IL-31RA and TRPV1 Expression in Atopic Dermatitis Induced with Trinitrochlorobenzene in Nc/Nga Mice
Abstract
:1. Introduction
2. Results
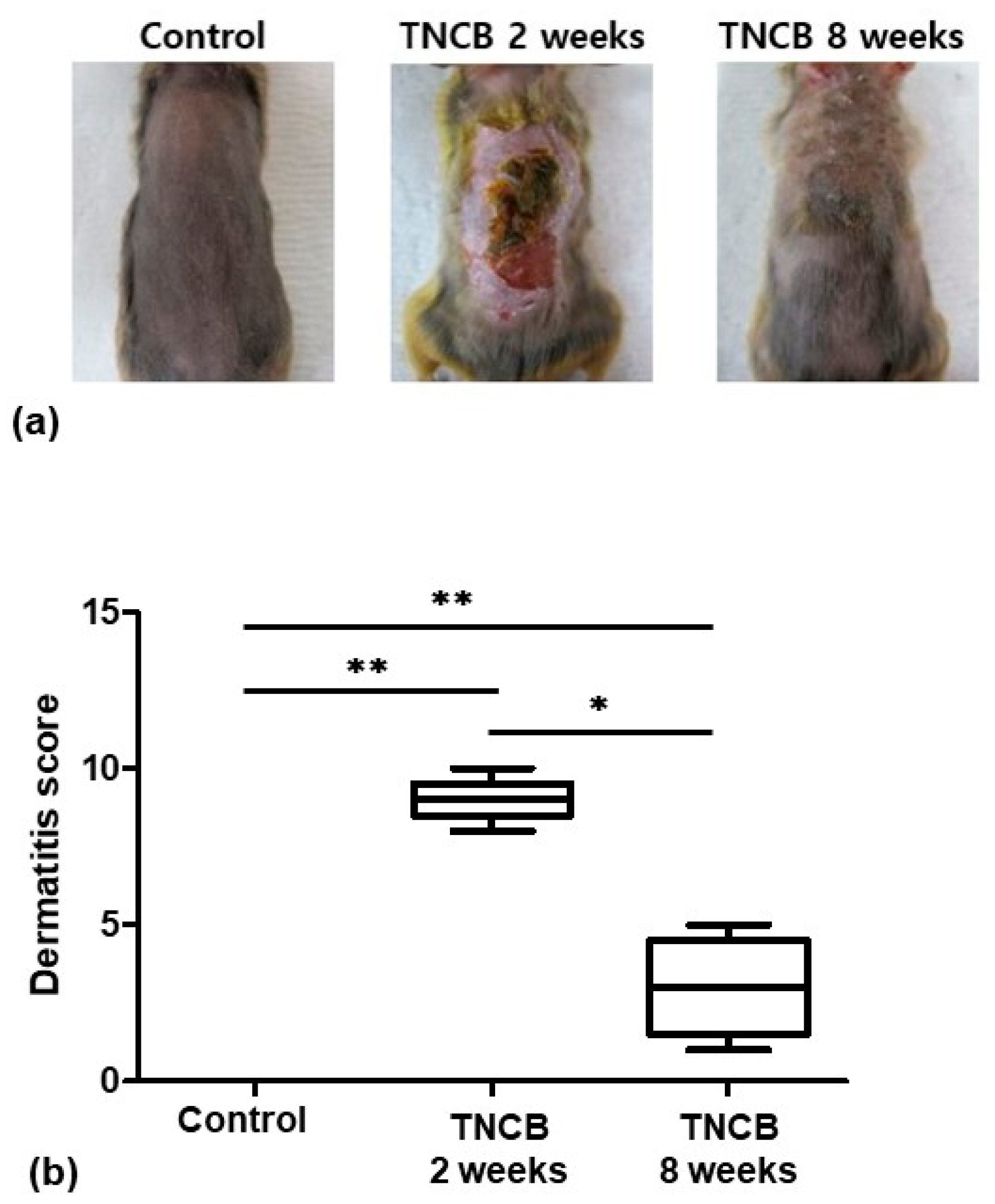
2.1. Evaluation of Skin Lesions
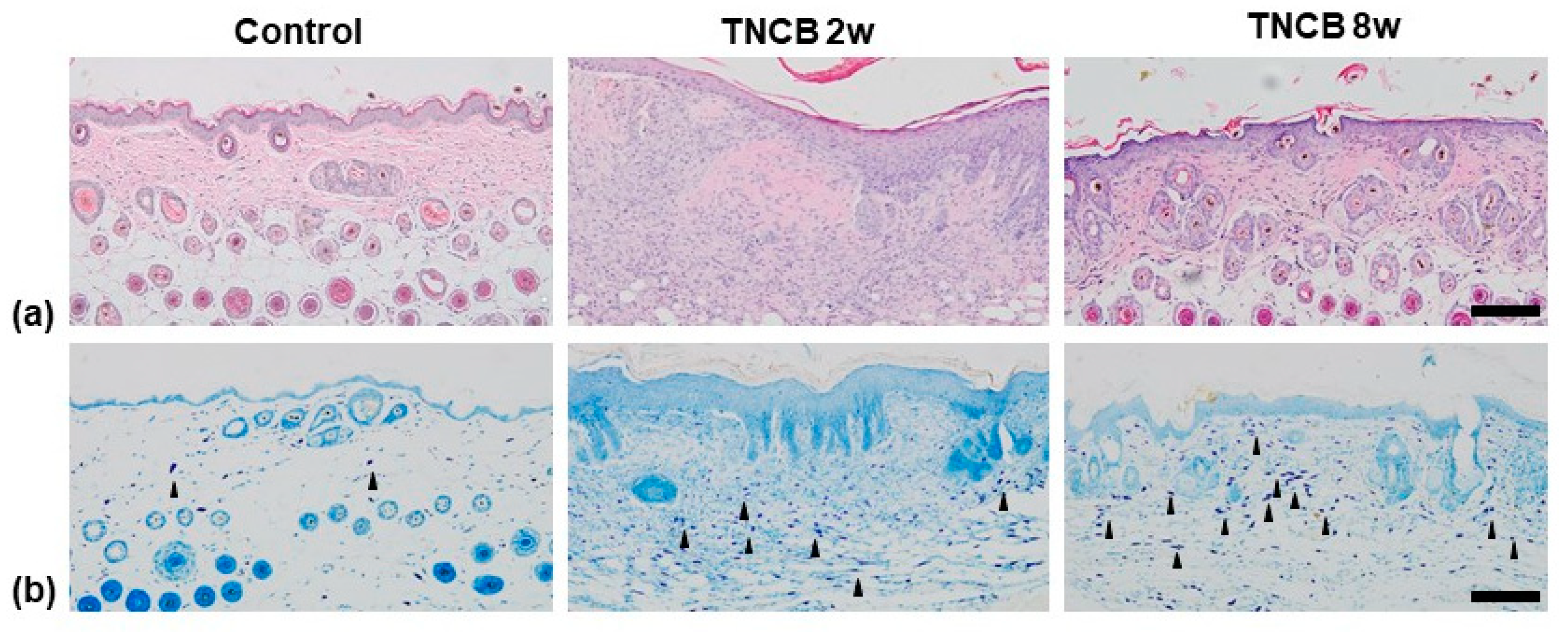
2.2. Histological Analysis
2.3. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
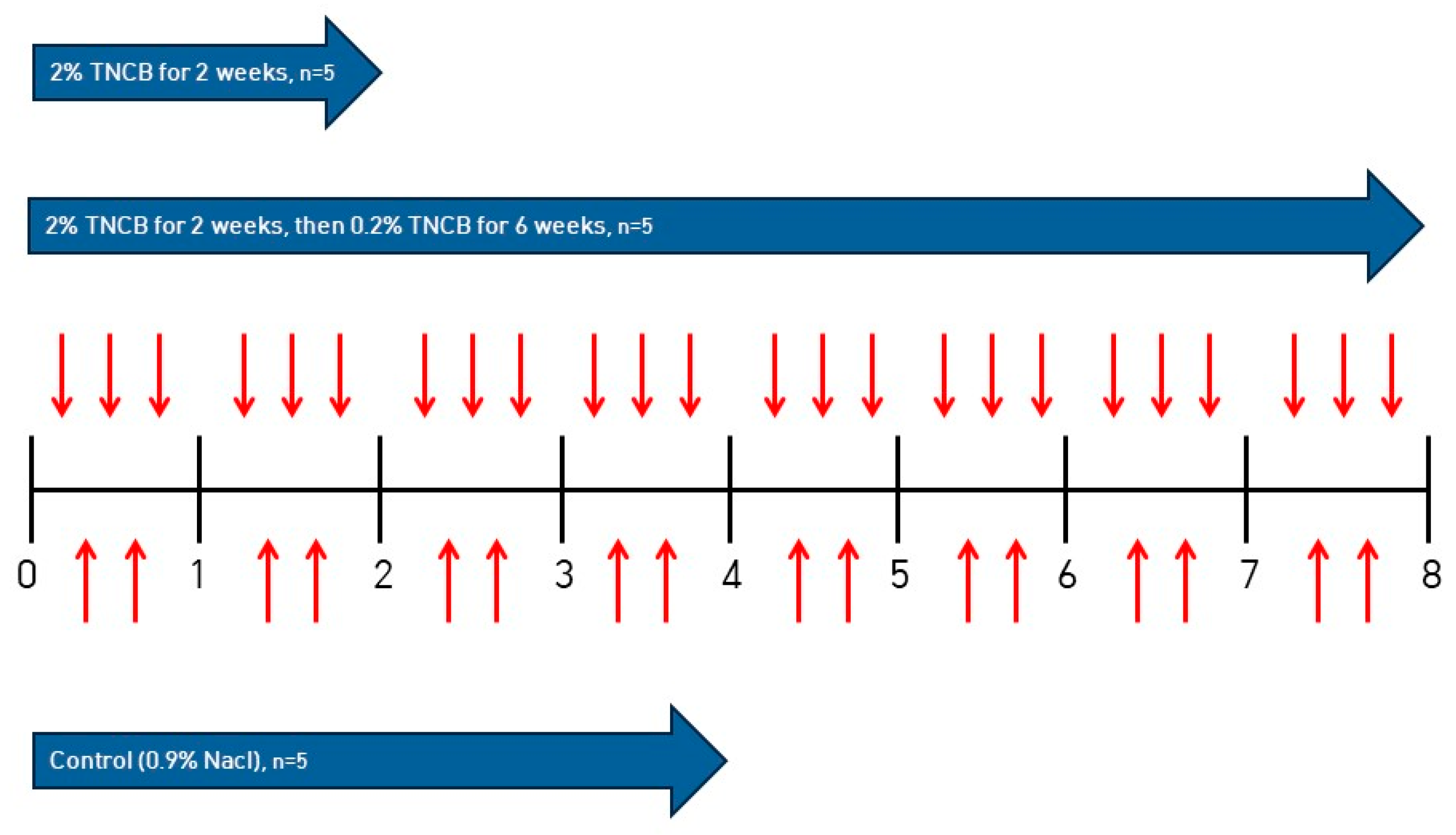
4.2. Inducing AD in NC/Nga Mice
4.3. Evaluation of Skin Lesions
4.4. Histological Analysis
4.5. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.D.; McLean, W.H.; Leung, D.Y. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Lowe, A.J.; Matheson, M.C.; Burgess, J.A.; Allen, K.J.; Abramson, M.J. Atopic dermatitis and the atopic march revisited. Allergy 2014, 69, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.J.; Leung, D.Y.M.; Tang, M.L.K.; Su, J.C.; Allen, K.J. The skin as a target for prevention of the atopic march. Ann. Allergy Asthma Immunol. 2018, 120, 145–151. [Google Scholar] [CrossRef]
- Grewe, M.; Bruijnzeel-Koomen, C.A.; Schopf, E.; Thepen, T.; Langeveld-Wildschut, A.G.; Ruzicka, T.; Krutmann, J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol. Today 1998, 19, 359–361. [Google Scholar] [CrossRef]
- Han, H.W.; Roan, F.; Ziegler, S.F. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef]
- Nakajima, S.; Tie, D.; Nomura, T.; Kabashima, K. Novel pathogenesis of atopic dermatitis from the view of cytokines in mice and humans. Cytokine 2021, 148, 155664. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Papoiu, A.D.P. What causes itch in atopic dermatitis? Curr. Allergy Asthma Rep. 2008, 8, 306–311. [Google Scholar] [CrossRef]
- Buddenkotte, J.; Steinhoff, M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy 2010, 65, 805–821. [Google Scholar] [CrossRef]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef]
- Ferretti, E.; Corcione, A.; Pistoia, V. The IL-31/IL-31 receptor axis: General features and role in tumor microenvironment. J. Leukoc. Biol. 2017, 102, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Scalvenzi, M.; Ruggiero, A.; Potestio, L.; Battista, T.; Megna, M. Hidradenitis Suppurativa and JAK Inhibitors: A Review of the Published Literature. Medicina 2023, 59, 801. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Genco, L. Rapid improvement in pruritus in atopic dermatitis patients treated with upadactinib: A real-life experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1497–1498. [Google Scholar] [CrossRef]
- Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhe, V.; Plee-Gautier, E.; Carre, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, F.; Luo, H.; Liu, F.Y.; Han, J.S.; Xing, G.G.; Wan, Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol. Pain 2008, 4, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Aioi, A.; Tonogaito, H.; Suto, H.; Hamada, K.; Ra, C.R.; Ogawa, H.; Maibach, H.; Matsuda, H. Impairment of skin barrier function in NC/Nga Tnd mice as a possible model for atopic dermatitis. Br. J. Dermatol. 2001, 144, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kobayashi, T.; Nagao, K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. J. Invest. Dermatol. 2019, 139, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Mizukoshi, K.; Oyobikawa, M.; Ohshima, H.; Tagami, H. Establishment of an atopic dermatitis-like skin model in a hairless mouse by repeated elicitation of contact hypersensitivity that enables to conduct functional analyses of the stratum corneum with various non-invasive biophysical instruments. Skin Res. Technol. 2004, 10, 122–129. [Google Scholar] [CrossRef]
- Man, M.Q.; Hatano, Y.; Lee, S.H.; Man, M.; Chang, S.; Feingold, K.R.; Leung, D.Y.M.; Holleran, W.; Uchida, Y.; Elias, P.M. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: Structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J. Invest. Dermatol. 2008, 128, 79–86. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Invest. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, T.H.; Kang, M.S.; Ahn, J.O.; Choi, J.H.; Chung, J.Y. Comparison of the presentation of atopic dermatitis induced by trinitrochlorobenzene and house dust mite in NC/Nga mice. J. Vet. Sci. 2020, 21, e59. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediators Inflamm. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takamori, A.; Nambu, A.; Sato, K.; Yamaguchi, S.; Matsuda, K.; Numata, T.; Sugawara, T.; Yoshizaki, T.; Arae, K.; Morita, H.; et al. IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity. Sci. Rep. 2018, 8, 6639. [Google Scholar] [CrossRef]
- Grimstad, O.; Sawanobori, Y.; Vestergaard, C.; Bilsborough, J.; Olsen, U.B.; Gronhoj-Larsen, C.; Matsushima, K. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: A model of atopic dermatitis. Exp. Dermatol. 2009, 18, 35–43. [Google Scholar] [CrossRef]
- Yun, J.W.; Seo, J.A.; Jang, W.H.; Koh, H.J.; Bae, I.H.; Park, Y.H.; Lim, K.M. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J. Invest. Dermatol. 2011, 131, 1576–1579. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, C.S.; Bae, I.H.; Choi, J.K.; Park, Y.H.; Park, M. A novel, topical, nonsteroidal, TRPV1 antagonist, PAC-14028 cream improves skin barrier function and exerts anti-inflammatory action through modulating epidermal differentiation markers and suppressing Th2 cytokines in atopic dermatitis. J. Dermatol. Sci. 2018, 91, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Imamachi, N.; Park, G.H.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335. [Google Scholar] [CrossRef]
- Kajihara, Y.; Murakami, M.; Imagawa, T.; Otsuguro, K.; Ito, S.; Ohta, T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience 2010, 166, 292–304. [Google Scholar] [CrossRef]
- Takaoka, A.; Arai, I.; Sugimoto, M.; Honma, Y.; Futaki, N.; Nakamura, A.; Nakaike, S. Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Exp. Dermatol. 2006, 15, 161–167. [Google Scholar] [CrossRef]
- Stander, S.; Moormann, C.; Schumacher, M.; Buddenkotte, J.; Artuc, M.; Shpacovitch, V.; Brzoska, T.; Lippert, U.; Henz, B.M.; Luger, T.A.; et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 2004, 13, 129–139. [Google Scholar] [CrossRef]
- Kubanov, A.A.; Katunina, O.R.; Chikin, V.V. Expression of Neuropeptides, Neurotrophins, and Neurotransmitters in the Skin of Patients with Atopic Dermatitis and Psoriasis. Bull. Exp. Biol. Med. 2015, 159, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Rodriguez, R.; Kaushik, S.B.; Yosipovitch, G. Transient receptor potential channels and dermatological disorders. Curr. Top. Med. Chem. 2013, 13, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Southall, M.D.; Li, T.; Gharibova, L.S.; Pei, Y.; Nicol, G.D.; Travers, J.B. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J. Pharmacol. Exp. Ther. 2003, 304, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Suto, H.; Matsuda, H.; Mitsuishi, K.; Hira, K.; Uchida, T.; Unno, T.; Ogawa, H.; Ra, C. NC/Nga mice: A mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999, 120, 70–75. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lim, N.Y.; Kang, M.S.; Jeong, Y.; Ahn, J.-O.; Choi, J.H.; Chung, J.-Y. IL-31RA and TRPV1 Expression in Atopic Dermatitis Induced with Trinitrochlorobenzene in Nc/Nga Mice. Int. J. Mol. Sci. 2023, 24, 13521. https://doi.org/10.3390/ijms241713521
Lee S, Lim NY, Kang MS, Jeong Y, Ahn J-O, Choi JH, Chung J-Y. IL-31RA and TRPV1 Expression in Atopic Dermatitis Induced with Trinitrochlorobenzene in Nc/Nga Mice. International Journal of Molecular Sciences. 2023; 24(17):13521. https://doi.org/10.3390/ijms241713521
Chicago/Turabian StyleLee, Seokwoo, Na Yeon Lim, Min Soo Kang, Yunho Jeong, Jin-Ok Ahn, Jung Hoon Choi, and Jin-Young Chung. 2023. "IL-31RA and TRPV1 Expression in Atopic Dermatitis Induced with Trinitrochlorobenzene in Nc/Nga Mice" International Journal of Molecular Sciences 24, no. 17: 13521. https://doi.org/10.3390/ijms241713521
APA StyleLee, S., Lim, N. Y., Kang, M. S., Jeong, Y., Ahn, J.-O., Choi, J. H., & Chung, J.-Y. (2023). IL-31RA and TRPV1 Expression in Atopic Dermatitis Induced with Trinitrochlorobenzene in Nc/Nga Mice. International Journal of Molecular Sciences, 24(17), 13521. https://doi.org/10.3390/ijms241713521





