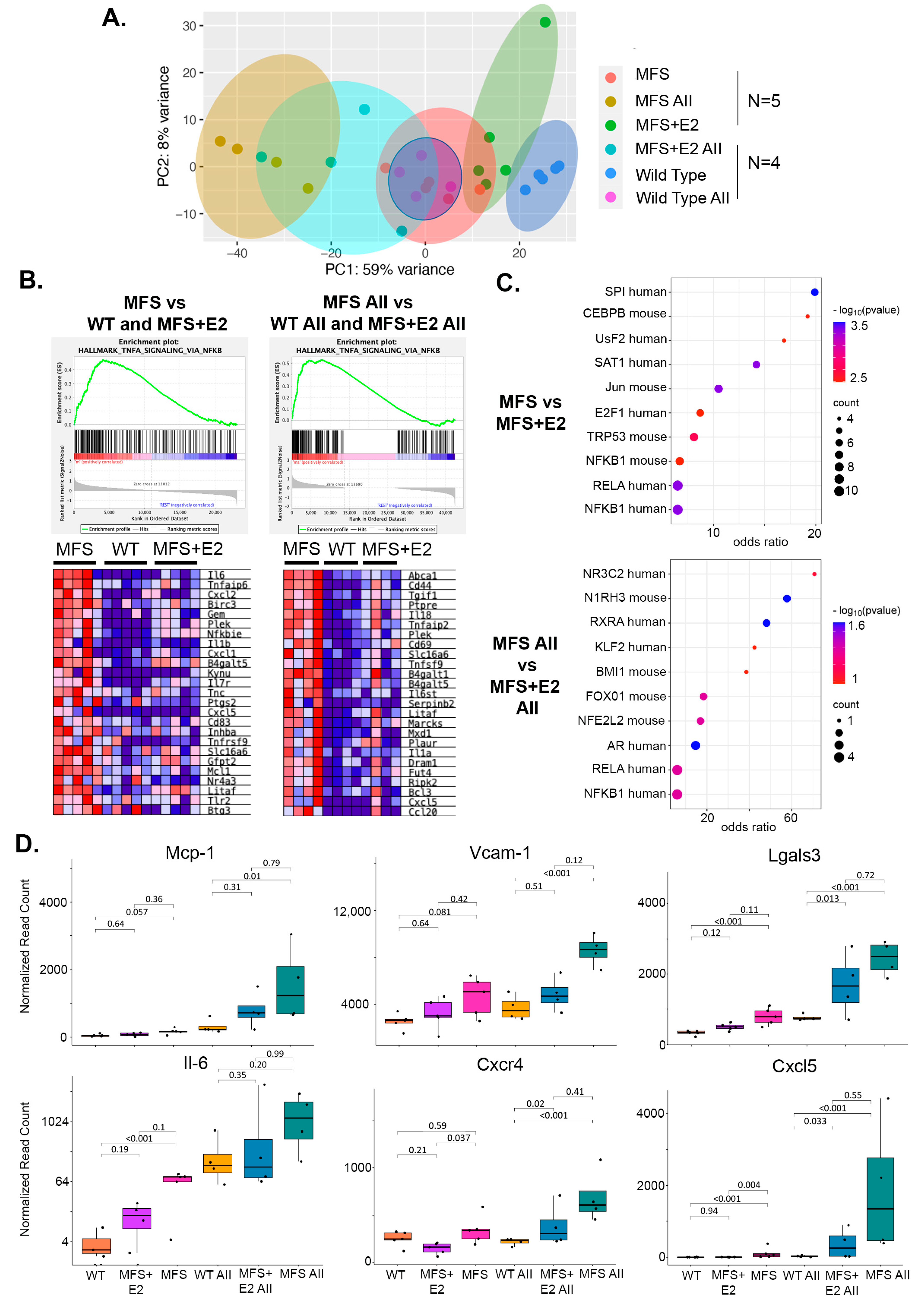
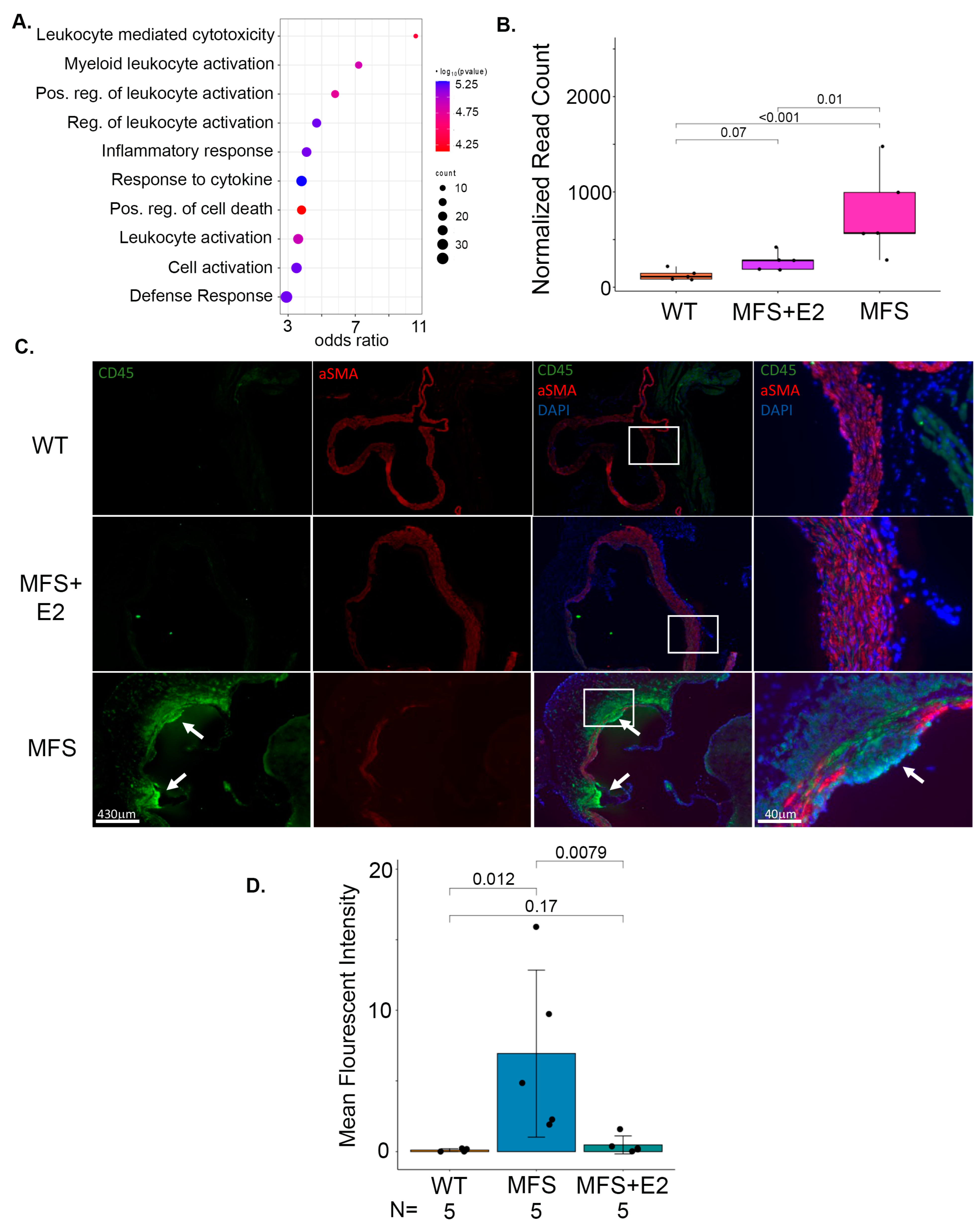
2.3. 17 β-Estradiol Attenuates an Inflammatory Molecular Phenotype in Male Marfan Mice
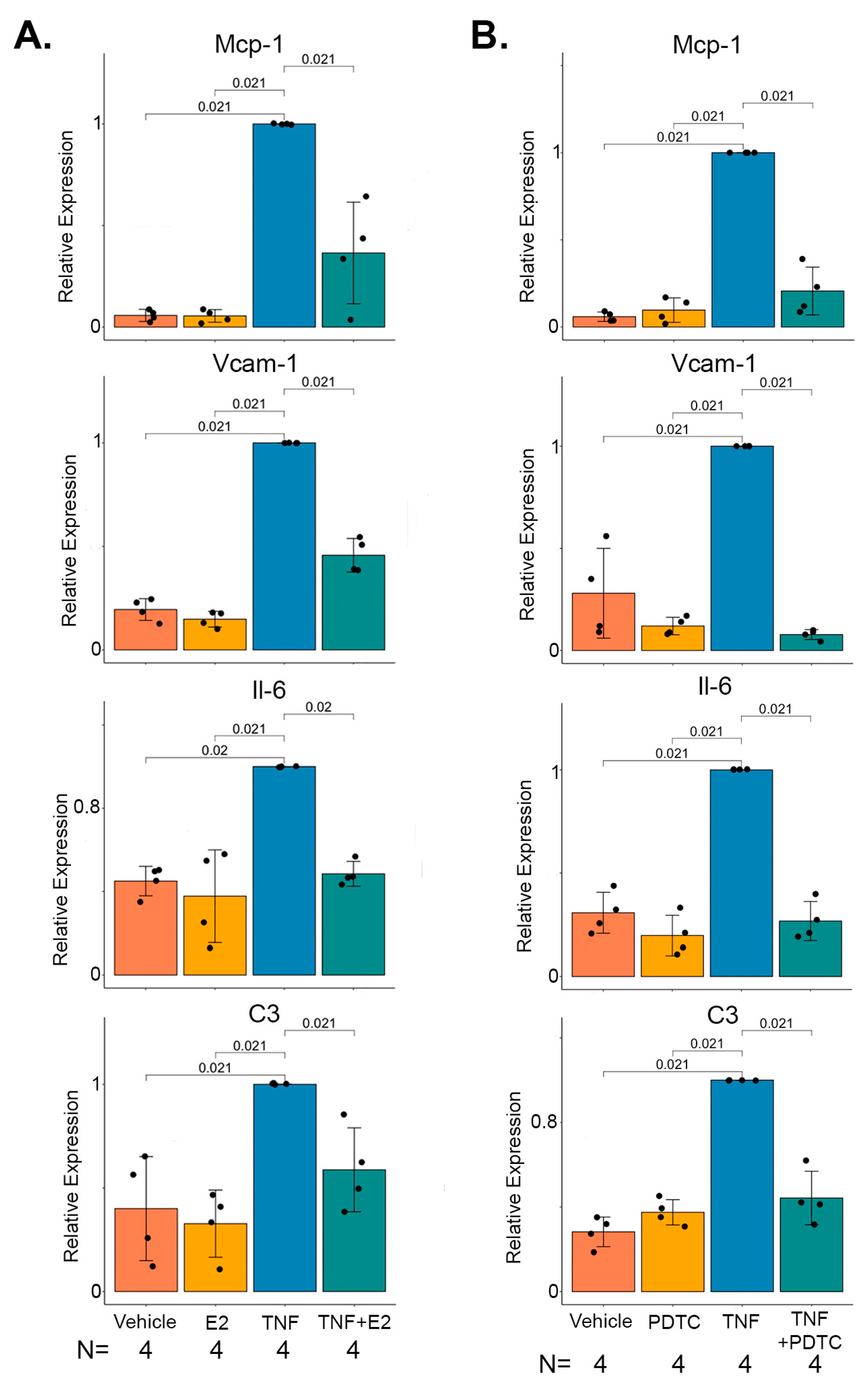
2.4. 17 β-Estradiol Inhibits TNFα Mediated NF-κB Target Gene Expression in Marfan Aortic Smooth Muscle Cells
2.5. 17 β-Estradiol Prevents Inflammatory Foci in the Adventitia of Marfan Aortic Roots
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Histology
4.3. Western Blot
4.4. Cell Culture
4.5. RNA Isolation and Quantitative Real-Time PCR (qPCR)
4.6. RNA-Seq
4.7. Immunofluorescence
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J. Am. Coll. Cardiol. 2010, 55, e27–e129, Correction in J. Am. Coll. Cardiol. 2013, 62, 1039–1040. [Google Scholar] [CrossRef]
- Cheung, K.; Boodhwani, M.; Chan, K.-L.; Beauchesne, L.; Dick, A.; Coutinho, T. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J. Am. Heart Assoc. 2017, 6, e003792. [Google Scholar] [CrossRef] [PubMed]
- Grubb, K.J.; Kron, I.L. Sex and gender in thoracic aortic aneurysms and dissection. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Eliason, J.L.; Roelofs, K.J.; Sinha, I.; Hannawa, K.K.; Kaldjian, E.P.; Lu, G.; Henke, P.K.; Stanley, J.C.; Weiss, S.J.; et al. Gender differences in experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Martin-McNulty, B.; Tham, D.M.; Cunha, V.d.; Ho, J.J.; Wilson, D.W.; Rutledge, J.C.; Deng, G.G.; Vergona, R.; Sullivan, M.E.; Wang, Y.-X. B-Estradiol Attenuates Development of Angiotensin II Induced Aortic Abdominal Aneurysm in Apolipoprotein E Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1627–1632. [Google Scholar] [CrossRef]
- Chen, X.; Rateri, D.L.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Cassis, L.A.; Daugherty, A. TGF-β Neutralization Enhances AngII-Induced Aortic Rupture and Aneurysm in Both Thoracic and Abdominal Regions. PLoS ONE 2016, 11, e0153811. [Google Scholar] [CrossRef] [PubMed]
- Groth, K.A.; Stochholm, K.; Hove, H.; Kyhl, K.; Gregersen, P.A.; Vejlstrup, N.; Østergaard, J.R.; Gravholt, C.H.; Andersen, N.H. Aortic events in a nationwide Marfan syndrome cohort. Clin. Res. Cardiol. 2017, 106, 105–112. [Google Scholar] [CrossRef]
- Gharraee, N.; Sun, Y.; Swisher, J.A.; Lessner, S.M. Age and sex dependency of thoracic aortopathy in a mouse model of Marfan syndrome. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H44–H56. [Google Scholar] [CrossRef]
- Jiménez-Altayó, F.; Siegert, A.M.; Bonorino, F.; Meirelles, T.; Barberà, L.; Dantas, A.P.; Vila, E.; Egea, G. Differences in the Thoracic Aorta by Region and Sex in a Murine Model of Marfan Syndrome. Front. Physiol. 2017, 8, 933. [Google Scholar] [CrossRef]
- Albornoz, G.; Coady, M.A.; Roberts, M.; Davies, R.R.; Tranquilli, M.; Rizzo, J.A.; Elefteriades, J.A. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann. Thorac. Surg. 2006, 82, 1400–1405. [Google Scholar] [CrossRef]
- Tashima, Y.; He, H.; Cui, J.Z.; Pedroza, A.J.; Nakamura, K.; Yokoyama, N.; Iosef, C.; Burdon, G.; Koyano, T.; Yamaguchi, A.; et al. Androgens Accentuate TGF-β Dependent Erk/Smad Activation During Thoracic Aortic Aneurysm Formation in Marfan Syndrome Male Mice. J. Am. Heart Assoc. 2020, 9, e015773. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Yang, S.; Choudhry, M.A.; Yu, H.P.; Bland, K.I.; Schwacha, M.G.; Chaudry, I.H. Flutamide restores cardiac function after trauma-hemorrhage via an estrogen-dependent pathway through upregulation of PGC-1. Am. J. Physiology. Heart Circ. Physiol. 2006, 290, H416–H423. [Google Scholar] [CrossRef]
- Metzger, D.L.; Kerrigan, J.R. Androgen receptor blockade with flutamide enhances growth hormone secretion in late pubertal males: Evidence for independent actions of estrogen and androgen. J. Clin. Endocrinol. Metab. 1993, 76, 1147–1152. [Google Scholar] [CrossRef]
- Boese, A.C.; Kim, S.C.; Yin, K.J.; Lee, J.P.; Hamblin, M.H. Sex differences in vascular physiology and pathophysiology: Estrogen and androgen signaling in health and disease. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H524–H545. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Nozell, S.; Chen, Y.-F.; Hage, F.; Oparil, S. Estrogen and Mechanisms of Vascular Protection. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and Cellular Immune Responses in Abdominal Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef]
- He, R.; Guo, D.C.; Sun, W.; Papke, C.L.; Duraisamy, S.; Estrera, A.L.; Safi, H.J.; Ahn, C.; Buja, L.M.; Arnett, F.C.; et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J. Thorac. Cardiovasc. Surg. 2008, 136, 922–929.e1. [Google Scholar] [CrossRef]
- Guo, G.; Booms, P.; Halushka, M.; Dietz, H.C.; Ney, A.; Stricker, S.; Hecht, J.; Mundlos, S.; Robinson, P.N. Induction of Macrophage Chemotaxis by Aortic Extracts of the mgR Marfan Mouse Model and a GxxPG-Containing Fibrillin-1 Fragment. Circulation 2006, 114, 1855–1862. [Google Scholar] [CrossRef]
- Cavanaugh, N.B.; Qian, L.; Westergaard, N.M.; Kutschke, W.J.; Born, E.J.; Turek, J.W. A Novel Murine Model of Marfan Syndrome Accelerates Aortopathy and Cardiomyopathy. Ann. Thorac. Surg. 2017, 104, 657–665. [Google Scholar] [CrossRef]
- Xing, D.; Feng, W.; Miller, A.P.; Weathington, N.M.; Chen, Y.F.; Novak, L.; Blalock, J.E.; Oparil, S. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2607–H2612. [Google Scholar] [CrossRef]
- Villard, C.; Eriksson, P.; Kronqvist, M.; Lengquist, M.; Jorns, C.; Hartman, J.; Roy, J.; Hultgren, R. Differential expression of sex hormone receptors in abdominal aortic aneurysms. Maturitas 2017, 96, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Sokolis, D.P.; Iliopoulos, D.C. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J. Mech. Behav. Biomed. Mater. 2014, 34, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Oparil, S.; Yu, H.; Gong, K.; Feng, W.; Black, J.; Chen, Y.F.; Nozell, S. Estrogen modulates NFκB signaling by enhancing IκBα levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-β. PLoS ONE 2012, 7, e36890. [Google Scholar] [CrossRef] [PubMed]
- Ghisletti, S.; Meda, C.; Maggi, A.; Vegeto, E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol. Cell Biol. 2005, 25, 2957–2968. [Google Scholar] [CrossRef]
- Belguise, K.; Sonenshein, G.E. PKCtheta promotes c-Rel-driven mammary tumorigenesis in mice and humans by repressing estrogen receptor alpha synthesis. J. Clin. Investig. 2007, 117, 4009–4021. [Google Scholar] [CrossRef]
- Nakamura, K.; Dalal, A.R.; Yokoyama, N.; Pedroza, A.J.; Kusadokoro, S.; Mitchel, O.; Gilles, C.; Masoudian, B.; Leipzig, M.; Casey, K.M.; et al. Lineage-Specific Induced Pluripotent Stem Cell-Derived Smooth Muscle Cell Modeling Predicts Integrin Alpha-V Antagonism Reduces Aortic Root Aneurysm Formation in Marfan Syndrome Mice. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1134–1153. [Google Scholar] [CrossRef]
- Alexander, M.R.; Murgai, M.; Moehle, C.W.; Owens, G.K. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol. Genom. 2012, 44, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Starke, R.M.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Owens, G.K.; Koch, W.J.; Greig, N.H.; Dumont, A.S. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm pathology. J. Cereb. Blood Flow Metab. 2013, 33, 1564–1573. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef]
- Parodi, F.E.; Mao, D.; Ennis, T.L.; Bartoli, M.A.; Thompson, R.W. Suppression of experimental abdominal aortic aneurysms in mice by treatment with pyrrolidine dithiocarbamate, an antioxidant inhibitor of nuclear factor-kappaB. J. Vasc. Surg. 2005, 41, 479–489. [Google Scholar] [CrossRef]
- Majesky, M.W.; Horita, H.; Ostriker, A.; Lu, S.; Regan, J.N.; Bagchi, A.; Dong, X.R.; Poczobutt, J.; Nemenoff, R.A.; Weiser-Evans, M.C. Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by Klf4. Circ. Res. 2017, 120, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Gensicke, N.M.; Cavanaugh, N.B.; Andersen, N.D.; Huang, T.; Qian, L.; Dyle, M.C.; Turek, J.W. Accelerated Marfan syndrome model recapitulates established signaling pathways. J. Thorac. Cardiovasc. Surg. 2020, 159, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Yang, H.; Tang, Q.; Gong, Y.C.; Fu, Y.H.; Wan, F.; Yang, B.; Guo, R.; Zhong, Y.L.; Zhu, J.M.; et al. Identification of Vinculin as a Potential Diagnostic Biomarker for Acute Aortic Dissection Using Label-Free Proteomics. BioMed Res. Int. 2020, 2020, 7806409. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, E.G.; Parker, S.J.; Shin, J.Y.; Kang, B.E.; Ziegler, S.G.; Creamer, T.J.; Bagirzadeh, R.; Bedja, D.; Chen, Y.; Calderon, J.F.; et al. Lineage-specific events underlie aortic root aneurysm pathogenesis in Loeys-Dietz syndrome. J. Clin. Investig. 2019, 129, 659–675. [Google Scholar] [CrossRef]
- Lu, S.; Sun, X.; Hong, T.; Song, K.; Yang, S.; Wang, C. Isolation and culture of smooth muscle cells from human acute type A aortic dissection. J. Cardiothorac. Surg. 2013, 8, 83. [Google Scholar] [CrossRef]
- FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 December 2022).
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2019, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio Team: Boston, MA, USA, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saddic, L.; Escopete, S.; Zilberberg, L.; Kalsow, S.; Gupta, D.; Eghbali, M.; Parker, S. 17 β-Estradiol Impedes Aortic Root Dilation and Rupture in Male Marfan Mice. Int. J. Mol. Sci. 2023, 24, 13571. https://doi.org/10.3390/ijms241713571
Saddic L, Escopete S, Zilberberg L, Kalsow S, Gupta D, Eghbali M, Parker S. 17 β-Estradiol Impedes Aortic Root Dilation and Rupture in Male Marfan Mice. International Journal of Molecular Sciences. 2023; 24(17):13571. https://doi.org/10.3390/ijms241713571
Chicago/Turabian StyleSaddic, Louis, Sean Escopete, Lior Zilberberg, Shannon Kalsow, Divya Gupta, Mansoureh Eghbali, and Sarah Parker. 2023. "17 β-Estradiol Impedes Aortic Root Dilation and Rupture in Male Marfan Mice" International Journal of Molecular Sciences 24, no. 17: 13571. https://doi.org/10.3390/ijms241713571
APA StyleSaddic, L., Escopete, S., Zilberberg, L., Kalsow, S., Gupta, D., Eghbali, M., & Parker, S. (2023). 17 β-Estradiol Impedes Aortic Root Dilation and Rupture in Male Marfan Mice. International Journal of Molecular Sciences, 24(17), 13571. https://doi.org/10.3390/ijms241713571




