Abstract
Dysregulation of clusterin (CLU) has been demonstrated in many cancers and has been proposed as a regulator of carcinogenesis. However, the roles of CLU in gliomas remain unclear. The expression of CLU was assessed using TIMER2.0, GEPIA2, and R package 4.2.1 software, leveraging data from TCGA and/or GTEx databases. Survival analysis and Cox regression were employed to investigate the prognostic significance of CLU. Immune infiltration was evaluated utilizing TIMER2.0, ESTIMATE, and CIBERSORT. The findings reveal the dysregulated expression of CLU in many cancers, with a marked increase observed in glioblastoma and lower-grade glioma (LGG). High CLU expression indicated worse survival outcomes and was an independent risk factor for the prognosis in LGG patients. CLU was involved in immune status as evidenced by its strong correlations with immune and stromal scores and the infiltration levels of multiple immune cells. Additionally, CLU was co-expressed with multiple immune-related genes, and high CLU expression was associated with the activation of immune-related pathways, such as binding to the antigen/immunoglobulin receptor and aiding the cytokine and cytokine receptor interaction. In conclusion, CLU appears to play crucial roles in tumor immunity within gliomas, highlighting its potential as a biomarker or target in glioma immunotherapy.
1. Introduction
Gliomas are the most common malignant primary brain tumors, accounting for 80.9% of malignant tumors in the central nervous system [1]. Glioblastoma (GBM, WHO grade IV), comprising 59.2% of gliomas [1], represents the most aggressive subtype, characterized by a 5-year survival rate of just 9.8% [2]. In contrast, lower-grade glioma (LGG, WHO grades II and III) exhibits a comparatively more favorable survival prognosis when compared to GBM. The currently standard treatment for gliomas is still confined to surgical resection and adjuvant chemotherapy with temozolomide combined with radiotherapy [2]. Although the standard treatment has been demonstrated to improve the survival of patients, these improvements are limited; the recurrence and progression of the tumor are still ineluctable, and the prognosis of patients remains unsatisfactory [3]. Immunotherapy is an innovative approach to cancer treatment that has demonstrated success across various cancer types [4,5]. Although there are still several challenges for the successful establishment of immunotherapy for gliomas, a better understanding of the immune infiltrates in the tumor microenvironment (TME) of gliomas may contribute to the development of more refined immunotherapies for the treatment of gliomas [6,7].
Clusterin (CLU) is an omnipresent conserved glycoprotein commonly secreted by cells that has been described as a stress-activated, ATP-independent molecular chaperone involved in a wide variety of pathological and physiological processes [8,9]. CLU is considered to be a regulator of carcinogenesis [10]. Elevated levels of CLU are observed in a variety of cancers, which exhibit close correlations with the risk for developing several cancers [11]. In addition, increasing evidence has demonstrated that CLU modulates a variety of cellular events associated with cancers, such as cancer stemness, epithelial–mesenchymal transition, cell survival, and treatment resistance, thus mediating the progression of many cancers, including breast carcinoma, renal cell carcinoma, bladder cancer, prostate cancer, hepatocellular carcinoma, esophageal cancer, colorectal cancer, ovarian cancer, and lung cancer [8,12,13,14,15,16]. Moreover, CLU seems to modulate tumor progression and metastasis by mediating the components of the TME. For example, CLU modulates the chemotactic migration and polarization of tumor-associated macrophages (TAM) by regulating the secretion of chemotactic cytokines [17], and it modulates the recruitment of dendritic cells (DCs) by regulating the expression of chemokine CCL20 [18]. Such properties potentialize CLU to be a promising target in cancer therapy. In central nervous system tumors, CLU has been observed to exhibit high expression levels in pituitary adenomas compared to its expression in non-neoplastic adenohypophyses [19]. Additionally, it has been identified as a tumor suppressor in neuroblastomas [20]. However, the specific functions of CLU in gliomas have been scarcely explored.
To this end, we designed this study to conduct a comprehensive investigation on the roles of CLU in gliomas in terms of three major aspects. First, expression of CLU in gliomas and its correlations with clinicopathologic features and prognosis were investigated. Second, the associations between CLU expression and the infiltration of immune cells as well as immune-checkpoint levels in the TME were explored. Third, the associations between CLU expression and the functional pathways were further investigated to uncover the underlying molecular mechanism. This study will provide evidence for the roles of CLU in gliomas and its potential to be a prognostic biomarker or therapeutic target in gliomas.
2. Results
2.1. Expression Pattern of CLU in Pan-Cancers
To assess the potential of clusterin (CLU) as a therapeutic target, the expression pattern of CLU was initially investigated in various types of cancers, comparing tumor tissues to their corresponding matched normal tissues. Based on the results obtained from TIMER2.0, we found that the expression of CLU was significantly reduced in the tumor tissue of a majority of cancers compared to their matched normal controls, such as bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), and colorectal (COAD and READ) and lung (LUSC and LUAD) cancers. In contrast, CLU exhibited elevated expression levels in kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), thyroid carcinoma (THCA), and GBM when compared to tissues from matched control samples (Figure 1A).
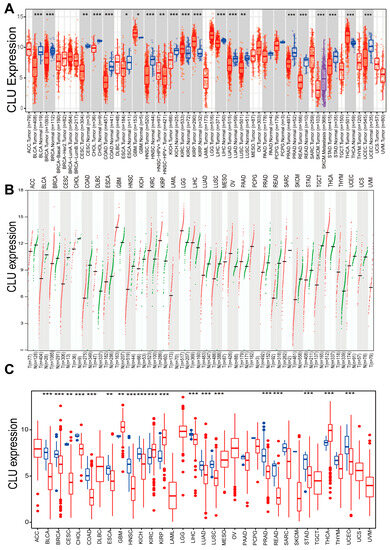
Figure 1.
Expression of CLU in pan-cancers. Expression levels of CLU in distinct cancers or tumor tissues and neighboring non-tumor tissues analyzed using TIMER2.0 ((A), red boxes represent tumor tissues, and green boxes represent normal tissues). GEPIA2 ((B), each dot represents the expression profile in one sample) and R package based on TCGA and/or GTEx databases (C). * p < 0.05, ** p < 0.01, *** p < 0.001.
The Gene Expression Profiling Interactive Analysis (GEPIA), which relies on tumor tissues from The Cancer Genome Atlas (TCGA) and normal tissues from the Genotype-Tissue Expression (GTEx) project, unveiled that the expression patterns of CLU exhibited tumor specificity. Among 33 different cancer types, its highest expression was observed in GBM and LGG (Figure 1B). Additionally, expression levels of CLU across 33 cancer types from TCGA database were further analyzed using R package, and similar results were observed with the findings of TIMER2.0 (Figure 1C). Expression of CLU was significantly reduced in tumor tissue in a majority of cancers, while it was significantly elevated in the tumor tissues of KIRC, KIRP, THCA, and GBM.
2.2. Expression Pattern of CLU in Gliomas
We further investigated the expression distributions of CLU in gliomas. According to the expression profiles in TCGA, we found that CLU expression was associated with the tumor grades of gliomas, with markedly higher expression in GBM than in LGG (Figure 2A). CLU expression had been demonstrated to increase in tumor tissue of GBM samples than normal tissue, Figure 1A–C, thus the expression of GLU in LGG were investigated primarily in the following analyses. Normal tissues of LGG samples were absent in TCGA database (Figure 2B), therefore expression of CLU in LGG was analyzed based on the tumor tissues in TCGA and the normal tissue in GTEx, and elevated expression of CLU in tumor samples than normal samples was observed (Figure 2C). GEPIA analysis revealed consistent results that CLU was highly expressed in tumor samples than normal samples in LGG (Figure 2D). Protein expression of CLU in LGG and normal tissue samples were further verified using immunohistochemical staining (Figure 2E). Consistently, protein expression of CLU was markedly higher in LGG tissue samples in comparison with that of normal tissue samples (Figure 2F). These findings indicated a significantly elevated CLU expression at both mRNA and protein levels in LGG tissue samples.
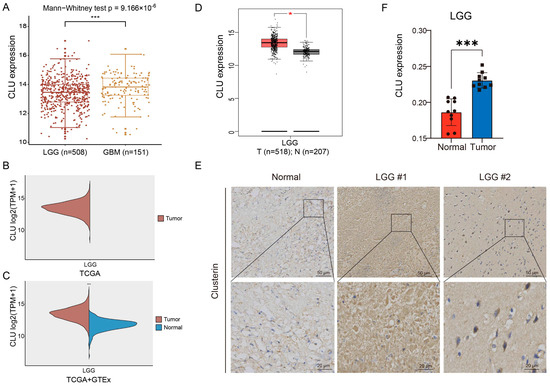
Figure 2.
Expression pattern of CLU in gliomas. (A) Differential CLU expression between LGG (WHO grade II and III) and GBM (WHO grade IV). (B) Levels of CLU in LGG tissue samples in TCGA. (C) Differential expression of CLU between LGG tissue in TCGA and normal tissue samples in GTEx. (D) Expression levels of CLU in between LGG tissue and normal tissue analyzed using GEPIA2. (E,F) Representative images and quantification of immunohistochemical staining for CLU in LGG tissue and normal tissue. * p < 0.05, *** p < 0.001.
2.3. CLU Expression Independently Associated with Prognosis of Patients with LGG
Prognostic value of CLU in LGG was explored using survival analyses on four survival outcomes. LGG patients with high CLU expression had significantly worse overall survival (OS, p < 0.001), disease-specific survival (DSS, p < 0.001), and progression-free interval (PFI, p < 0.001) in comparison with patients with low CLU expression (Figure 3A–C), indicating the associations of CLU expression with survival outcomes of LGG patients. However, no significant associations between CLU expression and disease-free interval (DFI, p = 0.342) of LGG patients were observed (Figure 3D). The independent force of CLU expression and three clinical factors were further explored. Forest plots revealed that both CLU expression and tumor grades were independently associated with the prognosis of LGG patients (Figure 3E,F). High CLU expression (hazard ratio of 1.451) and high glioma grades (hazard ratio of 3.145) were identified as two risk factors for prognosis of LGG patients.
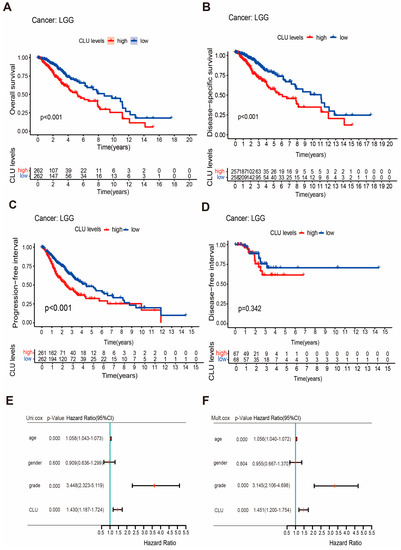
Figure 3.
Prognostic value of CLU in LGG. (A–D) Survival curves showing the differences on overall survival (A), disease-specific survival (B), progression-free interval (C), and disease-free interval (D) between high and low CLU expression; (E,F), forest plots showing the results of univariable (E) and multivariate (F) Cox regression.
2.4. Association of CLU Expression with Immune Infiltrates in LGG and GBM
The involvements of CLU in immune infiltrates in TME of LGG and GBM were further investigated using different methods. TIMER2.0 was applied to explore the correlations of CLU expression with the infiltration fractions of six main types of immune cells. LGG patients with high infiltration abundance of these six immune cells seemed to have worse cumulative survival in comparison with those patients with low infiltration abundance (Figure 4A). In addition to CD8 T cells, CLU expression showed outstanding positive correlations with the other five immune cells, including DCs (r = 0.341, p = 2.03 × 10−14), B cells (r = 0.183, p = 5.56 × 10−5), neutrophils (r = 0.328, p = 2.37 × 10−13), CD4+ T cells (r = 0.342, p = 1.54 × 10−14), and macrophages (r = 0.401, p = 1.15 × 10−19), which determined a negative correlation (r = −0.341, p = 1.54 × 10−14) of CLU expression with tumor purity in LGG (Figure 4B). Additionally, prominent positive correlations of CLU expression with stromal (r = 0.51, p < 2.2 × 10−16) and immune (r = 0.5, p < 2.2 × 10−16) scores were observed in LGG (Figure 4C,D). Moreover, correlation analysis of CLU expression with infiltration abundance of ten immune cells types inferred using CIBERSORT was further calculated (Figure 4E–N), and the results indicated that CLU expression positively correlated with CD8 T cells (r = 0.18, p = 0.00083), resting memory CD4 T cells (r = 0.21, p = 0.00012), macrophages M1 (r = 0.14, p = 0.0096), and resting mast cells (r = 0.15, p = 0.0068), while negatively correlated with activated mast cells (r = −0.15, p = 0.0051) in LGG.
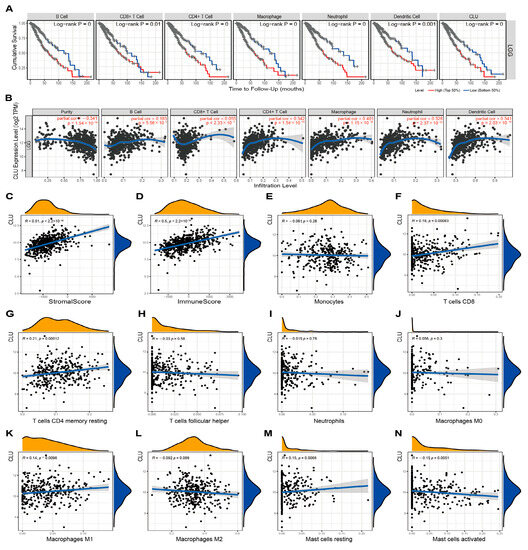
Figure 4.
Associations of CLU with immune infiltration in LGG. (A) Cumulative survival of LGG patients with high and low infiltrating levels of six immune cells in TIMER2.0. (B) Correlations between CLU expression and infiltrating levels of six immune cells in TIMER2.0. (C,D) Correlations of CLU expression with stromal (C) and immune (D) scores. (E–N) Correlations between CLU expression and infiltrating levels of immune cells using CIBERSORT.
In terms of GBM, infiltration abundance of six immune cells in TIMER2.0 showed no significant associations with cumulative survival of patients, in addition to DCs (Figure 5A). Similar to the findings in LGG, CLU expression showed outstanding positive correlations with five immune cells (all p < 0.05), except CD8 T cells in TIMER2.0 analysis (Figure 5B), and showed prominent positive correlations with stromal and immune scores (all r > 0.3, p < 0.05, Figure 5C,D). Additionally, CLU expression exhibited positive correlations with monocytes (r = 0.3, p = 0.00012) and resting memory CD4 T cells (r = 0.26, p = 0.00069), while negative correlations with macrophage M0 (r = −0.17, p = 0.031) and M2 (r = −0.33, p = 1.7 × 10−5) in GBM, which were inconsistent with the findings in LGG, and the detailed comparison of various parameters between LGG and GBM is shown in Table 1 and Table 2.
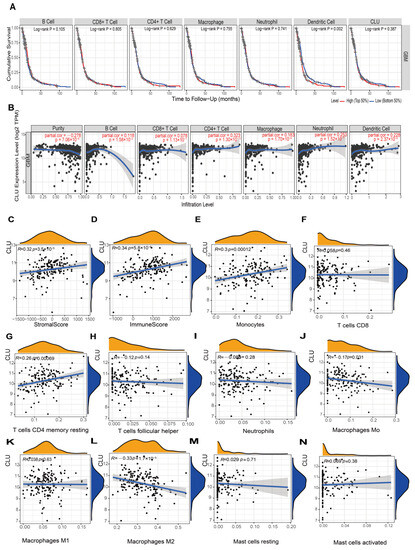
Figure 5.
Associations of CLU with immune infiltration in GBM. (A) Cumulative survival of GBM patients with high and low infiltrating levels of six immune cells in TIMER2.0. (B) Correlations between CLU expression and infiltrating levels of six immune cells in TIMER2.0. (C,D) Correlations of CLU expression with stromal (C) and immune (D) scores. (E–N) Correlations between CLU expression and infiltrating levels of the immune cells inferred using CIBERSORT.

Table 1.
Correlation analysis between CLU and immune cells using CIBERSORT in LGG and GBM.

Table 2.
Correlation analysis between CLU and immune cells using TIMER in LGG and GBM.
2.5. Associations of CLU Expression with Immune Status-Related Genes
To further uncover the possible mechanisms of CLU involved in immune infiltration, associations of CLU expression with several groups of immune-related genes were explored. There were prominent positive correlations between CLU expression and expression of almost all immunosuppressive genes and most immune-activated genes, such as CD274, CTLA4, and PDCD1LG2 (Figure 6A,B). Moreover, CLU expression positively correlated with several chemokine ligands and receptors, such as CCR1/2/3/4/5, CXCR2/3/4, and CCL3/4/5, whereas negatively correlated with chemokine ligands and receptors, such as CCR6, CXCR5, and CCL1 (Figure 6C,D). We further investigated the expression of eight immune-checkpoints (Figure 6E) and found that expression of all these immune-checkpoints (e.g., CD274, PDCD1, and CTLA4, all p < 0.05) were markedly elevated in LGG patients with high CLU expression in comparison to SIGLEC15 (p = 0.43). These findings highlighted the involvements of CLU in immune status and its potential value in immunotherapy.

Figure 6.
Associations of CLU expression with immune status-related genes. (A–D) Co-expression of CLU expression with immune activation (A), immunosuppressive (B), chemokine receptors (C), and chemokine (D) genes. (E) Boxplots showing the differential expression of immune-checkpoints in high and low CLU expression groups. * p < 0.05, ** p < 0.01, *** p < 0.001.
2.6. CLU Expression-Associated Functional Pathways
Gene set enrichment analysis (GSEA) was applied to investigate the significantly functional pathways associated with CLU expression. Several immune-related gene ontology (GO) terms, such as immunoglobulin complex, phagocytosis recognition, binding to antigen/immunoglobulin receptor, were found to be associated with high CLU expression (Figure 7A). Additionally, immune-related Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, such as primary immunodeficiency and cytokine and cytokine receptor interaction, were activated with high CLU expression (Figure 7B). These results might uncover the potential mechanism of CLU involvement in immune status.
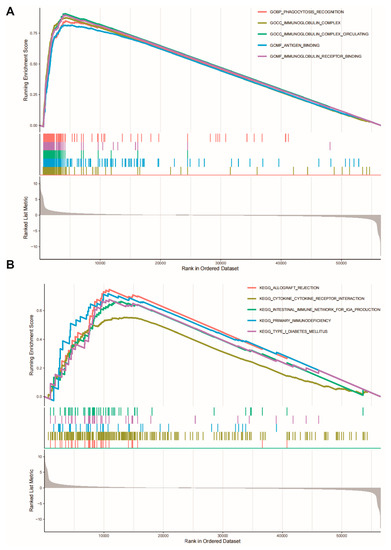
Figure 7.
Gene set enrichment analysis. CLU expression significantly associated with gene ontology terms (A) and KEGG pathways (B).
3. Discussion
Gliomas are the most common type of malignant intracranial tumors with extremely worse prognosis. Even with standard treatment, the recurrence and progression of tumor are still ineluctable for patients [21]. Increasing attention has been paid to prognosis-associated molecular markers in gliomas, such as 1p19q co-deletion, IDH mutations, and p53 mutation, as their application in clinical practice for gliomas has been demonstrated [22]. In addition, promising novel therapies, such as gene therapy and immunotherapy, are constantly being established [23,24]. Thus, it is crucial to identify additional prognostic biomarkers and develop new treatment strategy to optimize treatment in gliomas.
CLU has gained increasing attention due to its paradoxical and multifunctional properties in various pathologies [9] and is regarded as a regulator of carcinogenesis and a therapeutic target in cancers [8,10]. However, expression and the specific roles of CLU in glioma are rarely investigated. To this end, we conducted this study to conduct a comprehensive investigation on the roles of CLU in gliomas.
Expression of CLU in pan-cancers was first investigated, and it was found that CLU was down-regulated in most cancers. This seemed to be inconsistent with the previous reports that expression of CLU was elevated in many cancers [8,11], and this might be explained partly by the sample difference and the paradoxical property of CLU. The differential expression of CLU in a variety of cancers highlighted the importance of CLU in cancers, and the potential of CLU as a therapeutic target. Among 33 types of cancers, CLU showed highest expression in gliomas, including both GBM and LGG. In gliomas, CLU expression was markedly elevated in tumor tissue than in normal tissue, and immunohistochemical staining of CLU in clinical samples verified the high expression of CLU in tumor tissue. High CLU expression was found to be associated with worse survival outcomes. We found that CLU expression was associated with glioma grades, with higher expression levels in GBM than in LGG. Further Cox regression analyses indicated that high CLU expression was an independent risk factor for prognosis of LGG patients. Such findings indirectly explained why LGG patients with high CLU expression had a worse survival outcome. Effective prognostic biomarkers are important for the clinical management and making treatment decisions for patients because they provide key information in terms of tumor progression or clinical outcome [25,26]. Our analyses demonstrated that CLU was an independent prognostic biomarker in gliomas, associated with glioma grades and worse survival outcomes in gliomas.
Diverse immunotherapies have been developed in gliomas; however, unlike its successful use in other tumors, the effects of several immunotherapy strategies appear to be limited in gliomas [24]. The effect of immunotherapy relies mainly on the infiltrating immune cells within the TME of tumor, which are the crucial part of TME that can modulate the progression of tumors by their dynamic and extensive crosstalk with tumor cells [27,28]. Several molecules have been demonstrated to participate in such intercellular crosstalk [29,30]. Results from our analyses indicated that high CLU expression positively correlated with the infiltrating abundance of different immune cells, such as macrophages, DCs, and CD4+ T cells, which suggest that CLU might modulate the recruitment of immune cells in TME of gliomas to some extent. We further found that high CLU expression strongly correlated with multiple immune checkpoints and immune status-related genes as well as chemokine-related genes. Consistently, studies have reported that CLU modulates the chemotactic migration and polarization of TAM by regulating the secretion of chemotactic cytokines [17], and modulates the recruitment of DCs by regulating expression of chemokine CCL20 [18]. In addition, Yang et al. revealed that CLU was involved in the recruitment of immune cells in breast tumors, and its elevated expression was closely related to multiple specific immune cell subset-associated molecular markers [31]. Additionally, GSEA indicated that multiple immune-related biological processes and pathways were activated in LGG patients with high CLU expression. All these findings emphasized the close involvement of CLU in immune status of gliomas. Thus, we speculated that CLU might have the potential to be a biomarker or a specific target in glioma immunotherapy. Even though this study is preliminary, it still has several limitations about how CLU can be adapted and modulated with immunotherapy. Then, more mechanical and functional experiments should be performed to explore the effects of CLU on the immune cells within the tumor microenvironment in gliomas, in order to develop personalized approaches such as vaccination and explore multiple clinical trials investigating immunotherapy combination studies.
4. Materials and Methods
4.1. Data Sources and Differential Analysis of CLU Expression
Expression of CLU between tumor tissues and the corresponding normal tissues in pan-cancer were analyzed using the “Gene_DE Module” of TIMER2.0 (http://timer.cistrome.org/ 2022 accessed on 14 August 2023) [32] and GEPIA (version 2, http://gepia.cancer-pku.cn/ 2022 accessed on 14 August 2023) [33]. In addition, the transcriptome data of 33 pan-cancer types in the TCGA (https://tcga.xenahubs.net 2022 accessed on 14 August 2023) [34] were downloaded, and the differential expression of CLU was analyzed with the aid of Wilcoxon test provided in R package. Through these three ways, data were divided into high and low expression groups based on the median expression value of CLU expression. False Discovery Rate < 0.05 indicated statistical significance. The abbreviations and full names of the these tumor types are shown in Abbreviation Table.
Genes expression profiles of both LGG and GBM in TCGA, and genes expression profiles of normal samples in the GTEx (http://commonfund.nih.gov/GTEx/, accessed on 14 August 2023) [35] database were downloaded to conduct the differential analysis of CLU expression between tumor and normal tissues using Wilcoxon test. Verification of the differential expressions of CLU in LGG and normal tissues was conducted using GEPIA. FDR < 0.05 indicated statistical significance. Expression distributions of CLU were visualized in the form of a boxplot with the aid of “ggpubr” R package.
4.2. Immunohistochemical Staining
LGG (n = 10) and normal (n = 10) tissue samples were collected at the First Affiliated Hospital of Yan’an University from November 2020 to April 2023. The clinical characteristics of patients and healthy controls are detailed in Table 3. All LGG tissue samples were confirmed by pathologists. Protein expression of CLU in these tissue samples were analyzed utilizing immunohistochemical staining. In short, the tissues were firstly prepared as 3.5 µm-thick paraffin sections. Following deparaffinization, rehydration, and antigen retrieval, the sections were incubated with anti-CLU (SC-166907, 1:500, Santa Cruz, CA, USA) at 4 °C overnight. All slides were covered with poly-HRR goat anti-body and counter-stained with diaminobenzidine solution (3–5 min) and hematoxylin. Finally, the section was examined under a fluorescent microscope and analyzed using Image-Pro plus 6.0 (Media Cybernetics, Inc., Rockville, Maryland). The study was approved by the Research Ethics Committee of Northwest University, and all participants provided informed consent in accordance with the Declaration of Helsinki.

Table 3.
Clinical characteristics of LGG patients and healthy controls for validation.
4.3. Association between CLU Expression and Prognosis in LGG
The clinicopathological and survival data of LGG samples were downloaded from TCGA database. To assess the prognostic value of CLU in LGG, all the LGG samples were assigned into high CLU expression and low CLU expression on the basis of their median expression values. Then, survival analysis, including OS, DSS, PFI, and DFI, were assessed utilizing R survival package and were visualized as Kaplan–Meier curves with log-rank p-values. Additionally, the independent prognostic value of CLU and clinical factors, including age, gender, and tumor grades, were assessed using univariate and multivariate Cox regression analyses. The results were displayed in the form of forest plots using “forestplot” R package.
4.4. Immune Infiltration in LGG and GBM
The correlation between CLU and immune cell infiltration was assessed using the “Gene Module” of TIMER2.0 [32], which generated scatter plots of Spearman’s correlations between CLU expression and tumor purity as well as the abundance of six immune cells types, including DCs, B cells, neutrophils, CD4+ T cells, macrophages, and CD8+ T cells. Stromal and immune scores were calculated using R “ESTIMATE” package, and the sum of these two score was the ESTIMATE score, which could indirectly reflect the tumor purity [36]. The infiltrating abundance of immune cells in LGG and GBM tissues were estimated using the CIBERSORT algorithm, a computational method for quantifying fractions of 22 cell types from bulk tissue gene expression profiles. The correlations of CLU expression with stromal and immune scores as well as infiltration abundance of immune cells were further analyzed using R packages “ggplot2,” “ggpubr,” and “ggExtra”.
4.5. Co-Expression Analysis and Immune-Checkpoint Analysis in LGG
Co-expression analysis between CLU expression and the expression of immunosuppressive and immune activation genes and chemokine and chemokine receptor-related genes was conducted using the R “limma” package. Expression data of immune-checkpoint genes were extracted, and their expression in two LGG groups stratified by high and low CLU expression was analyzed utilizing R “ggplot2” package.
4.6. Gene Set Enrichment Analysis (GSEA)
With the predefined GO and KEGG gene sets in GSEA website as an enrichment reference, GSEA was conducted to analyze the GO annotations terms and KEGG pathways that were significantly associated with CLU expression using the R package “enrichplot”.
5. Conclusions
In conclusion, this was the first study to investigate the expression and specific roles of CLU in gliomas. This study provided evidences that CLU was highly expressed in gliomas and might be an independent indicator to predict worse survival outcomes for LGG patients. We confirmed the close involvement of CLU in immune status of gliomas, which could be used as a biomarker or a specific target in glioma immunotherapy. Further investigations focusing on the roles of CLU in gliomas are needed in future.
Author Contributions
X.R. conceived the study and wrote the paper. P.Y., C.C., X.Z., Y.W. and T.Q. performed the experiments and data analysis. F.G. and X.L. supervised the research. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Science Foundation of China (No. 32071274, 82100148, 31971211), Science Foundation for Distinguished Young Scholars of Shaanxi Province (2021JC-39), the Natural Science Foundation of Shaanxi Province (2021SF-294), and the Youth Innovation Team of Shaanxi Universities.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Research Ethics Committee of Northwest University (protocol code: 210307005-1).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Publicly available datasets were used in this study. The TCGA gene expressions along with the clinical datasets were downloaded from the TCGA hub (https://tcga.xenahubs.net accessed on 14 August 2023) and GTEx database (http://commonfund.nih.gov/GTEx/ accessed on 14 August 2023).
Conflicts of Interest
The authors have declared no competing interest.
Abbreviations
| ACC | Adrenocortical carcinoma |
| BLCA | Bladder urothelial carcinoma |
| BRCA | Breast invasive carcinoma |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma |
| CHOL | Cholangiocarcinoma |
| COAD | Colon adenocarcinoma |
| DLBC | Lymphoid neoplasm diffuse large B-cell lymphoma |
| ESCA | Esophageal carcinoma |
| GBM | Glioblastoma multiforme |
| HNSC | Head and neck squamous cell carcinoma |
| KICH | Kidney chromophobe |
| KIRC | Kidney renal clear cell carcinoma |
| KIRP | Kidney renal papillary cell carcinoma |
| LAML | Acute myeloid leukemia |
| LGG | Brain lower grade glioma |
| LIHC | Liver hepatocellular carcinoma |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| MESO | Mesothelioma |
| OV | Ovarian serous cystadenocarcinoma |
| PAAD | Pancreatic adenocarcinoma |
| PCPG | Pheochromocytoma and paraganglioma |
| PRAD | Prostate adenocarcinoma |
| READ | Rectum adenocarcinoma |
| SARC | Sarcoma |
| SKCM | Skin cutaneous melanoma |
| STAD | Stomach adenocarcinoma |
| TGCT | Testicular germ cell tumors |
| THCA | Thyroid carcinoma |
| THYM | Thymoma |
| UCEC | Uterine corpus endometrial carcinoma |
| UCS | Uterine carcinosarcoma |
| UVM | Uveal melanoma |
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Camacho, A.; Flores-Vázquez, J.G.; Moscardini-Martelli, J.; Torres-Ríos, J.A.; Olmos-Guzmán, A.; Ortiz-Arce, C.S.; Cid-Sánchez, D.R.; Pérez, S.R.; Macías-González, M.D.S.; Hernández-Sánchez, L.C.; et al. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 7207. [Google Scholar] [CrossRef]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Franson, A.; McClellan, B.L.; Varela, M.L.; Comba, A.; Syed, M.F.; Banerjee, K.; Zhu, Z.; Gonzalez, N.; Candolfi, M.; Lowenstein, P.; et al. Development of immunotherapy for high-grade gliomas: Overcoming the immunosuppressive tumor microenvironment. Front. Med. 2022, 9, 966458. [Google Scholar] [CrossRef] [PubMed]
- Bunse, L.; Bunse, T.; Krämer, C.; Chih, Y.-C.; Platten, M. Clinical and Translational Advances in Glioma Immunotherapy. Neurotherapeutics 2022, 19, 1799–1817. [Google Scholar] [CrossRef]
- Wilson, M.R.; Zoubeidi, A. Clusterin as a therapeutic target. Expert Opin. Ther. Targets 2017, 21, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rivera, C.; Garcia, M.M.; Molina-Álvarez, M.; González-Martín, C.; Goicoechea, C. Clusterin: Always protecting. Synthesis, function and potential issues. Biomed. Pharmacother. 2021, 134, 111174. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, P.P.; Patra, S.; Panigrahi, D.P.; Patra, S.K.; Bhutia, S.K. Clusterin as modulator of carcinogenesis: A potential avenue for targeted cancer therapy. Biochim. Biophys. Acta BBA Rev. Cancer 2021, 1875, 188500. [Google Scholar] [CrossRef] [PubMed]
- Namdar, A.B.; Kabiri, M.; Mozaffari, H.M.; Aminifar, E.; Mehrad-Majd, H. Circulating Clusterin Levels and Cancer Risk: A Systematic Review and Meta-Analysis. Cancer Control 2022, 29, 10732748211038437. [Google Scholar]
- Fu, N.; Du, H.; Li, D.; Lu, Y.; Li, W.; Wang, Y.; Kong, L.; Du, J.; Zhao, S.; Ren, W.; et al. Clusterin contributes to hepatitis C virus-related hepatocellular carcinoma by regulating autophagy. Life Sci. 2020, 256, 117911. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Ma, X.; Chen, L. Inhibition Lysosomal Degradation of Clusterin by Protein Kinase D3 Promotes Triple-Negative Breast Cancer Tumor Growth. Adv. Sci. 2021, 8, 2003205. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, X.; Chen, L.; Liu, Y. The role and function of CLU in cancer biology and therapy. Clin. Exp. Med. 2022, 23, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Guo, W.; Yang, S.; Han, D.; Li, H. The multiple roles and therapeutic potential of clusterin in non-small-cell lung cancer: A narrative review. Transl. Lung Cancer Res. 2021, 10, 2683–2697. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Deng, J.; Zhou, S.; Tao, T.; Su, Q.; Xue, Y.; Yang, X. The role of Clusterin in cancer metastasis. Cancer Manag. Res. 2019, 11, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.-J.; Kang, B.-H.; Choi, B.-K.; Park, I.-S.; Min, B.-H. Clusterin induces the secretion of TNF-α and the chemotactic migration of macrophages. Biochem. Biophys. Res. Commun. 2012, 422, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.H.; Kwon, H.S.; Moon, K.A.; Park, S.Y.; Park, S.; Lee, K.Y.; Ha, E.H.; Kim, T.B.; Moon, H.B.; Lee, H.K.; et al. Clusterin Modulates Allergic Airway Inflammation by Attenuating CCL20-Mediated Dendritic Cell Recruit-ment. J. Immunol. 2016, 196, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Ekici, A.I.D.; Eren, B.; Türkmen, N.; Çomunoğlu, N.; Fedakar, R. Clusterin expression in non-neoplastic adenohypophyses and pituitary adenomas: Cytoplasmic clusterin localization in adenohypophysis is related to aging. Endocr. Pathol. 2008, 19, 47–53. [Google Scholar] [CrossRef]
- Chayka, O.; Corvetta, D.; Dews, M.; Caccamo, A.E.; Piotrowska, I.; Santilli, G.; Gibson, S.; Sebire, N.J.; Himoudi, N.; Hogarty, M.D.; et al. Clusterin, a haploinsufficient tumor suppressor gene in neuroblastomas. J. Natl. Cancer Inst. 2009, 101, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.; Chen, Z.; Zhang, S.; Li, S.; Wageh, S.; Al-Hartomy, O.A.; Al-Sehemi, A.G.; Xie, Z.; Kankala, R.K.; et al. Glioma diagnosis and therapy: Current challenges and nanomaterial-based solutions. J. Control. Release 2022, 352, 338–370. [Google Scholar] [CrossRef] [PubMed]
- Mischkulnig, M.; Kiesel, B.; Rötzer-Pejrimovsky, T.; Borkovec, M.; Lang, A.; Millesi, M.; Wadiura, L.I.; Hervey-Jumper, S.; Penninger, J.M.; Berger, M.S.; et al. The impact of heme biosynthesis regulation on glioma aggressiveness: Correlations with diagnostic molecu-lar markers. Front. Mol. Neurosci. 2022, 15, 928355. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.L.; Comba, A.; Faisal, S.M.; Argento, A.; Franson, A.; Barissi, M.N.; Sachdev, S.; Castro, M.G.; Lowenstein, P.R. Gene Therapy for High Grade Glioma: The Clinical Experience. Expert Opin. Biol. Ther. 2023, 23, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tang, L.; Li, X.; Fan, F.; Liu, Z. Immunotherapy for glioma: Current management and future application. Cancer Lett. 2020, 476, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jelski, W.; Mroczko, B. Molecular and Circulating Biomarkers of Brain Tumors. Int. J. Mol. Sci. 2021, 22, 7039. [Google Scholar] [CrossRef] [PubMed]
- Senhaji, N.; Houssaini, A.S.; Lamrabet, S.; Louati, S.; Bennis, S. Molecular and Circulating Biomarkers in Patients with Glioblastoma. Int. J. Mol. Sci. 2022, 23, 7474. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kose, K.; Kraehenbuehl, L.; Byers, C.; Holland, A.; Tembo, T.; Santella, A.; Alfonso, A.; Li, M.; Cordova, M.; et al. In vivo tumor immune microenvironment phenotypes correlate with inflammation and vasculature to predict immunotherapy response. Nat. Commun. 2022, 13, 5312. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Yang, P.; Qin, H.; Li, Y.; Xiao, A.; Zheng, E.; Zeng, H.; Su, C.; Luo, X.; Lu, Q.; Liao, M.; et al. CD36-mediated metabolic crosstalk between tumor cells and macrophages affects liver metastasis. Nat. Commun. 2022, 13, 5782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Liu, Y.; Jiang, J.; Tang, Y.J.; Tang, Y.L.; Liang, X.H. Extracellular vesicle long non-coding RNA-mediated crosstalk in the tumor microenvironment: Tiny molecules, huge roles. Cancer Sci. 2020, 111, 2726–2735. [Google Scholar] [CrossRef]
- Yang, P.; Yang, Z.; Dong, Y.; Yang, L.; Peng, S.; Yuan, L.; Hu, X.; Chen, S.; Tang, H.; Yang, X.; et al. Clusterin is a biomarker of breast cancer prognosis and correlated with immune microenvironment. Transl. Cancer Res. 2023, 12, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Blum, A.; Wang, P.; Zenklusen, J.C. SnapShot: TCGA-Analyzed Tumors. Cell 2018, 173, 530. [Google Scholar] [CrossRef]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).