PD-L1 Downregulation and DNA Methylation Inhibition for Molecular Therapy against Cancer Stem Cells in Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
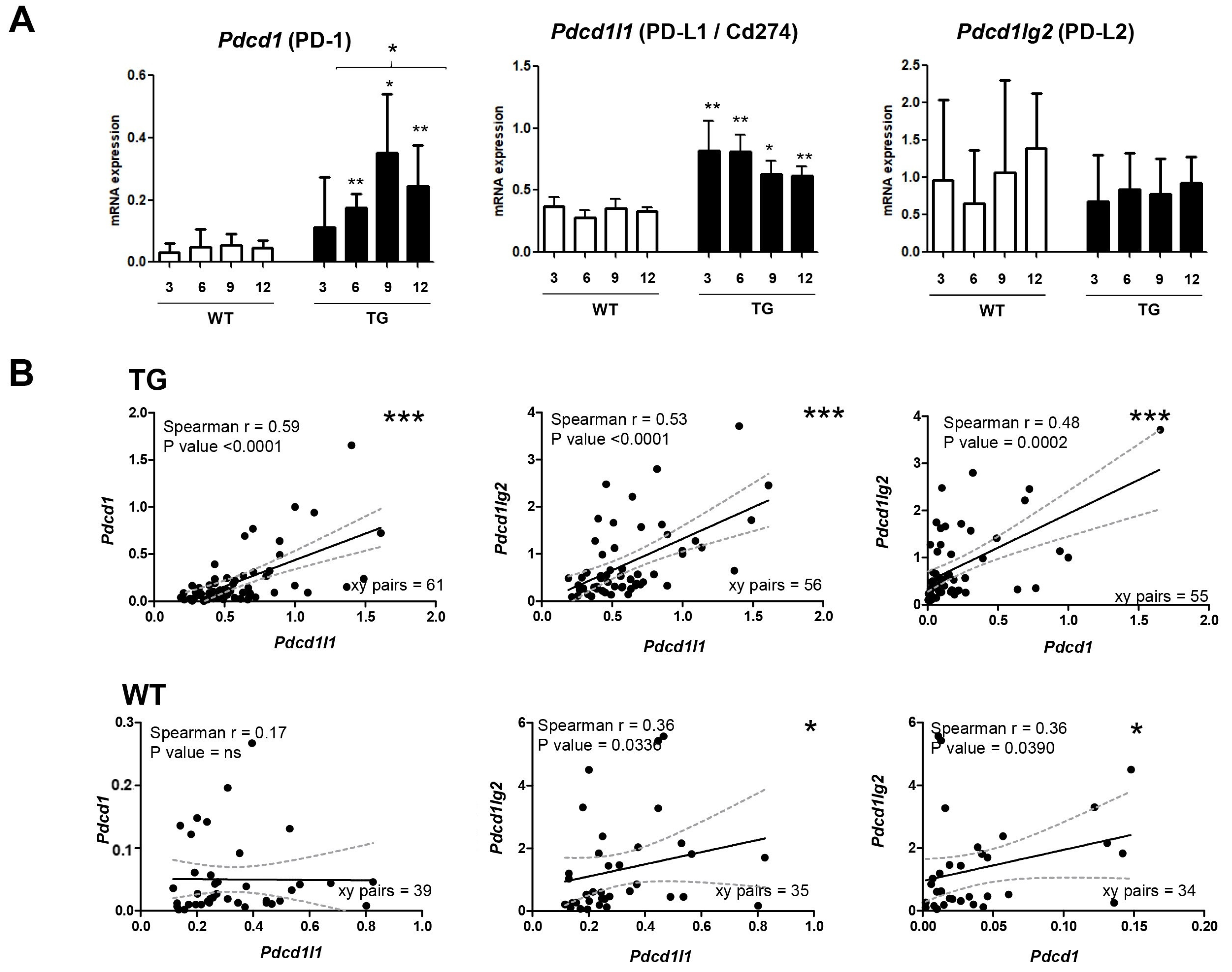
2.1. Dynamics of PD-1/PD-L1 during Hepatocarcinogenesis
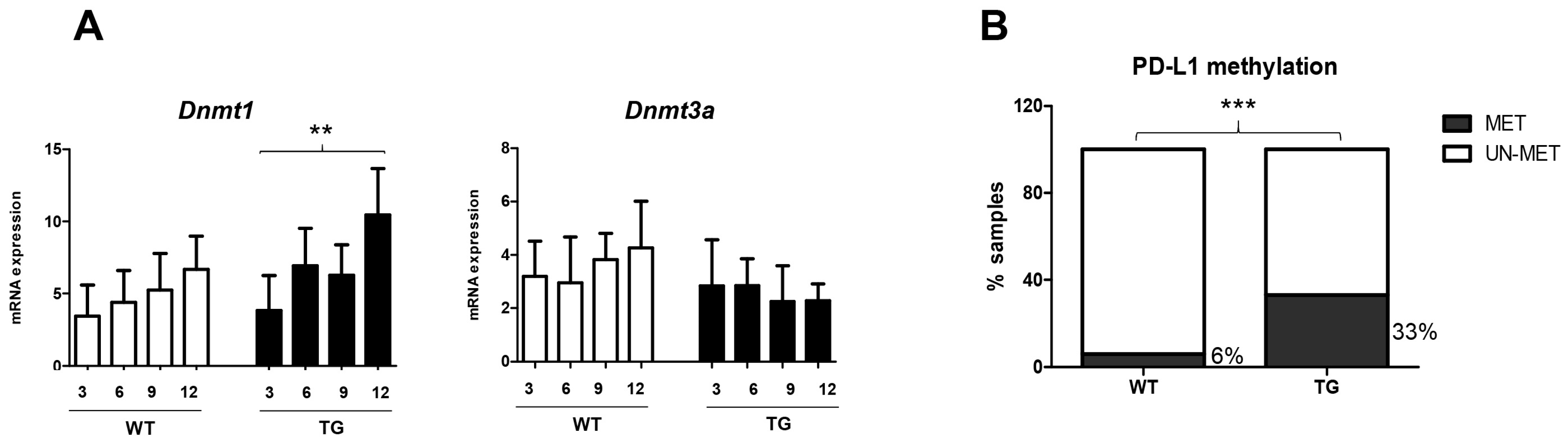
2.2. Relevance of DNA Methylation during Hepatocarcinogenesis
2.3. Silencing of PD-L1 mRNA in Human HCC Cell Lines
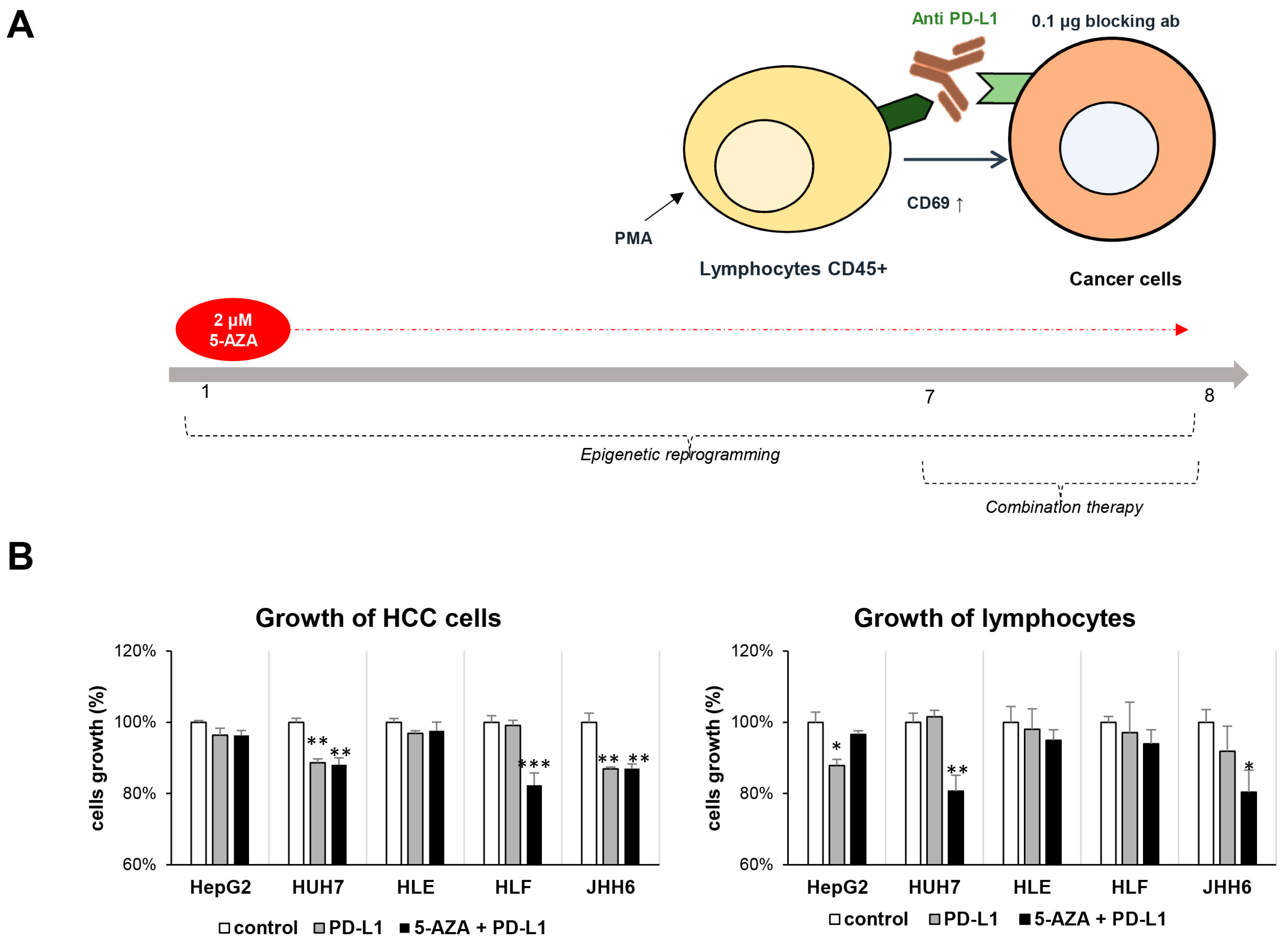
2.4. Combination Therapy Inhibits HCC Growth
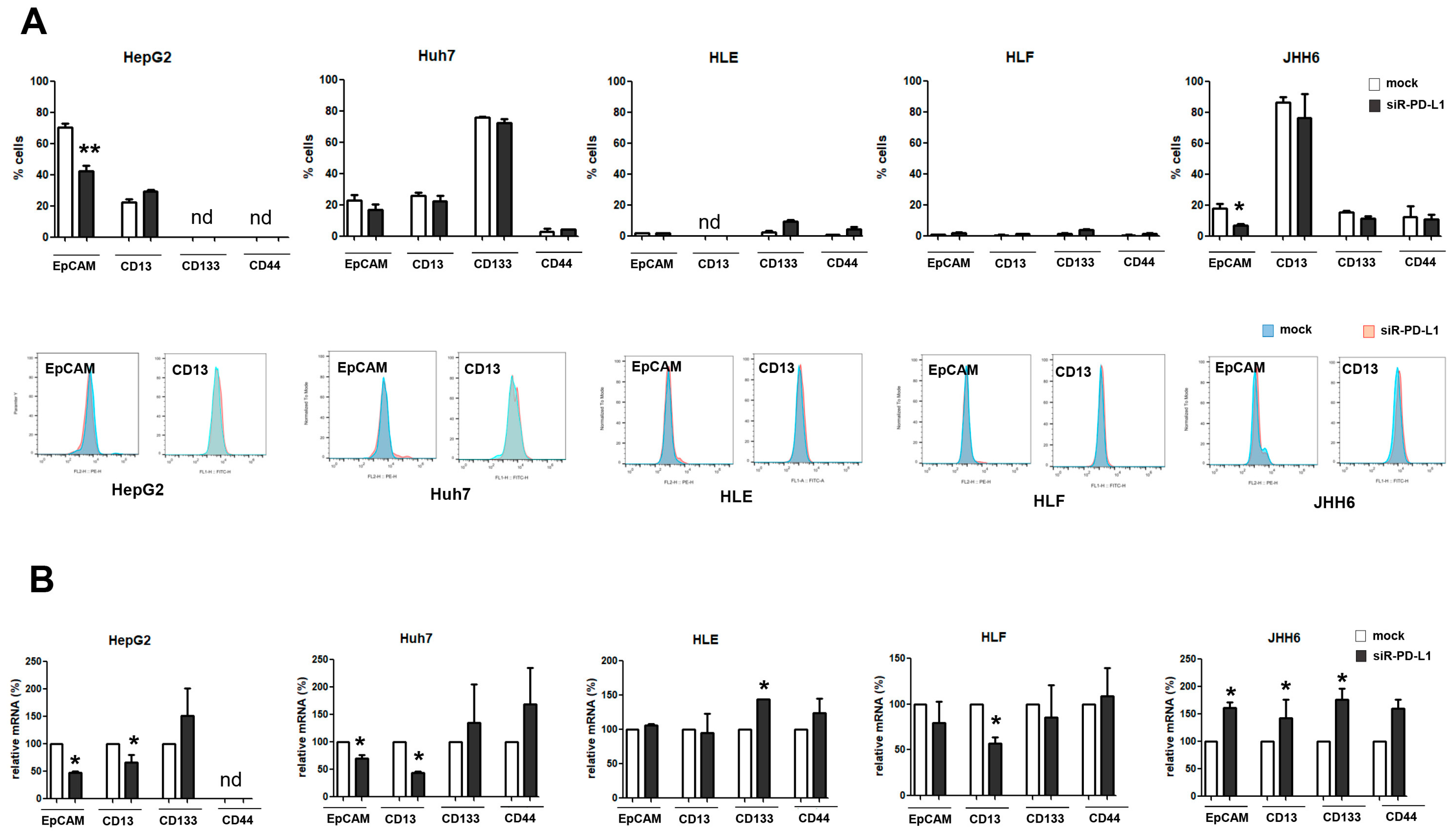
2.5. The Expression of PD-L1 Is Related to That of CSC Marker EpCAM
3. Discussion
4. Materials and Methods
4.1. Transgenic Mouse Model
4.2. In Vitro Models
4.3. Reverse Transcription–Quantitative Real Time PCR (RT–qPCR)
4.4. DNA Isolation and Methylation-Specific PCR (MS-PCR)
4.5. PD-L1 RNA Silencing
4.6. Flow Cytometry
4.7. Immunofluorescence
4.8. Co-Treatment of Anti PD-L1 and DNMT1 Inhibitor
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.K.D.; Tiribelli, C.; Sukowati, C.H.C. Sorafenib Resistance in Hepatocellular Carcinoma: The Relevance of Genetic Heterogeneity. Cancers 2020, 12, 1576. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Toor, S.M.; Nair, V.S.; Elkord, E. Role of Epigenetic Modifications in Inhibitory Immune Checkpoints in Cancer Development and Progression. Front. Immunol. 2020, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Yeon, M.; Kim, Y.; Jung, H.S.; Jeoung, D. Histone Deacetylase Inhibitors to Overcome Resistance to Targeted and Immuno Therapy in Metastatic Melanoma. Front. Cell Dev. Biol. 2020, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.K.D.; Reyes, P.A.C.; Crocè, L.S.; Tiribelli, C.; Sukowati, C.H.C. The Relevance of SOCS1 Methylation and Epigenetic Therapy in Diverse Cell Populations of Hepatocellular Carcinoma. Diagnostics 2021, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Gailhouste, L.; Liew, L.C.; Yasukawa, K.; Hatada, I.; Tanaka, Y.; Nakagama, H.; Ochiya, T. Differentiation Therapy by Epigenetic Reconditioning Exerts Antitumor Effects on Liver Cancer Cells. Mol. Ther. 2018, 26, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Gu, M.; Wang, Y.; Ban, D.; Ji, H. Oxymatrine reduces expression of programmed death-ligand 1 by promoting DNA demethylation in colorectal cancer cells. Clin. Transl. Oncol. 2021, 23, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sang, M.; Liu, F.; Zhang, J.; Li, W.; Li, Z.; Gu, L.; Zheng, Y.; Li, J.; Shan, B. Epigenetic modulation combined with PD-1/PD-L1 blockade enhances immunotherapy based on MAGE-A11 antigen-specific CD8+T cells against esophageal carcinoma. Carcinogenesis 2020, 41, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Sukowati, C.H.C.; El-Khobar, K.E.; Tiribelli, C. Immunotherapy against programmed death-1/programmed death ligand 1 in hepatocellular carcinoma: Importance of molecular variations, cellular heterogeneity, and cancer stem cells. World J. Stem Cells 2021, 13, 795–824. [Google Scholar] [CrossRef]
- Chisari, F.V.; Klopchin, K.; Moriyama, T.; Pasquinelli, C.; Dunsford, H.A.; Sell, S.; Pinkert, C.A.; Brinster, R.L.; Paimiter, R.D. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 1989, 59, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Anfuso, B.; El-Khobar, K.E.; Ie, S.I.; Avellini, C.; Radillo, O.; Raseni, A.; Tiribelli, C.; Sukowati, C.H.C. Activation of hepatic stem cells compartment during hepatocarcinogenesis in a HBsAg HBV-transgenic mouse model. Sci. Rep. 2018, 8, 13168. [Google Scholar] [CrossRef]
- Wang, B.-J.; Bao, J.-J.; Wang, J.-Z.; Wang, Y.; Jiang, M.; Xing, M.-Y.; Zhang, W.-G.; Qi, J.-Y.; Roggendorf, M.; Lu, M.-J.; et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J. Gastroenterol. 2011, 17, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Semaan, A.; Dietrich, D.; Bergheim, D.; Dietrich, J.; Kalff, J.C.; Branchi, V.; Matthaei, H.; Kristiansen, G.; Fischer, H.-P.; Goltz, D. CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Arch. 2016, 470, 185–196. [Google Scholar] [CrossRef]
- Chen, C.-L.; Pan, Q.-Z.; Zhao, J.-J.; Wang, Y.; Li, Y.-Q.; Wang, Q.-J.; Pan, K.; Weng, D.-S.; Jiang, S.-S.; Tang, Y.; et al. PD-L1 expression as a predictive biomarker for cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. OncoImmunology 2016, 5, e1176653. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.I.; Jeong, D.; Ji, S.; Ahn, T.S.; Bae, S.H.; Chin, S.; Chung, J.C.; Kim, H.C.; Lee, M.S.; Baek, M.-J. Overexpression of PD-L1 and PD-L2 Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. Treat. 2017, 49, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Spano, D.; D’apolito, M.; Centra, M.; Lasalandra, C.; Capasso, M.; Di Leo, A.; Volinia, S.; Arcelli, D.; Rosso, N.; et al. Gene Expression Analysis in HBV Transgenic Mouse Liver: A Model to Study Early Events Related to Hepatocarcinogenesis. Mol. Med. 2006, 12, 115–123. [Google Scholar] [PubMed]
- Kaji, K.; Factor, V.M.; Andersen, J.B.; Durkin, M.E.; Tomokuni, A.; Marquardt, J.U.; Matter, M.S.; Hoang, T.; Conner, E.A.; Thorgeirsson, S.S. DNMT1 is a required genomic regulator for murine liver histogenesis and regeneration. Hepatology 2016, 64, 582–598. [Google Scholar] [CrossRef]
- Cha, J.-H.; Chan, L.-C.; Li, C.-W.; Hsu, J.L.; Hung, M.-C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar]
- Caruso, S.; Calatayud, A.-L.; Pilet, J.; La Bella, T.; Rekik, S.; Imbeaud, S.; Letouzé, E.; Meunier, L.; Bayard, Q.; Rohr-Udilova, N.; et al. Analysis of Liver Cancer Cell Lines Identifies Agents with Likely Efficacy Against Hepatocellular Carcinoma and Markers of Response. Gastroenterology 2019, 157, 760–776. [Google Scholar] [PubMed]
- Asgarova, A.; Asgarov, K.; Godet, Y.; Peixoto, P.; Nadaradjane, A.; Boyer-Guittaut, M.; Galaine, J.; Guenat, D.; Mougey, V.; Perrard, J.; et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology 2018, 7, e1423170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Meng, L.; Liu, K.; Ji, B. Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma. Oncol. Rep. 2017, 38, 899–907. [Google Scholar]
- Ralser, D.J.; Klümper, N.; Gevensleben, H.; Zarbl, R.; Kaiser, C.; Landsberg, J.; Hölzel, M.; Strieth, S.; Faridi, A.; Abramian, A.; et al. Molecular and Immune Correlates of PDCD1 (PD-1), PD-L1 (CD274), and PD-L2 (PDCD1LG2) DNA Methylation in Triple Negative Breast Cancer. J. Immunother. 2021, 44, 319–324. [Google Scholar] [PubMed]
- Del Castellanos, M.C.; López-Giral, S.; López-Cabrera, M.; de Landázuri, M.O. Multiple cis-acting elements regulate the expression of the early T cell activation antigen CD69. Eur. J. Immunol. 2002, 32, 3108–3117. [Google Scholar] [CrossRef]
- Yang, S.-C.; Wang, W.-Y.; Zhou, J.-J.; Wu, L.; Zhang, M.-J.; Yang, Q.-C.; Deng, W.-W.; Sun, Z.-J. Inhibition of DNMT1 potentiates antitumor immunity in oral squamous cell carcinoma. Int. Immunopharmacol. 2022, 111, 109113. [Google Scholar]
- Li, Y.; Hong, Y.K.; Wang, X.; Pandit, H.; Zheng, Q.; Yu, Y.; Shi, X.; Chen, Y.; Tan, M.; Pulliam, Z.; et al. Epigenetic modulation enhances immunotherapy for pancreatic ductal adenocarcinoma. Clin. Transl. Immunol. 2022, 11, e1430. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Li, Y.; Pandit, H.; Li, S.; Pulliam, Z.; Zheng, Q.; Yu, Y.; Martin, R.C. Epigenetic modulation enhances immunotherapy for hepatocellular carcinoma. Cell. Immunol. 2019, 336, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kimura, O.; Kondo, Y.; Kogure, T.; Kakazu, E.; Ninomiya, M.; Iwata, T.; Morosawa, T.; Shimosegawa, T. Expression of EpCAM Increases in the Hepatitis B Related and the Treatment-Resistant Hepatocellular Carcinoma. BioMed Res. Int. 2014, 2014, 172913. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.; Noh, S.J.; Bae, J.S.; Park, H.S.; Jang, K.Y.; Chung, M.J.; Moon, W.S. Immunohistochemical Expression and Clinical Significance of Suggested Stem Cell Markers in Hepatocellular Carcinoma. J. Pathol. Transl. Med. 2016, 50, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Almozyan, S.; Colak, D.; Mansour, F.; Alaiya, A.; Al-Harazi, O.; Qattan, A.; Al-Mohanna, F.; Al-Alwan, M.; Ghebeh, H. PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int. J. Cancer 2017, 141, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Mansour, F.A.; Al-Mazrou, A.; Al-Mohanna, F.; Al-Alwan, M.; Ghebeh, H. PD-L1 is overexpressed on breast cancer stem cells through notch3/mTOR axis. Oncoimmunology 2020, 9, 1729299. [Google Scholar] [CrossRef]
- Nishida, N.; Sakai, K.; Morita, M.; Aoki, T.; Takita, M.; Hagiwara, S.; Komeda, Y.; Takenaka, M.; Minami, Y.; Ida, H.; et al. Association between Genetic and Immunological Background of Hepatocellular Carcinoma and Expression of Programmed Cell Death-1. Liver Cancer 2020, 9, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Luong, A.B.; Do, H.Q.; Tarchi, P.; Bonazza, D.; Bottin, C.; Cabral, L.K.D.; Tran, L.D.C.; Doan, T.P.T.; Crocè, L.S.; Pham, H.L.; et al. The mRNA Distribution of Cancer Stem Cell Marker CD90/Thy-1 Is Comparable in Hepatocellular Carcinoma of Eastern and Western Populations. Cells 2020, 9, 2672. [Google Scholar] [CrossRef]
- Cabral, L.K.D.; Giraudi, P.J.; Giannelli, G.; Dituri, F.; Negro, R.; Tiribelli, C.; Sukowati, C.H.C. Network Analysis for the Discovery of Common Oncogenic Biomarkers in Liver Cancer Experimental Models. Biomedicines 2023, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-C.; Dahiya, R. MethPrimer: Designing primers for methylation PCRs. Bioinformatics 2002, 18, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Uhercik, M.; Sanders, A.J.; Owen, S.; Davies, E.L.; Sharma, A.K.; Jiang, W.G.; Mokbel, K. Clinical Significance of PD1 and PDL1 in Human Breast Cancer. Anticancer Res. 2017, 37, 4249–4254. [Google Scholar]
- Hassan, S.S.; Akram, M.; King, E.C.; Dockrell, H.M.; Cliff, J.M. PD-1, PD-L1 and PD-L2 Gene Expression on T-Cells and Natural Killer Cells Declines in Conjunction with a Reduction in PD-1 Protein during the Intensive Phase of Tuberculosis Treatment. PLoS ONE 2015, 10, e0137646. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, F.; Yang, L.; Guo, C.; Wan, R.; Ke, A.; Xu, L.; Hu, G.; Xu, X.; Shen, J.; et al. Expression of DNMT1 and DNMT3a Are Regulated by GLI1 in Human Pancreatic Cancer. PLoS ONE 2011, 6, e27684. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Spandidos, A.; Wang, H.; Seed, B. PrimerBank: A PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012, 40, D1144–D1149. [Google Scholar] [CrossRef] [PubMed]
- Amani Abkenari, S.; Safdarian, L.; Amidi, F.; Hosseini, A.; Aryanpour, R.; Salahi, E.; Sobhani, A. Metformin improves epigenetic modification involved in oocyte growth and embryo development in polycystic ovary syndrome mice model. Mol. Reprod. Dev. 2021, 88, 817–829. [Google Scholar] [CrossRef] [PubMed]

| TG | WT | |||||
|---|---|---|---|---|---|---|
| PD-1 | PD-L1 | PD-L2 | PD-1 | PD-L1 | PD-L2 | |
| Thy-1 | XY pairs: 39 | XY pairs: 42 | XY pairs: 34 | XY pairs: 35 | XY pairs: 39 | XY pairs: 31 |
| R: 0.15 | R: 0.26 | R: 0.04 | R: 0.69 | R: 0.31 | R: 0.21 | |
| p: ns | p: ns | p: ns | p < 0.0001 | p: 0.0546 | p: ns | |
| Prom-1 | XY pairs: 60 | XY pairs: 62 | XY pairs: 54 | XY pairs: 39 | XY pairs: 43 | XY pairs: 35 |
| R: −0.11 | R: 0.04 | R: −0.10 | R: −0.01 | R: −0.00 | R: −0.05 | |
| p: ns | p: ns | p: ns | p: ns | p: ns | p: ns | |
| Epcam | XY pairs: 40 | XY pairs: 41 | XY pairs: 38 | XY pairs: 26 | XY pairs: 28 | XY pairs: 25 |
| R: 0.59 | R: 0.40 | R: 0.30 | R: 0.20 | R: −0.21 | R: −0.22 | |
| p: 0.0010 | p: 0.0101 | p: 0.0633 | p: ns | p: ns | p: ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukowati, C.; Cabral, L.K.D.; Anfuso, B.; Dituri, F.; Negro, R.; Giannelli, G.; Tiribelli, C. PD-L1 Downregulation and DNA Methylation Inhibition for Molecular Therapy against Cancer Stem Cells in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 13357. https://doi.org/10.3390/ijms241713357
Sukowati C, Cabral LKD, Anfuso B, Dituri F, Negro R, Giannelli G, Tiribelli C. PD-L1 Downregulation and DNA Methylation Inhibition for Molecular Therapy against Cancer Stem Cells in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2023; 24(17):13357. https://doi.org/10.3390/ijms241713357
Chicago/Turabian StyleSukowati, Caecilia, Loraine Kay D. Cabral, Beatrice Anfuso, Francesco Dituri, Roberto Negro, Gianluigi Giannelli, and Claudio Tiribelli. 2023. "PD-L1 Downregulation and DNA Methylation Inhibition for Molecular Therapy against Cancer Stem Cells in Hepatocellular Carcinoma" International Journal of Molecular Sciences 24, no. 17: 13357. https://doi.org/10.3390/ijms241713357
APA StyleSukowati, C., Cabral, L. K. D., Anfuso, B., Dituri, F., Negro, R., Giannelli, G., & Tiribelli, C. (2023). PD-L1 Downregulation and DNA Methylation Inhibition for Molecular Therapy against Cancer Stem Cells in Hepatocellular Carcinoma. International Journal of Molecular Sciences, 24(17), 13357. https://doi.org/10.3390/ijms241713357








