Tear Biomarkers and Alzheimer’s Disease
Abstract
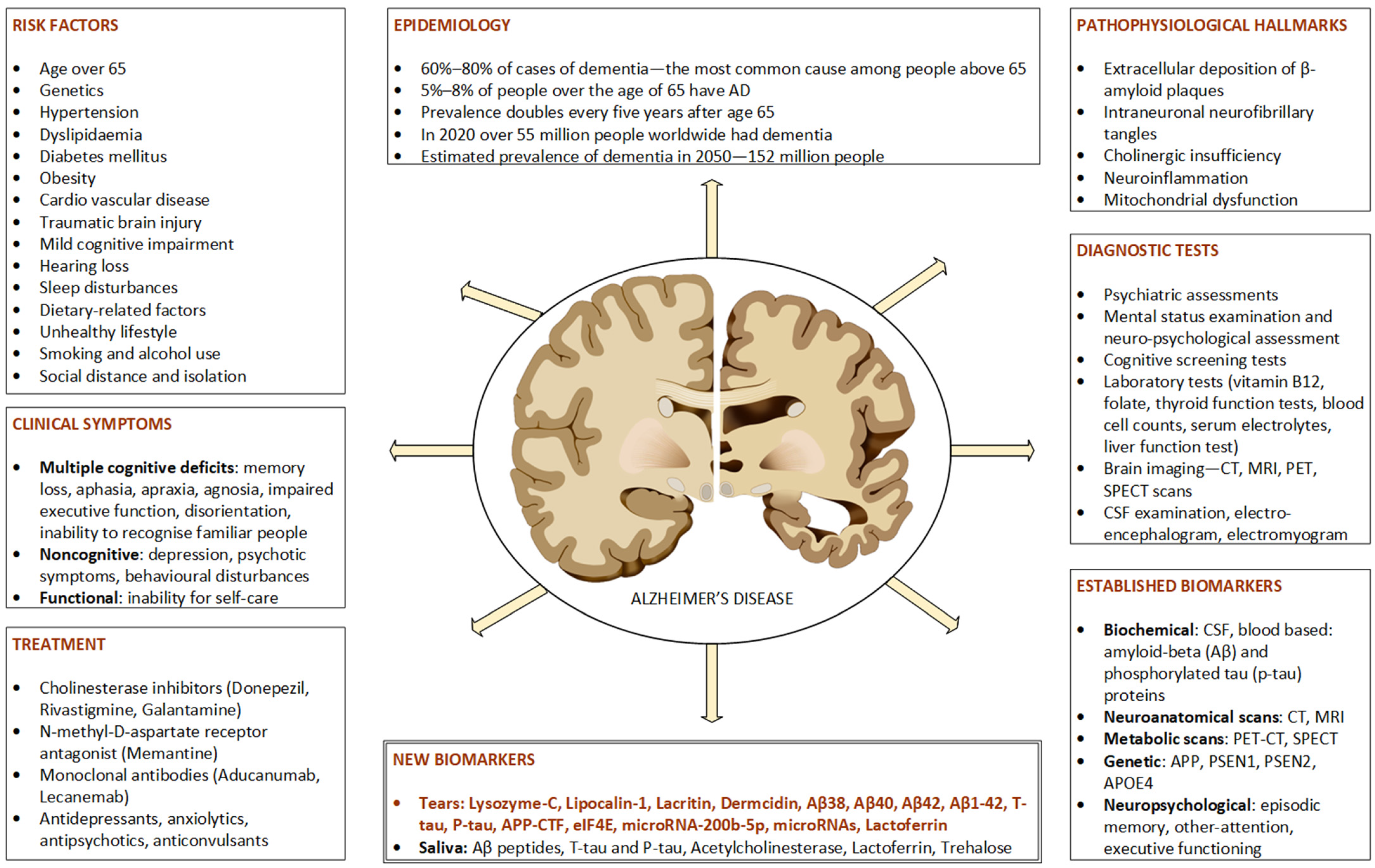
1. Introduction
2. Alzheimer’s Disease and the Visual System
3. Biomarkers for Alzheimer’s Disease
4. Tears as a Source of Biomarkers
Collection and Analysis of Tears
5. Tear Biomarkers in Alzheimer’s Disease
5.1. Current Research
5.2. MicroRNAs as Potential Biomarkers for AD
5.3. Lactoferrin as a Potential Biomarker for AD
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hane, F.T.; Robinson, M.; Lee, B.Y.; Bai, O.; Leonenko, Z.; Albert, M.S. Recent Progress in Alzheimer’s Disease Research, Part 3: Diagnosis and Treatment. J. Alzheimers Dis. 2017, 57, 645–665. [Google Scholar]
- Majeed, A.; Marwick, B.; Yu, H.; Fadavi, H.; Tavakoli, M. Ophthalmic Biomarkers for Alzheimer’s Disease: A Review. Front. Aging Neurosci. 2021, 13, 720167. [Google Scholar] [CrossRef]
- Mahajan, D.; Votruba, M. Can the retina be used to diagnose and plot the progression of Alzheimer’s disease? Acta Ophthalmol. 2017, 95, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Król-Grzymała, A.; Sienkiewicz-Szłapka, E.; Fiedorowicz, E.; Rozmus, D.; Cieślińska, A.; Grzybowski, A. Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). Int. J. Mol. Sci. 2022, 23, 10123. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, S.; Didier, M.A.; Vinjamuri, S. The Who, When, Why, and How of PET Amyloid Imaging in Management of Alzheimer’s Disease-Review of Literature and Interesting Images. Diagnostics 2019, 9, 65. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Body Fluid Biomarkers for Alzheimer’s Disease-an up-to-Date Overview. Biomedicines 2020, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.M.; Vinters, H.V. Pathologic lesions in neurodegenerative diseases. Prog. Mol. Biol. Transl. Sci. 2012, 107, 1–40. [Google Scholar] [CrossRef]
- Dhiman, K.; Gupta, V.B.; Villemagne, V.L.; Eratne, D.; Graham, P.L.; Fowler, C.; Bourgeat, P.; Li, Q.-X.; Collins, S.; Bush, A.I.; et al. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12005. [Google Scholar] [CrossRef]
- Zilkova, M.; Koson, P.; Zilka, N. The hunt for dying neurons: Insight into the neuronal loss in Alzheimer’s disease. Bratisl. Lek. Listy 2006, 107, 366–373. [Google Scholar]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Gong, N.J.; Dibb, R.; Bulk, M.; van derWeerd, L.; Liu, C. Imaging beta amyloid aggregation and iron accumulation in Alzheimer’sdisease using quantitative susceptibility mapping MRI. Neuroimage 2019, 191, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hondius, D.C.; Koopmans, F.; Leistner, C.; Pita-Illobre, D.; Peferoen-Baert, R.M.; Marbus, F.; Paliukhovich, I.; Li, K.W.; Rozemuller, A.J.; Hoozemans, J.J. The proteome of granulovacuolar degeneration and neurofibrillary tangles in Alzheimer’s disease. Acta Neuropathol. 2021, 141, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Romaus-Sanjurjo, D.; Regueiro, U.; López-López, M.; Vázquez-Vázquez, L.; Ouro, A.; Lema, I.; Sobrino, T. Alzheimer’s Disease Seen through the Eye: Ocular Alterations and Neurodegeneration. Int. J. Mol Sci. 2022, 23, 2486. [Google Scholar] [CrossRef]
- Morris, J.K.; Honea, R.A.; Vidoni, E.D.; Swerdlow, R.H.; Burns, J.M. Is Alzheimer’s Disease a Systemic Disease? Biochim. Biophys. Acta 2014, 1842, 1340–1349. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Yu, C.; He, Y.; Zhou, Y.; Liang, M.; Wang, L.; Jiang, T. Regional homogeneity, functional connectivity and imaging markers of Alzheimer’s disease: A review of resting-state fMRI studies. Neuropsychologia 2008, 46, 1648–1656. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Rosen, H.J.; Alkalay, A.; Kornak, J.; Furst, A.J.; Agarwal, N.; Mormino, E.C.; O’Neil, J.P.; Janabi, M.; Karydas, A.; et al. Amyloid vs. FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011, 77, 2034–2042. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef]
- Hansson, O.; Zetterberg, H.; Buchhave, P.; Londos, E.; Blennow, K.; Minthon, L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006, 5, 228–234. [Google Scholar] [CrossRef]
- Rosen, C.; Hansson, O.; Blennow, K.; Zetterberg, H. Fluid biomarkers in Alzheimer’s disease—Current concepts. Mol. Neurodegener. 2013, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Picanco, L.C.; Ozela, P.F.; de Fatima de Brito Brito, M.; Pinheiro, A.A.; Padilha, E.C.; Braga, F.S.; de Paula da Silva, C.; Dos Santos, C.; Rosa, J.; da Silva Hage-Melim, L.I. Alzheimer’s Disease: A Review from the Pathophysiology to Diagnosis, New Perspectives for Pharmacological Treatment. Curr. Med. Chem. 2018, 25, 3141–3159. [Google Scholar] [CrossRef] [PubMed]
- Rampa, A.; Gobbi, S.; Belluti, F.; Bisi, A. Tackling Alzheimer’s Disease with Existing Drugs: A Promising Strategy for Bypassing Obstacles. Curr. Med. Chem. 2021, 28, 2305–2327. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Wong, J.H.; Ng, T.B.; Tsui, S.K.W.; Zuo, T. Drugs for Targeted Therapies of Alzheimer’s Disease. Curr. Med. Chem. 2019, 26, 335–359. [Google Scholar] [CrossRef]
- Walsh, S.; Merrick, R.; Milne, R.; Brayne, C. Aducanumab for Alzheimer’s disease? BMJ 2021, 374, n1682. [Google Scholar] [CrossRef]
- Reiss, A.B.; Muhieddine, D.; Jacob, B.; Mesbah, M.; Pinkhasov, A.; Gomolin, I.H.; Stecker, M.M.; Wisniewski, T.; De Leon, J. Alzheimer’s Disease Treatment: The Search for a Breakthrough. Medicina 2023, 59, 1084. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, Q.; Chen, Y.X.; Zhao, Y.F.; Li, Y.M. Aβ 42 and Aβ 40: Similarities and Differences. J. Pept. Sci. 2015, 21, 522–529. [Google Scholar] [CrossRef]
- World Alzheimer Report 2021: Journey through the Diagnosis of Dementia. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf (accessed on 25 September 2022).
- Kim, D.H.; Yeo, S.H.; Park, J.M.; Choi, J.Y.; Lee, T.H.; Park, S.Y.; Ock, M.S.; Eo, J.; Kim, H.S.; Cha, H.J. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene 2014, 545, 185–193. [Google Scholar] [CrossRef]
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Gerrish, A.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; Dowzell, K.; Williams, A.; et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [CrossRef]
- Koníčková, D.; Menšíková, K.; Tučková, L.; Hényková, E.; Strnad, M.; Friedecký, D.; Stejskal, D.; Matěj, R.; Kaňovský, P. Biomarkers of Neurodegenerative Diseases: Biology, Taxonomy, Clinical Relevance, and Current Research Status. Biomedicines 2022, 10, 1760. [Google Scholar] [CrossRef]
- Ausó, E.; Gómez-Vicente, V.; Esquiva, G. Biomarkers for Alzheimer’s Disease Early Diagnosis. J. Pers. Med. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; O’Bryant, S.E.; Molinuevo, J.L.; Zetterberg, H.; Masters, C.L.; Lista, S.; Kiddle, S.J.; Batrla, R.; Blennow, K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018, 14, 639–652. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Zetterberg, H.; Blennow, K. Salivary Biomarkers for Alzheimer’s Disease and Related Disorders. Neurol. Ther. 2019, 8 (Suppl. S2), 83–94. [Google Scholar] [CrossRef]
- Pomilio, A.B.; Vitale, A.A.; Lazarowski, A.J. Uncommon Noninvasive Biomarkers for the Evaluation and Monitoring of the Etiopathogenesis of Alzheimer’s Disease. Curr. Pharm. Des. 2022, 28, 1152–1169. [Google Scholar] [CrossRef]
- van de Sande, N.; Ramakers, I.H.G.B.; Visser, P.J.; Verhey, F.R.J.; Verbraak, F.D.; Bouwman, F.H.; Berendschot, T.T.J.M.; Nuijts, R.M.M.A.; Webers, C.A.B.; Gijs, M. Tear biomarkers for Alzheimer’s disease screening and diagnosis (the TearAD study): Design and rationale of an observational longitudinal multicenter study. BMC Neurol. 2023, 23, 293. [Google Scholar] [CrossRef]
- Dor, M.; Eperon, S.; Lalive, P.H.; Guex-Crosier, Y.; Hamedani, M.; Salvisberg, C.; Turck, N. Investigation of the Global Protein Content from Healthy Human Tears. Exp. Eye Res. 2019, 179, 64–74. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. The Power of Tears: How Tear Proteomics Research Could Revolutionize the Clinic. Expert Rev. Proteom. 2017, 14, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Rentka, A.; Koroskenyi, K.; Harsfalvi, J.; Szekanecz, Z.; Szucs, G.; Szodoray, P.; Kemeny-Beke, A. Evaluation of commonly used tear sampling methods and their relevance in subsequent biochemical analysis. Ann. Clin. Biochem. 2017, 54, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear Fluid Biomarkers in Ocular and Systemic Disease: Potential Use for Predictive, Preventive and Personalised Medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef]
- Sperandio, B.; Fischer, N.; Sansonetti, P.J. Mucosal physical and chemical innate barriers: Lessons from microbial evasion strategies. Semin. Immunol. 2015, 27, 111–118. [Google Scholar] [CrossRef]
- Glinská, G.; Krajčíková, K.; Tomečková, V. Diagnostic potential of tears in ophthalmology. Ceska Slov. Oftalmol. Cas. Ceske Oftalmol. Spol. Slov. Oftalmol. Spol. 2017, 73, 101–108. [Google Scholar]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Gverović Antunica, A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem. Med. 2020, 30, 030502. [Google Scholar] [CrossRef]
- Soria, J.; Durán, J.A.; Etxebarria, J.; Merayo, J.; González, N.; Reigada, R.; García, I.; Acera, A.; Suárez, T. Tear Proteome and Protein Network Analyses Reveal a Novel Pentamarker Panel for Tear Film Characterization in Dry Eye and Meibomian Gland Dysfunction. J. Proteom. 2013, 78, 94–112. [Google Scholar] [CrossRef]
- Runström, G.; Mann, A.; Tighe, B. The fall and rise of tear albumin levels: A multifactorial phenomenon. Ocul. Surf. 2013, 11, 165–180. [Google Scholar] [CrossRef]
- Uchino, E.; Sonoda, S.; Kinukawa, N.; Sakamoto, T. Alteration pattern of tear cytokines during the course of a day: Diurnal rhythm analyzed by multicytokine assay. Cytokine 2006, 33, 36–40. [Google Scholar] [CrossRef]
- Ayaki, M.; Tachi, N.; Hashimoto, Y.; Kawashima, M.; Tsubota, K.; Negishi, K. Diurnal variation of human tear meniscus volume measured with tear strip meniscometry self-examination. PLoS ONE 2019, 14, e0215922. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Zhu, D.; Shen, M.; Cui, L.; Wang, J. Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens. 2012, 38, 282–287. [Google Scholar] [CrossRef]
- Vu, C.H.V.; Kawashima, M.; Nakamura, W.; Nakamura, T.J.; Tsubota, K. Circadian clock regulates tear secretion in the lacrimal gland. Exp. Eye Res. 2021, 206, 108524. [Google Scholar] [CrossRef]
- Benito, M.J.; González-García, M.J.; Tesón, M.; García, N.; Fernández, I.; Calonge, M.; Enríquez-de-Salamanca, A. Intra- and inter-day variation of cytokines and chemokines in tears of healthy subjects. Exp. Eye Res. 2014, 120, 43–49. [Google Scholar] [CrossRef]
- Alves, M.; Novaes, P.; Morraye Mde, A.; Reinach, P.S.; Rocha, E.M. Is dry eye an environmental disease? Arq. Bras. Oftalmol. 2014, 77, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S. Biochemistry of human tear film: A review. Exp. Eye Res. 2022, 220, 109101. [Google Scholar] [CrossRef] [PubMed]
- Fagehi, R. Impact of environmental adaptation on tear film assessments. J. Français Ophtalmol. 2018, 41, 231–237. [Google Scholar] [CrossRef]
- Kaštelan, S.; Bakija, I.; Bogadi, M.; Orešković, I.; Kasun, B.; Gotovac, M.; Gverović Antunica, A. Psychiatric Disorders and Dry Eye Disease—A Transdisciplinary Approach. Psychiatr. Danub. 2021, 33 (Suppl. S4), 580–587. [Google Scholar]
- Kaštelan, S.; Lukenda, A.; Salopek-Rabatić, J.; Pavan, J.; Gotovac, M. Dry eye symptoms and signs in long-term contact lens wearers. Coll. Antropol. 2013, 37 (Suppl. S1), 199–203. [Google Scholar]
- Bachhuber, F.; Huss, A.; Senel, M.; Tumani, H. Diagnostic biomarkers in tear fluid: From sampling to preanalytical processing. Sci. Rep. 2021, 11, 10064. [Google Scholar] [CrossRef]
- Acera, A.; Rocha, G.; Vecino, E.; Lema, I.; Durán, J.A. Inflammatory Markers in the Tears of Patients with Ocular Surface Disease. Ophthalmic. Res. 2008, 40, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Uchino, E.; Nakao, K.; Sakamoto, T. Inflammatory Cytokine of Basal and Reflex Tears Analysed by Multicytokine Assay. Br. J. Ophthalmol. 2006, 90, 120–122. [Google Scholar] [CrossRef][Green Version]
- Moreddu, R.; Elsherif, M.; Adams, H.; Moschou, D.; Cordeiro, M.F.; Wolffsohn, J.S.; Vigolo, D.; Butt, H.; Cooper, J.M.; Yetisen, A.K. Integration of paper microfluidic sensors into contact lenses for tear fluid analysis. Lab. Chip. 2020, 20, 3970–3979. [Google Scholar] [CrossRef]
- Örnek, N.; Dag, E.; Örnek, K. Corneal Sensitivity and Tear Function in Neurodegenerative Diseases. Curr. Eye Res. 2015, 40, 423–428. [Google Scholar] [CrossRef]
- Tamhane, M.; Cabrera-Ghayouri, S.; Abelian, G.; Viswanath, V. Review of Biomarkers in Ocular Matrices: Challenges and Opportunities. Pharm. Res. 2019, 36, 40. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Duan, X.; Petznick, A.; Wenk, M.R.; Shui, G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid. Res. 2014, 55, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Tomić, M.; Salopek-Rabatić, J.; Novak, B. Diagnostic procedures and management of dry eye. Biomed. Res. Int. 2013, 2013, 309723. [Google Scholar] [CrossRef]
- Yu, M.D.; Park, J.K.; Kossler, A.L. Stimulating Tear Production: Spotlight on Neurostimulation. Clin. Ophthalmol. 2021, 15, 4219–4226. [Google Scholar] [CrossRef] [PubMed]
- Erdinest, N.; Pincovich, S.; London, N.; Solomon, A. Neurostimulation for dry eye disease. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 328–334. [Google Scholar] [CrossRef]
- Kalló, G.; Emri, M.; Varga, Z.; Ujhelyi, B.; Tozsér, J.; Csutak, A.; Csosz, É. Changes in the Chemical Barrier Composition of Tears in Alzheimer’s Disease Reveal Potential Tear Diagnostic Biomarkers. PLoS ONE 2016, 11, e0158000. [Google Scholar] [CrossRef]
- Kenny, A.; Jiménez-Mateos, E.M.; Zea-Sevilla, M.A.; Rábano, A.; Gili-Manzanaro, P.; Prehn, J.H.; Henshall, D.C.; Ávila, J.; Engel, T.; Hernández, F. Proteins and MicroRNAs Are Differentially Expressed in Tear Fluid from Patients with Alzheimer’s Disease. Sci. Rep. 2019, 9, 15437. [Google Scholar] [CrossRef]
- Del Prete, S.; Marasco, D.; Sabetta, R.; Del Prete, A.; Marino, F.Z.; Franco, R.; Troisi, S.; Troisi, M.; Cennamo, G. Tear Liquid for Predictive Diagnosis of Alzheimer’s Disease. Reports 2021, 4, 26. [Google Scholar] [CrossRef]
- Gijs, M.; Ramakers, I.H.G.B.; Visser, P.J.; Verhey, F.R.J.; van de Waarenburg, M.P.H.; Schalkwijk, C.G.; Nuijts, R.M.M.A.; Webers, C.A.B. Association of Tear Fluid Amyloid and Tau Levels with Disease Severity and Neurodegeneration. Sci. Rep. 2021, 11, 22675. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chuang, H.C.; Tripathi, A.; Wang, Y.L.; Ko, M.L.; Chuang, C.C.; Chen, J.C. High-Sensitivity and Trace-Amount Specimen Electrochemical Sensors for Exploring the Levels of β-Amyloid in Human Blood and Tears. Anal. Chem. 2021, 93, 8099–8106. [Google Scholar] [CrossRef]
- Gharbiya, M.; Visioli, G.; Trebbastoni, A.; Albanese, G.M.; Colardo, M.; D’Antonio, F.; Segatto, M.; Lambiase, A. Beta-Amyloid Peptide in Tears: An Early Diagnostic Marker of Alzheimer’s Disease Correlated with Choroidal Thickness. Int. J. Mol. Sci. 2023, 24, 2590. [Google Scholar] [CrossRef]
- Grimmer, T.; Riemenschneider, M.; Förstl, H.; Henriksen, G.; Klunk, W.E.; Mathis, C.A.; Shiga, T.; Wester, H.J.; Kurz, A.; Drzezga, A. Beta Amyloid in Alzheimer’s Disease: Increased Deposition in Brain Is Reflected in Reduced Concentration in Cerebrospinal Fluid. Biol. Psychiatry 2009, 65, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Calais, G.; Forzy, G.; Crinquette, C.; MacKowiak, A.; de Seze, J.; Blanc, F.; Lebrun, C.; Heinzlef, O.; Clavelou, P.; Moreau, T.; et al. Tear Analysis in Clinically Isolated Syndrome as New Multiple Sclerosis Criterion. Mult. Scler. J. 2009, 16, 87–92. [Google Scholar] [CrossRef]
- Serafini, B.; Rosicarelli, B.; Magliozzi, R.; Stigliano, E.; Aloisi, F. Detection of Ectopic B-Cell Follicles with Germinal Centers in the Meninges of Patients with Secondary Progressive Multiple Sclerosis. Brain Pathol. 2004, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Van Setten, G.B.; Nilsson, L.; Hahne, S.; Johnston, J.A.; Kvanta, A.; Gandy, S.E.; Näslund, J.; Nordstedt, C. Beta-Amyloid Protein Protein Precursor Expression in Lacrimal Glands and Tear Fluid. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2585–2593. [Google Scholar]
- Selkoe, D.J. Cell Biology of the Amyloid Beta-Protein Precursor and the Mechanism of Alzheimer’s Disease. Annu. Rev. Cell Biol. 1994, 10, 373–403. [Google Scholar] [CrossRef]
- Altman, J.; Jones, G.; Ahmed, S.; Sharma, S.; Sharma, A. Tear Film MicroRNAs as Potential Biomarkers: A Review. Int. J. Mol. Sci. 2023, 24, 3694. [Google Scholar] [CrossRef]
- Ravishankar, P.; Daily, A. Tears as the Next Diagnostic Biofluid: A Comparative Study between Ocular Fluid and Blood. Appl. Sci. 2022, 12, 2884. [Google Scholar] [CrossRef]
- Higaki, S.; Muramatsu, M.; Matsuda, A.; Matsumoto, K.; Satoh, J.-I.; Michikawa, M.; Niida, S. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models. PLoS ONE 2018, 13, e0196929. [Google Scholar] [CrossRef]
- Liu, C.-G.; Wang, J.-L.; Li, L.; Wang, P.-C. MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer’s disease. Int. J. Mol. Med. 2014, 34, 160–166. [Google Scholar] [CrossRef]
- Wijesinghe, P.; Xi, J.; Cui, J.; Campbell, M.; Pham, W.; Matsubara, J.A. MicroRNAs in tear fluids predict underlying molecular changes associated with Alzheimer’s disease. Life Sci. Alliance 2023, 6, e202201757. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Poon, C.H.; Zhang, Z.; Yue, M.; Chen, R.; Zhang, Y.; Hossain, M.F.; Pan, Y.; Zhao, J.; Rong, L.; et al. MicroRNA-128 suppresses tau phosphorylation and reduces amyloid-beta accumulation by inhibiting the expression of GSK3β, APPBP2, and mTOR in Alzheimer’s disease. CNS Neurosci. Ther. 2023, 29, 1848–1864. [Google Scholar] [CrossRef]
- Park, H.; Lee, Y.B.; Chang, K.A. miR-200c suppression increases tau hyperphosphorylation by targeting 14-3-3γ in early stage of 5xFAD mouse model of Alzheimer’s disease. Int. J. Biol. Sci. 2022, 18, 2220–2234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Cui, J.G.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. Increased expression of miRNA-146a in Alzheimer’s disease transgenic mouse models. Neurosci. Lett. 2011, 487, 94–98. [Google Scholar] [CrossRef][Green Version]
- He, B.; Chen, W.; Zeng, J.; Tong, W.; Zheng, P. MicroRNA-326 decreases tau phosphorylation and neuron apoptosis through inhibition of the JNK signaling pathway by targeting VAV1 in Alzheimer’s disease. J. Cell Physiol. 2020, 235, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Veerakumarasivam, A.; Lim, W.L.; Chew, J. Neuroprotective Effects of Lactoferrin in Alzheimer’s and Parkinson’s Diseases: A Narrative Review. ACS Chem. Neurosci. 2023, 14, 1342–1355. [Google Scholar] [CrossRef]
- Bruno, F.; Malvaso, A.; Canterini, S.; Bruni, A.C. Antimicrobial Peptides (AMPs) in the Pathogenesis of Alzheimer’s Disease: Implications for Diagnosis and Treatment. Antibiotics 2022, 11, 726. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C.; Zhang, J. Lactoferrin and Its Detection Methods: A Review. Nutrients 2021, 13, 2492. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. Biometals 2004, 17, 189–196. [Google Scholar] [CrossRef]
- Kawamata, T.; Tooyama, I.; Yamada, T.; Walker, D.G.; McGeer, P.L. Lactotransferrin immunocytochemistry in Alzheimer and normal human brain. Am. J. Pathol. 1993, 142, 1574–1585. [Google Scholar] [PubMed]
- Wang, L.; Sato, H.; Zhao, S.; Tooyama, I. Deposition of lactoferrin in fibrillar-type senile plaques in the brains of transgenic mouse models of Alzheimer’s disease. Neurosci. Lett. 2010, 481, 164–167. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Li, F.; Zhou, Y.; Qi, L.; Liu, L.; Chen, Z. Lactoferrin improves cognitive function and attenuates brain senescence in aged mice. J. Funct. Foods 2020, 65, 103736. [Google Scholar] [CrossRef]
- Guo, C.; Yang, Z.H.; Zhang, S.; Chai, R.; Xue, H.; Zhang, Y.H.; Li, J.Y.; Wang, Z.Y. Intranasal Lactoferrin Enhances α-Secretase-Dependent Amyloid Precursor Protein Processing via the ERK1/2-CREB and HIF-1α Pathways in an Alzheimer’s Disease Mouse Model. Neuropsychopharmacology 2017, 42, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.; Jung, C.G.; Zhou, C.; Abdullah, M.; Nakano, M.; Wakabayashi, H.; Abe, F.; Michikawa, M. Dietary Lactoferrin Suppl.ementation Prevents Memory Impairment and Reduces Amyloid-β Generation in J20 Mice. J. Alzheimers Dis. 2020, 74, 245–259. [Google Scholar] [CrossRef]
- Antequera, D.; Moneo, D.; Carrero, L.; Bartolome, F.; Ferrer, I.; Proctor, G.; Carro, E. Salivary Lactoferrin Expression in a Mouse Model of Alzheimer’s Disease. Front. Immunol. 2021, 12, 749468. [Google Scholar] [CrossRef]
- Carro, E.; Bartolomé, F.; Bermejo-Pareja, F.; Villarejo-Galende, A.; Molina, J.A.; Ortiz, P.; Orive, G. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 131–138. [Google Scholar] [CrossRef]
- González-Sánchez, M.; Bartolome, F.; Antequera, D.; Puertas-Martín, V.; González, P.; Gómez-Grande, A.; Llamas-Velasco, S.; Herrero-San Martín, A.; Pérez-Martínez, D.; Villarejo-Galende, A.; et al. Decreased salivary lactoferrin levels are specific to Alzheimer’s disease. EBioMedicine 2020, 57, 102834. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mim, S.A.; Islam, M.R.; Parvez, A.; Islam, F.; Uddin, M.B.; Rahaman, M.S.; Shuvo, P.A.; Ahmed, M.; Greig, N.H.; et al. Exploring the Recent Trends in Management of Dementia and Frailty: Focus on Diagnosis and Treatment. Curr. Med. Chem. 2022, 29, 5289–5314. [Google Scholar] [CrossRef]
- Wood, H. Alzheimer disease: Could tear proteins be biomarkers for Alzheimer disease? Nat. Rev. Neurol. 2016, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Bogadi, M.; Bakija, I. Eyes as the Window to the Brain—A Key to the Schizophrenia Puzzle. Psychiatr. Danub. 2022, 34, 107–108. [Google Scholar] [PubMed]
- Winiarczyk, M.; Biela, K.; Michalak, K.; Winiarczyk, D.; Mackiewicz, J. Changes in Tear Proteomic Profile in Ocular Diseases. Int. J. Environ. Res. Public Health 2022, 19, 13341. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.; Winblad, B.; O’Brien, J. Revised NIA-AA criteria for the diagnosis of Alzheimer’s disease: A step forward but not yet ready for widespread clinical use. Int. Psychogeriatr. 2011, 23, 1191–1196. [Google Scholar] [CrossRef]
- Chaitanuwong, P.; Singhanetr, P.; Chainakul, M.; Arjkongharn, N.; Ruamviboonsuk, P.; Grzybowski, A. Potential Ocular Biomarkers for Early Detection of Alzheimer’s Disease and Their Roles in Artificial Intelligence Studies. Neurol. Ther. 2023, 12, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Advantages | Limitations |
|---|---|---|
| Genetic | Insights into an individual’s genetic predisposition Insights into pathogenesis Early detection and risk assessment Development of personalized treatment strategies Clinical trial recruitment | Incomplete penetrance and gene expression Complex interactions with other factors Limited predictive accuracy Cost and accessibility Lack of treatment options Rarely used for routine clinical diagnosis of AD |
| Brain imaging CT | Provide detailed structural information Preferable to MRI for non-collaborative patients Relatively low cost Greater accessibility in developing countries | Not sensitive to early changes Limited quantitative measurements Lack of functional information Radiation exposure Inferior soft tissue detail Not considered as a standard biomarker for early diagnosis of AD |
| Brain imaging MRI | Insight into the microstructural, functional and molecular alterations occurring in the brain (DTI, MRS, fMRI) No radiation exposure Early diagnosis Quantification of brain atrophy Longitudinal monitoring of disease progression Distinguishing AD from other neurodegenerative disorders | Cost and availability Time consuming Patient cooperation Complexity of interpretation: expertise is required for the analysis of images Limited molecular information in comparison to PET scans Limited specificity and overlap with ageing Potentially missing early disease-related alterations Contrast side effects |
| Brain imaging PET scan | Early detection allowing for timely intervention and treatment planning Objective measurement of the extent and distribution of beta-amyloid and tau pathology in the brain Differentiation of AD from other forms of dementia Tracking disease progression Clinical trial recruitment | Cost and availability Exposition to ionizing radiation Time consuming Possibility of false positive and false negative results Ethical considerations in asymptomatic individuals Complexity of interpretation: expertise is required for the analysis of images Contrast side effects |
| Cerebrospinal fluid | Proximity to the brain—contains brain proteins High concentration of biomarkers Capacity to test numerous potential biomarkers Standardized methodology High accuracy in the diagnostic procedures Potential detection of AD in its early stages, even before significant clinical symptoms Longitudinal monitoring of disease progression Can be used as outcome measures to assess the efficacy of potential therapies | Invasive procedure Requires hospitalization Need for specialized expertise and equipment for collection and analysis Relatively high cost Risk of complications (infection, headache) A less acceptable procedure among the general population A risk of inducing harm, fear and anxiety in the patient Complex interpretation, as biomarker levels may vary due to age, gender and underlying health conditions |
| Blood | Minimally invasive and simple sampling Cost- and time-efficient Widespread use Reproducible and simple to measure Easy to implement in large populations Ability to test a large number of biomarkers The possibility of repeated sampling and measurements Initial diagnostic examination in a complex diagnostic procedure | Relatively low concentration of the potential biomarkers due to the presence of the blood–brain barrier Significant dilution of analytes caused by the volume ratio between the blood and the CSF Unreliability of findings—blood is a complex fluid—non-specific biomarkers may be expressed from sources other than the CNS Possible influence on the concentration levels of biomarkers due to liver or plasma proteolytic degradation, plasma protein or blood cell adhesion and kidney excretion The sensitivity and specificity of blood biomarkers for AD are still considerably low |
| Saliva | Easily accessible and non-invasive collection technique Repeatable collection—opportunity to monitor biomarker fluctuations Cost-effective and minimally stressful Without risk of infection Suitable for a wide range of individuals Convenient and reproducible sample collection Accessibility without regard to location or time limitations | Lack of standardisation in collection procedures (stimulated versus unstimulated samples), pre-processing and storage of samples Lack of validated studies Lack of results replicated in broader, multicentre and longitudinal investigations Difficulties in collecting saliva due to poor compliance of elderly patients, particularly with AD Limited sensitivity and specificity Saliva is a pooled sample from different salivary glands—potential influence on the sample composition Influence of ageing, oral health, circadian variations, environmental factors, psychogenic disorders, medication use, local and systemic pathology and treatment |
| Tears | Close association between eye and the brain Easily accessible and non-invasive collection technique Repeatable collection—opportunity to monitor biomarker fluctuations Cost-effective and minimally stressful Without risk of infection Suitable for a wide range of individuals Convenient and reproducible sample collection Accessibility without regard to location or time limitations | Lack of standardisation in collection procedures, pre-processing and storage of samples Lack of validated studies Lack of results that have been replicated in broader, multicentre and longitudinal investigations. Small volume sample size Tear production and drainage can influence the concentration of biomarkers The influence of circadian rhythms and environmental factors |
| Sampling Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Schirmer’s strip | Strips of sterile filter paper with an imprinted graduated scale Placed inside the lower eyelid with or without anaesthetics (basal or reflex tears) | Simple application Available in routine clinical practice | Unsuitable for dry eye Causes discomfort Demanding sampling processing Requires tear fluid extraction Retention of proteins |
| Microcapillary tube | Small diameter thin-walled tubes with capillary action Collect a volume of 10 µL within 10 minutes Gently placed to the lower eyelid | Comfortable procedure less frequent and shorter sensation of foreign body Convenient sampling collection method Easy post-collection procedure No elution necessary | Unsuitable for dry eye Risk of reflex tearing Requires experience Time-consuming process |
| Cellulose micro-sponge | Sponges with a high absorption rate Insertion—an inferior cul-de-sac of the eye, the eye surface of the inferior lower lid | Comfortable procedure (children) Time-saving Highly efficient Good reproducibility | Unsuitable for dry eye Demanding sampling processing Retention of proteins Inability to accurately determine the volume of tears |
| Micropipette | Tear samples collected using a micropipette after a flush of sterile distilled water (20 µL) over the eye surface | Suitable for dry eye Comfortable procedure Time-saving | Questionable reproducibility Diluted sample |
| Author (Year) | Biomarker | AD Related Changes | Collection Method | Analytical Method | Results |
|---|---|---|---|---|---|
| Kallo et al. (2016) [67] | Lysozyme-C, Lipocalin-1 Lacritin Dermcidin | ↓ ↓ ↓ ↑ | Microcapillary tube | Electrophoresis LC–MS/MS analysis SRM-based targeted proteomics mass spectrometry | Significantly increased tear flow rates in AD patients. Significantly increased total protein concentration in tears of AD patients. Combination of lysozyme-C, lipocalin-1, lacritin and dermcidin could be a potential biomarker for AD, with 81% sensitivity and 77% specificity. |
| Kenny et. al. (2019) [68] | microRNAs microRNA-200b-5p eIF4E | ↑ ↑ Present | Schirmer’s strips | Reverse phase-liquid chromatography RP-LC–MS/MS analysis Genome-wide high-throughput qPCR-based microRNA platform (Open Array) | Total microRNA abundance higher in AD patients. Elevated microRNA-200b-5p levels in tears of AD patients—potential biomarker. eIF4E existing only in AD patients—potential biomarker. |
| Del Prete et al. (2021) [69] | Aβ42 | ↑ | Not reported | Immunocytochemistry assay | Aβ42 protein was found in the tear fluid of healthy subjects with a family history of AD. Relationship between the retinal plaques and the expression of Aβ42. |
| Gijs et al. (2021) [70] | Aβ38 Aβ40 Aβ42 T-tau P-tau | ↑ ↑ ↑ ↑ ↑ | Schirmer’s strips without topical anaesthesia | Multiplex immunoassay | Levels of Aβ40 and T-tau detectable in tear fluid of 94% of patients with cognitive impairment and correlated with cognitive decline. Tear T-tau levels elevated in patients with neurodegeneration. Tear T-tau concentrations were found to be ten times higher than measured in CSF. |
| Wang et al. (2021) [71] | Aβ40 Aβ42 | NA | Schirmer’s strips | Electrochemical immunosensor | New biosensor testing. The concentration of Aβ peptide in tears of healthy people (10 pg/mL) was 10 times higher than in blood (1 pg/mL). Aβ concentration in healthy subjects was inversely proportional to age. |
| Gharbiya et al. (2023) [72] | Aβ1-42 APP-CTF P-tau | ↓ NS NS | Micro-sponges | ELISA Western blot | Aβ1-42 levels in tears were lower in patients with mild cognitive impairment (p < 0.01) and with AD group (p < 0.001) compared to healthy controls. No differences were observed in the concentration of APP-CTF and P-tau in tears. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaštelan, S.; Braš, M.; Pjevač, N.; Bakija, I.; Tomić, Z.; Pjevač Keleminić, N.; Gverović Antunica, A. Tear Biomarkers and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 13429. https://doi.org/10.3390/ijms241713429
Kaštelan S, Braš M, Pjevač N, Bakija I, Tomić Z, Pjevač Keleminić N, Gverović Antunica A. Tear Biomarkers and Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(17):13429. https://doi.org/10.3390/ijms241713429
Chicago/Turabian StyleKaštelan, Snježana, Marijana Braš, Neda Pjevač, Ivana Bakija, Zora Tomić, Nada Pjevač Keleminić, and Antonela Gverović Antunica. 2023. "Tear Biomarkers and Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 17: 13429. https://doi.org/10.3390/ijms241713429
APA StyleKaštelan, S., Braš, M., Pjevač, N., Bakija, I., Tomić, Z., Pjevač Keleminić, N., & Gverović Antunica, A. (2023). Tear Biomarkers and Alzheimer’s Disease. International Journal of Molecular Sciences, 24(17), 13429. https://doi.org/10.3390/ijms241713429







