Vitamin D Receptor Gene Polymorphism Predicts the Outcome of Multidisciplinary Rehabilitation in Multiple Sclerosis Patients
Abstract
1. Introduction
2. Results
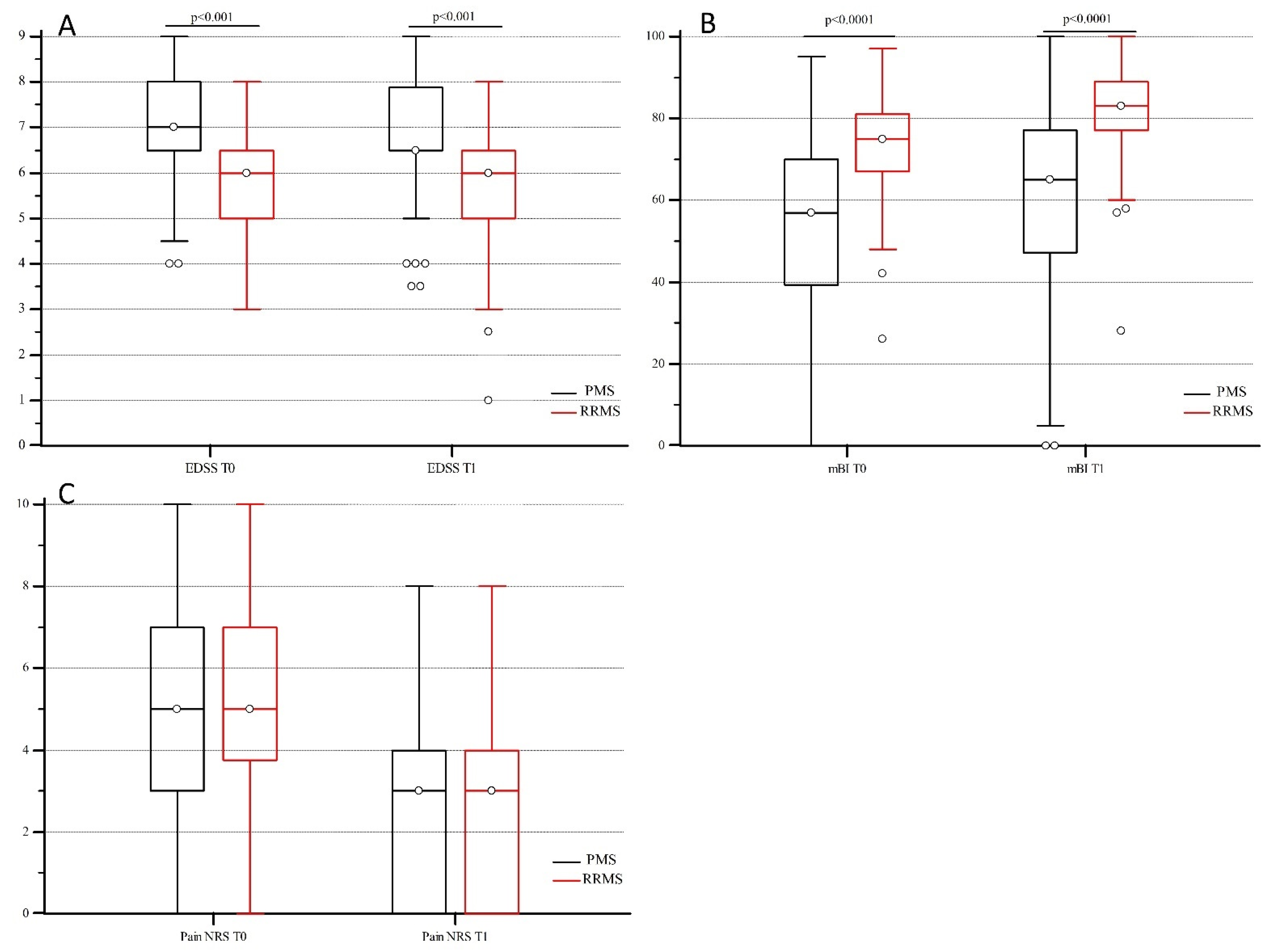
2.1. VDR Polymorphism Distribution and Disability Indexes in MS Patients
2.2. Disability Indexes and MDR Outcome Correlate with VDR TaqI–ApaI–FokI Polymorphisms
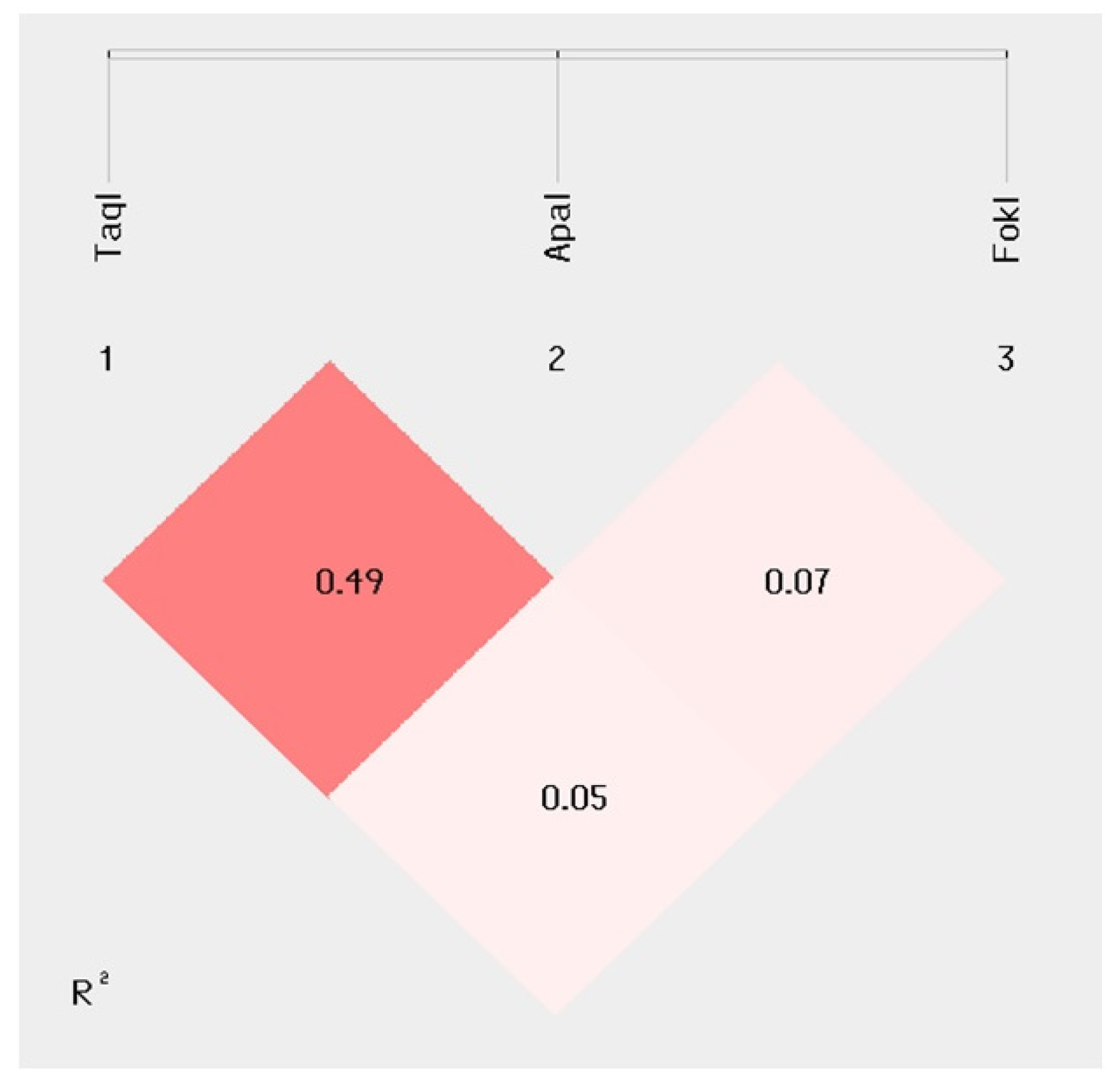
2.3. Disability Indexes and MDR Outcome Correlation with VDR TaqI–ApaI–FokI Haplotype Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Rehabilitation Treatment
4.3. Samples Collection and DNA Extraction
4.4. HLA-DRB1*15.01 Characterization
4.5. VDR Polymorphisms and Genotyping
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72 (Suppl. S1), 1–5. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed]
- Dighriri, I.M.; Aldalbahi, A.A.; Albeladi, F.; Tahiri, A.A.; Kinani, E.M.; Almohsen, R.A.; Alamoudi, N.H.; Alanazi, A.A.; Alkhamshi, S.J.; Althomali, N.A.; et al. An Overview of the History, Pathophysiology, and Pharmacological Interventions of Multiple Sclerosis. Cureus 2023, 15, e33242. [Google Scholar] [CrossRef]
- Goris, A.; Vandebergh, M.; McCauley, J.L.; Saarela, J.; Cotsapas, C. Genetics of multiple sclerosis: Lessons from polygenicity. Lancet Neurol. 2022, 21, 830–842. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Münz, C.; Cohen, J.I.; Ascherio, A. Epstein-Barr virus as a leading cause of multiple sclerosis: Mechanisms and implications. Nat. Rev. Neurol. 2023, 19, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; Silva, D.D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Agliardi, C.; Guerini, F.R.; Bolognesi, E.; Zanzottera, M.; Clerici, M. VDR Gene Single Nucleotide Polymorphisms and Autoimmunity: A Narrative Review. Biology 2023, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, C.; Souberbielle, J.C. Vitamin D and multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2017, 14, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Dyment, D.A.; Cader, M.Z.; Morrison, K.M.; Disanto, G.; Morahan, J.M.; Berlanga-Taylor, A.J.; Handel, A.; De Luca, G.C.; Sadovnick, A.D.; et al. Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann. Neurol. 2011, 70, 881–886. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Saresella, M.; Caputo, D.; Leone, M.A.; Zanzottera, M.; Bolognesi, E.; Marventano, I.; Barizzone, N.; Fasano, M.E.; et al. Vitamin D receptor (VDR) gene SNPs influence VDR expression and modulate protection from multiple sclerosis in HLA-DRB1*15-positive individuals. Brain Behav. Immun. 2011, 25, 1460–1467. [Google Scholar] [CrossRef]
- Scazzone, C.; Agnello, L.; Bivona, G.; Lo Sasso, B.; Ciaccio, M. Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem. Genet. 2021, 59, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Ciaccio, M. The immunological implication of the new vitamin D metabolism. Cent. Eur. J. Immunol. 2018, 43, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Goudarzvand, M.; Javan, M.; Mirnajafi-Zadeh, J.; Mozafari, S.; Tiraihi, T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol. Neurobiol. 2010, 30, 289–299. [Google Scholar] [CrossRef]
- Chiuso-Minicucci, F.; Ishikawa, L.L.; Mimura, L.A.; Fraga-Silva, T.F.; França, T.G.; Zorzella-Pezavento, S.F.; Marques, C.; Ikoma, M.R.; Sartori, A. Treatment with vitamin D/MOG association suppresses experimental autoimmune encephalomyelitis. PLoS ONE 2015, 10, e0125836. [Google Scholar] [CrossRef]
- Mimura, L.A.; Chiuso-Minicucci, F.; Fraga-Silva, T.F.; Zorzella-Pezavento, S.F.; França, T.G.; Ishikawa, L.L.; Penitenti, M.; Ikoma, M.R.; Sartori, A. Association of myelin peptide with vitamin D prevents autoimmune encephalomyelitis development. Neuroscience 2016, 317, 130–140. [Google Scholar] [CrossRef][Green Version]
- Matías-Guíu, J.; Oreja-Guevara, C.; Matias-Guiu, J.A.; Gomez-Pinedo, U. Vitamin D and remyelination in multiple sclerosis. Neurologia 2018, 33, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Faraco, J.H.; Morrison, N.A.; Baker, A.; Shine, J.; Frossard, P.M. ApaI dimorphism at the human vitamin D receptor gene locus. Nucleic Acids Res. 1989, 17, 2150. [Google Scholar] [CrossRef]
- Morrison, N.A.; Yeoman, R.; Kelly, P.J.; Eisman, J.A. Contribution of trans-acting factor alleles to normal physiological variability: Vitamin D receptor gene polymorphism and circulating osteocalcin. Proc. Natl. Acad. Sci. USA 1992, 89, 6665–6669. [Google Scholar] [CrossRef]
- Morrison, N.A.; Qi, J.C.; Tokita, A.; Kelly, P.J.; Crofts, L.; Nguyen, T.V.; Sambrook, P.N.; Eisman, J.A. Prediction of bone density from vitamin D receptor alleles. Nature 1994, 367, 284–287, Erratum in Nature 1997, 387, 106. [Google Scholar] [CrossRef]
- Jurutka, P.W.; Remus, L.S.; Whitfield, G.K.; Thompson, P.D.; Hsieh, J.C.; Zitzer, H.; Tavakkoli, P.; Galligan, M.A.; Dang, H.T.; Haussler, C.A.; et al. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol. Endocrinol. 2000, 14, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Groppo, E.; Signori, A.; Sormani, M.P.; Grosso, C.; Mantia, L.; Cattaneo, D.; Rovaris, M. Predictors of hospital-based multidisciplinary rehabilitation effects in persons with multiple sclerosis: A large-scale, single-centre study. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319843673. [Google Scholar] [CrossRef]
- Giordano, A.; Clarelli, F.; Cannizzaro, M.; Mascia, E.; Santoro, S.; Sorosina, M.; Ferrè, L.; Leocani, L.; Esposito, F. BDNF Val66Met Polymorphism Is Associated with Motor Recovery After Rehabilitation in Progressive Multiple Sclerosis Patients. Front Neurol. 2022, 13, 790360. [Google Scholar] [CrossRef]
- Mori, F.; Ljoka, C.; Nicoletti, C.G.; Kusayanagi, H.; Buttari, F.; Giordani, L.; Rossi, S.; Foti, C.; Centonze, D. CB1 receptor affects cortical plasticity and response to physiotherapy in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e48. [Google Scholar] [CrossRef] [PubMed]
- Hosking, L.; Lumsden, S.; Lewis, K.; Yeo, A.; McCarthy, L.; Bansal, A.; Riley, J.; Purvis, I.; Xu, C.F. Detection of genotyping errors by Hardy-Weinberg equilibrium testing. Eur. J. Hum. Genet. 2004, 12, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhang, L.; Chen, S.Y.; Yang, G.J.; Huang, X.L.; Duan, Y.; Yang, L.J.; Ye, D.Q.; Wang, J. Association between VDR polymorphisms and multiple sclerosis: Systematic review and updated meta-analysis of case-control studies. Neurol. Sci. 2018, 39, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Azarnezhad, A.; Khanbabaei, H.; Izadpanah, E.; Abdollahzadeh, R.; Barreto, G.E.; Sahebkar, A. Vitamin D receptor genetic polymorphisms and the risk of multiple sclerosis: A systematic review and meta-analysis. Steroids 2020, 158, 108615. [Google Scholar] [CrossRef]
- Imani, D.; Razi, B.; Motallebnezhad, M.; Rezaei, R. Association between vitamin D receptor (VDR) polymorphisms and the risk of multiple sclerosis (MS): An updated meta-analysis. BMC Neurol. 2019, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Zhang, M.L.; Zhu, L.; Peng, M.L.; Liu, F.Z.; Zhang, G.X.; Wang, L.M.; Zhao, J. Vitamin D receptor gene polymorphisms and the risk of multiple sclerosis: An updated meta-analysis. Microb. Pathog. 2017, 110, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, E.; Rafiei, A.; Yazdani, Y.; Eslami, M.; Saeedi, M. Association of serum levels and receptor genes BsmI, TaqI and FokI polymorphisms of vitamin D with the severity of multiple sclerosis. J. Clin. Neurosci. 2021, 84, 75–81. [Google Scholar] [CrossRef]
- Simon, K.C.; Munger, K.L.; Xing, Y.; Ascherio, A. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult. Scler. 2010, 16, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Pepineli, A.C.; Alves, H.V.; Tiyo, B.T.; Macedo, L.C.; Visentainer, L.; de Lima Neto, Q.A.; Zacarias, J.M.V.; Sell, A.M.; Visentainer, J.E.L. Vitamin D Receptor Gene Polymorphisms Are Associated with Leprosy in Southern Brazil. Front. Immunol. 2019, 10, 2157. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B. Rehabilitation in Multiple Sclerosis: A Systematic Review of Systematic Reviews. Arch. Phys. Med. Rehabil. 2017, 98, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Fortuna, D.; Giannı, C.; Leonardi, L.; Marchetti, M.R.; Pozzilli, C. Home-based balance training using the Wii balance board: A randomized, crossover pilot study in multiple sclerosis. Neurorehabil Neural Repair. 2013, 27, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; Dieberg, G.; Smart, N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: A meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1339–1348.e7. [Google Scholar] [CrossRef] [PubMed]
- Kubsik-Gidlewska, A.M.; Klimkiewicz, P.; Klimkiewicz, R.; Janczewska, K.; Woldańska-Okońska, M. Rehabilitation in multiple sclerosis. Adv. Clin. Exp. Med. 2017, 26, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Podda, J.; Tacchino, A.; Pedullà, L.; Monti Bragadin, M.; Battaglia, M.A.; Brichetto, G. Focus on neglected features of cognitive rehabilitation in MS: Setting and mode of the treatment. Mult. Scler. 2022, 28, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult. Scler. 2020, 26, 1459–1469. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, P.I.; McVean, G.; Sabeti, P.C.; Miretti, M.M.; Green, T.; Marchini, J.; Ke, X.; Monsuur, A.J.; Whittaker, P.; Delgado, M.; et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat. Genet. 2006, 38, 1166–1172. [Google Scholar] [CrossRef]
- Shi, Y.Y.; He, L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005, 15, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; He, Z.; Tang, W.; Li, T.; Zeng, Z.; He, L.; Shi, Y. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: Update of the SHEsis (http://analysis.bio-x.cn). Cell Res. 2009, 19, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, Z.; Chen, J.; Song, Z.; Zhou, Z.; Shi, Y. SHEsisPlus, a toolset for genetic studies on polyploid species. Sci. Rep. 2016, 6, 24095. [Google Scholar] [CrossRef]

| VDR | PPMS | RRMS | SPMS | Total MS | pc Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| rs731236 TaqI | N | % | N | % | N | % | N | % | |
| TT | 15 | 41.7 | 29 | 30.9 | 41 | 34.5 | 85 | 34.1 | |
| TC | 18 | 50 | 38 | 40.4 | 56 | 47.1 | 112 | 45 | |
| CC | 3 | 8.3 | 27 | 28.7 | 22 | 18.5 | 52 | 20.9 | |
| HWE ns | HWE ns | HWE ns | HWE ns | ns | |||||
| rs1544410 BsmI | |||||||||
| CC | 15 | 41.7 | 31 | 33 | 40 | 33.6 | 86 | 34.5 | |
| CT | 16 | 44.4 | 32 | 34 | 45 | 37.8 | 93 | 37.3 | |
| TT | 5 | 13.9 | 31 | 33 | 34 | 28.6 | 70 | 28.1 | |
| HWE ns | HWE p < 0.01 | HWE p < 0.01 | HWE p < 0.001 | ns | |||||
| rs7975232 ApaI | |||||||||
| AA | 7 | 19.4 | 34 | 36.2 | 40 | 33.6 | 80 | 32.1 | |
| AC | 20 | 55.6 | 42 | 44.7 | 57 | 47.9 | 119 | 47.8 | |
| CC | 9 | 25.0 | 18 | 19.1 | 22 | 18.5 | 50 | 20.1 | |
| HWE ns | HWE ns | HWE ns | HWE ns | ns | |||||
| rs10735810 FokI | |||||||||
| CC | 19 | 52.8 | 32 | 34.0 | 52 | 43.7 | 104 | 41.8 | |
| CT | 12 | 33.3 | 52 | 55.3 | 47 | 39.5 | 112 | 45 | |
| TT | 5 | 13.9 | 10 | 10.6 | 20 | 16.8 | 33 | 13.3 | |
| HWE ns | HWE ns | HWE ns | HWE ns | ns | |||||
| EDSS T0 | EDSS T1 | mBI T0 | mBI T1 | Pain NRS T0 | Pain NRS T1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p Value | Median | IQR | Median | IQR | p Value | Median | IQR | Median | IQR | p Value | |
| Total | 6.5 | 1.5 | 6.5 | 1.0 | <0.001 | 65.0 | 27.0 | 75.0 | 26.0 | <0.001 | 5.0 | 4.0 | 3.0 | 4.0 | <0.001 |
| PMS (N = 155) | 7.0 | 1.5 | 6.5 | 1.3 | <0.001 | 57.0 | 30.5 | 65.0 | 29.5 | <0.001 | 5.0 | 4.0 | 3.0 | 4.0 | <0.001 |
| RRMS (N = 94) | 6.0 | 1.5 | 6.0 | 1.5 | <0.001 | 75.0 | 14.0 | 83.0 | 11.8 | <0.001 | 5.0 | 3.0 | 3.0 | 4.0 | <0.001 |
| Delta mBI in RRMS Patients | Beta Value | Standard Error | t Value | p Value |
|---|---|---|---|---|
| Intercept | 35.91 | 6.75 | 5.32 | <0.0001 |
| Sex: Female vs. Male | 0.03 | 1.45 | 0.02 | 0.9851 |
| mBI T0 (1 unit more) | −0.23 | 0.06 | −3.94 | 0.0002 |
| Age (1 year more) | −0.22 | 0.07 | −3.06 | 0.003 |
| N of interventions (1 more) | −0.71 | 0.67 | −1.06 | 0.2925 |
| Disease duration (1 year more) | −0.01 | 0.07 | −0.01 | 0.9897 |
| hospitalization duration (1 day more) | −0.07 | 0.07 | −0.88 | 0.3827 |
| DRB1*15.01 positivity | 1.78 | 1.51 | 1.19 | 0.2379 |
| TaqI TT vs. (TC + CC) | 6.35 | 1.65 | 3.86 | 0.0002 |
| ApaI CC vs. (AC + AA) | 0.23 | 1.94 | 0.12 | 0.9064 |
| FokI TC vs. CC | 1.09 | 1.39 | 0.78 | 0.4357 |
| FokI TT vs. CC | 1.89 | 2.29 | 0.83 | 0.4096 |
| Haplotype Association with Delta mBI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PMS N = 155 | RRMS N = 94 | Haplotype | TaqI | ApaI | FokI | PMS | p Value | RRMS | p Value |
| freq | freq | Beta Value | Beta Value | ||||||
| 0.21 | 0.31 | VDR-1 | T | C | C | 0.23 | ns | 3.24 * | 0.007 * |
| 0.29 | 0.26 | VDR-2 | C | A | C | −1.37 | ns | −2.18 * | 0.04 * |
| 0.23 | 0.08 | VDR-3 | T | C | T | −0.44 | ns | 2.18 | ns |
| 0.09 | 0.20 | VDR-4 | C | A | T | 1.72 | ns | −2.34 | ns |
| 0.13 | 0.04 | VDR-5 | T | A | C | −0.88 | ns | −1.14 | ns |
| 0.03 | 0.08 | VDR-6 | T | A | T | 1.49 | ns | 3.15 | ns |
| PPMS | RRMS | SPMS | Total MS | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | 36 | 94 | 119 | 249 | |||||
| Female: N (%) | 17 * | (47.2) | 65 * | (69.1) | 68 | (57.1) | 150 | (60.2) | * = 0.02 |
| Age mean (SD) | 55.8 * | (12.2) | 45.4 *° | (9.7) | 53.7 ° | (12.3) | 50.8 | (12.1) | * < 0.001 °<0.001 |
| DRB1*15 positive: N (%) | 7 | (19.4) | 23 | (24.5) | 36 | (30.3) | 66 | (26.5) | ns |
| disease duration Years’ median (IQR) | 16.0 ^ | (12.5) | 17.5 ° | (13.0) | 24.0 °^ | (11.8) | 20.0 | (14.0) | ^ < 0.001 ° < 0.001 |
| Hospitalization Days’ median (IQR) | 31 | (14.3) | 34 | (13.0) | 35 | (12.5) | 35.0 | (13.0) | ns |
| Interventions N (IQR) | 4 | (1.5) | 4 | (2.0) | 4 | (2.0) | 4 | (2.0) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerini, F.R.; Agliardi, C.; Oreni, L.; Groppo, E.; Bolognesi, E.; Zanzottera, M.; Caputo, D.; Rovaris, M.; Clerici, M. Vitamin D Receptor Gene Polymorphism Predicts the Outcome of Multidisciplinary Rehabilitation in Multiple Sclerosis Patients. Int. J. Mol. Sci. 2023, 24, 13379. https://doi.org/10.3390/ijms241713379
Guerini FR, Agliardi C, Oreni L, Groppo E, Bolognesi E, Zanzottera M, Caputo D, Rovaris M, Clerici M. Vitamin D Receptor Gene Polymorphism Predicts the Outcome of Multidisciplinary Rehabilitation in Multiple Sclerosis Patients. International Journal of Molecular Sciences. 2023; 24(17):13379. https://doi.org/10.3390/ijms241713379
Chicago/Turabian StyleGuerini, Franca Rosa, Cristina Agliardi, Letizia Oreni, Elisabetta Groppo, Elisabetta Bolognesi, Milena Zanzottera, Domenico Caputo, Marco Rovaris, and Mario Clerici. 2023. "Vitamin D Receptor Gene Polymorphism Predicts the Outcome of Multidisciplinary Rehabilitation in Multiple Sclerosis Patients" International Journal of Molecular Sciences 24, no. 17: 13379. https://doi.org/10.3390/ijms241713379
APA StyleGuerini, F. R., Agliardi, C., Oreni, L., Groppo, E., Bolognesi, E., Zanzottera, M., Caputo, D., Rovaris, M., & Clerici, M. (2023). Vitamin D Receptor Gene Polymorphism Predicts the Outcome of Multidisciplinary Rehabilitation in Multiple Sclerosis Patients. International Journal of Molecular Sciences, 24(17), 13379. https://doi.org/10.3390/ijms241713379








