Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach—A Comprehensive Narrative Review
Abstract
1. Introduction
1.1. Microbiota and Neurodegeneration
1.2. Methodology of Search
2. Microbiota–Gut–Brain Axis in Neurodegenerative Diseases: Role of Microbial Metabolome
2.1. Eubiosis and Dysbiosis
2.2. Dysbiosis in Neurodegenerative Pathologies
2.2.1. Alzheimer’s Disease
Metabolites Altered in AD
- AD and Trimethylamine-N-oxide
- AD and Lipopolysaccharide
- AD and Amyloid
- AD and Neurotransmitters
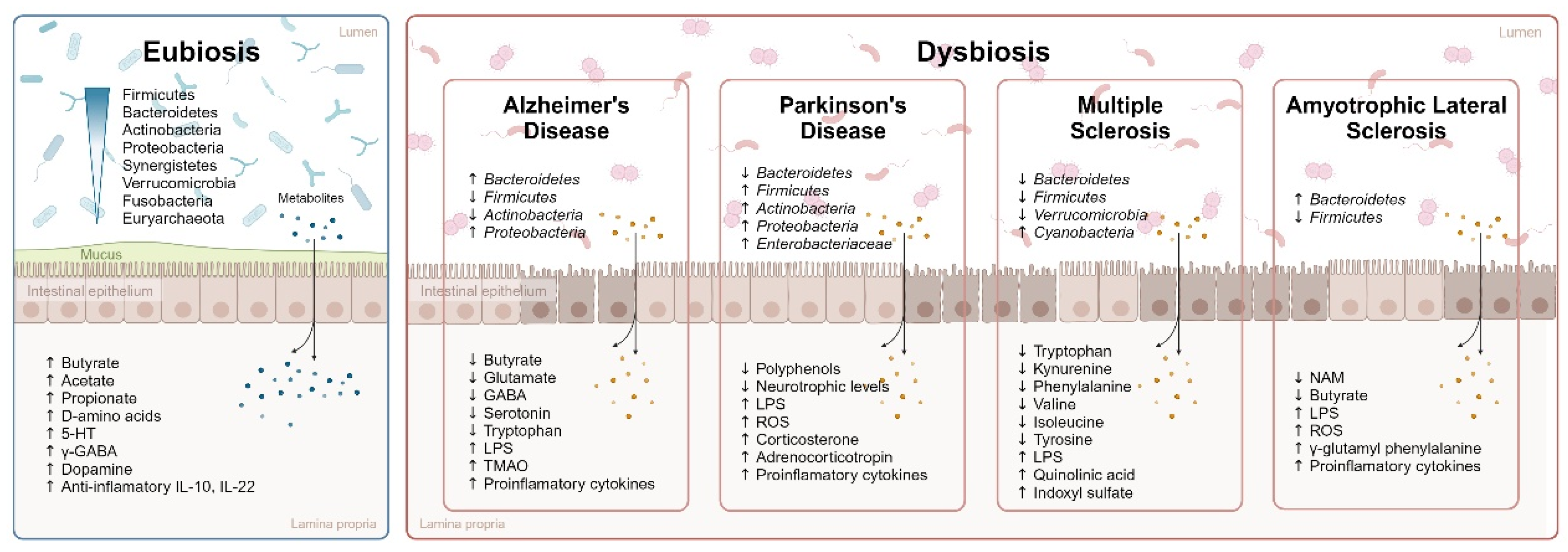
2.2.2. Parkinson’s Disease
Metabolites Altered in PD
- PD and Polyphenols
- PD and LPS
- PD and SCFAs
2.2.3. Multiple Sclerosis and Amyotrophic Lateral Sclerosis
Metabolites Altered in MS and ALS
- Multiple Sclerosis
- MS and Tryptophan
- MS and Phenols
- MS and Branched-Chain Amino Acids
- Amyotrophic Lateral Sclerosis
- ALS and SCFAs: Butyrate
- ALS and Tryptophan
3. Interventions That Improve Symptomatology through the Intestinal Microbiota
3.1. Alzheimer´s Disease
3.1.1. Fecal Microbiota Transplantation
3.1.2. Prebiotics and Probiotics
3.2. Parkinson’s Disease
3.2.1. Fecal Microbiota Transplantation
3.2.2. Prebiotics and Probiotics
3.3. MS and ALS
3.3.1. Fecal Microbiota Transplantation
3.3.2. Prebiotics and Probiotics
4. Conclusions
- There is growing evidence supporting that the onset and progression of neurodegenerative diseases are partially modulated by the gut microbiota. Both animal and human studies in AD, PD, MS, and ALS have shown an altered composition of the intestinal microbiota and its metabolites.
- There are some common metabolomic alterations in AD, PD, MS, and ALS, such as the presence of neurotoxins (i.e., LPS), a reduction in some neurotransmitters (e.g., GABA or serotonin) and anti-inflammatories cytokines (e.g., IL-10 and IL-22), an increase of pro-inflammatory cytokines, and upregulation and downregulation of different metabolites, such as SCFAs (specifically butyrate reduction). Moreover, these alterations can be moderated through the regulation of the microbiota.
- Among the microbiota reprogramming of interventions with positive results, fecal microbiota transplantation and psychobiotics are presented as potential therapeutic tools in AD, PD, MS, and ALS.
5. Limitations of Study
- This is a developing field of study. This means that in some pathologies, it is not yet possible to find many studies that consolidate the data found.
- The studies analyzed have different research methodologies, with different samples and models (human and animal). This could be a limitation for establishing representative results. However, the growing number of publications in recent years allows trends and relationships to be established.
- As indicated in the Section 4, in some cases it is not possible to determine whether microbial modifications are a cause or a consequence of the symptomatology associated with neurodegenerative pathologies. However, this interaction indicates that the microbiota–gut–brain axis may be used as a therapeutic target in these diseases.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. 2021, 28, 36967–36983. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Tong, Y.; Marion, T.; Schett, G.; Luo, Y.; Liu, Y. Microbiota and metabolites in rheumatic diseases. Autoimmun. Rev. 2020, 19, 102530. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Khan, I.; Usman, M.; Xiao Wei, Z.; Ping, X.; Khan, S.; Khan, F.; Jianye, Z.; Zhiqiang, L.; Lizhe, A. Circulating microbiota and metabolites: Insights into cardiovascular diseases. J. Clin. Lab. Anal. 2022, 36, e24779. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Allison, J.; Kaliszewska, A.; Uceda, S.; Reiriz, M.; Arias, N. Targeting DNA Methylation in the Adult Brain through Diet. Nutrients 2021, 13, 3979. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Nikolaidis, P.A.; Belando, N.; Tornero-Aguilera, J.F. Physical activity and COVID-19. The basis for an efficient intervention in times of COVID-19 pandemic. Physiol. Behav. 2022, 244, 113667. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martínez-González, M.B.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Beltran-Velasco, A.I.; Ruisoto, P.; Diaz Arroyo, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Impact of the COVID-19 Pandemic on Mental Disorders. A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 10041. [Google Scholar] [CrossRef]
- Reiriz, M.; Donoso-González, M.; Rodríguez-Expósito, B.; Uceda, S.; Beltrán-Velasco, A.I. Impact of COVID-19 Confinement on Mental Health in Youth and Vulnerable Populations: An Extensive Narrative Review. Sustainability 2023, 15, 3087. [Google Scholar] [CrossRef]
- Mishima, Y.; Ishihara, S. Molecular Mechanisms of Microbiota-Mediated Pathology in Irritable Bowel Syndrome. Int. J. Mol. Sci. 2020, 21, 8664. [Google Scholar] [CrossRef] [PubMed]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719.e6. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef]
- Yan, D.; Cao, L.; Zhou, M.; Mohimani, H. TransDiscovery: Discovering Biotransformation from Human Microbiota by Integrating Metagenomic and Metabolomic Data. Metabolites 2022, 12, 119. [Google Scholar] [CrossRef]
- Docimo, G.; Cangiano, A.; Romano, R.M.; Pignatelli, M.F.; Offi, C.; Paglionico, V.A.; Galdiero, M.; Donnarumma, G.; Nigro, V.; Esposito, D.; et al. The Human Microbiota in Endocrinology: Implications for Pathophysiology, Treatment, and Prognosis in Thyroid Diseases. Front. Endocrinol. 2020, 11, 586529. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Rodríguez, L.; Echeverry-Alzate, V.; Rincón-Pérez, I.; Calleja-Conde, J.; Bühler, K.M.; Giné, E.; Albert, J.; Hinojosa, J.A.; Huertas, E.; Gómez-Gallego, F.; et al. Gut microbiota and voluntary alcohol consumption. Transl. Psychiatry 2022, 12, 146. [Google Scholar] [CrossRef]
- Calleja-Conde, J.; Echeverry-Alzate, V.; Bühler, K.M.; Durán-González, P.; Morales-García, J.; Segovia-Rodríguez, L.; Rodríguez de Fonseca, F.; Giné, E.; López-Moreno, J.A. The Immune System through the Lens of Alcohol Intake and Gut Microbiota. Int. J. Mol. Sci. 2021, 22, 7485. [Google Scholar] [CrossRef] [PubMed]
- Munawar, N.; Ahmad, A.; Anwar, M.A.; Muhammad, K. Modulation of Gut Microbial Diversity through Non-Pharmaceutical Approaches to Treat Schizophrenia. Int. J. Mol. Sci. 2022, 23, 2625. [Google Scholar] [CrossRef]
- Miller, A.W.; Orr, T.; Dearing, D.; Monga, M. Loss of function dysbiosis associated with antibiotics and high fat, high sugar diet. ISME J. 2019, 13, 1379–1390. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic review: Gastric microbiota in health and disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Tovar, A.R.; Torres, N. Diet as Regulator of Gut Microbiota and its Role in Health and Disease. Arch. Med. Res. 2019, 50, 259–268. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Bacha, A.S.; Khan, I.; Hanif, A.; Li, W.; Guo, X. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. Int. J. Biol. Sci. 2021, 17, 818–833. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Oliveira, R.; Soares, R.; Barata, P. Influence of gut microbiota dysbiosis on brain function: A systematic review. Porto Biomed. J. 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Salguero, M.; Al-Obaide, M.; Singh, R.; Siepmann, T.; Vasylyeva, T. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients With Type 2 Diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- Blaser, M.J. The microbiome revolution. J. Clin. Investig. 2014, 124, 4162–4165. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Marć, M.A.; Jastrząb, R.; Mytych, J. Does the Gut Microbial Metabolome Really Matter? The Connection between GUT Metabolome and Neurological Disorders. Nutrients 2022, 14, 3967. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Yoo, J.; Groer, M.; Dutra, S.; Sarkar, A.; McSkimming, D. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.W.H.; Chen, H.C.; Chen, C.Y.; Yen, C.Y.T.; Lin, C.J.; Prajnamitra, R.P.; Chen, L.L.; Ruan, S.C.; Lin, J.H.; Lin, P.J.; et al. Loss of Gut Microbiota Alters Immune System Composition and Cripples Postinfarction Cardiac Repair. Circulation 2019, 139, 647–659. [Google Scholar] [CrossRef]
- Teixeira, P.J.P.; Colaianni, N.R.; Fitzpatrick, C.R.; Dangl, J.L. Beyond pathogens: Microbiota interactions with the plant immune system. Curr. Opin. Microbiol. 2019, 49, 7–17. [Google Scholar] [CrossRef]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef]
- Salzman, N.H. Microbiota–immune system interaction: An uneasy alliance. Curr. Opin. Microbiol. 2011, 14, 99–105. [Google Scholar] [CrossRef]
- Ancona, G.; Alagna, L.; Lombardi, A.; Palomba, E.; Castelli, V.; Renisi, G.; Dondossola, D.; Iavarone, M.; Muscatello, A.; Gori, A.; et al. The Interplay between Gut Microbiota and the Immune System in Liver Transplant Recipients and Its Role in Infections. Infect. Immun. 2021, 89, e0037621. [Google Scholar] [CrossRef]
- Konjevod, M.; Nikolac Perkovic, M.; Sáiz, J.; Svob Strac, D.; Barbas, C.; Rojo, D. Metabolomics analysis of microbiota-gut-brain axis in neurodegenerative and psychiatric diseases. J. Pharm. Biomed. Anal. 2021, 194, 113681. [Google Scholar] [CrossRef] [PubMed]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part III—Convergence toward clinical trials. Gut Pathog. 2013, 5, 4. [Google Scholar] [CrossRef]
- Yang, B.; Wei, J.; Ju, P.; Chen, J. Effects of regulating intestinal microbiota on anxiety symptoms: A systematic review. Gen. Psychiatry 2019, 32, e100056. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.T.; Zhang, Z.; Sun, Y.; Zhu, R.Y.; Wang, F.X.; Ma, L.J.; Jiang, L.; Liu, H.D. Neuroprotective Effects of Sodium Butyrate by Restoring Gut Microbiota and Inhibiting TLR4 Signaling in Mice with MPTP-Induced Parkinson’s Disease. Nutrients 2023, 15, 930. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. [Google Scholar] [CrossRef]
- Takizawa, C.; Thompson, P.L.; van Walsem, A.; Faure, C.; Maier, W.C. Epidemiological and Economic Burden of Alzheimer’s Disease: A Systematic Literature Review of Data across Europe and the United States of America. J. Alzheimer’s Dis. 2014, 43, 1271–1284. [Google Scholar] [CrossRef]
- Bengmark, S. Gut microbiota, immune development and function. Pharmacol. Res. 2013, 69, 87–113. [Google Scholar] [CrossRef]
- Liu, G.; Huang, W.; Moir, R.D.; Vanderburg, C.R.; Lai, B.; Peng, Z.; Tanzi, R.E.; Rogers, J.T.; Huang, X. Metal exposure and Alzheimer’s pathogenesis. J. Struct. Biol. 2006, 155, 45–51. [Google Scholar] [CrossRef]
- Grochowska, M.; Laskus, T.; Radkowski, M. Gut Microbiota in Neurological Disorders. Arch. Immunol. Ther. Exp. 2019, 67, 375–383. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Can a Bacterial Endotoxin be a Key Factor in the Kinetics of Amyloid Fibril Formation? J. Alzheimer’s Dis. 2014, 39, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xu, G.; Ran, M.; Luo, W.; Wang, H. APOE-ε4 Carrier Status and Gut Microbiota Dysbiosis in Patients With Alzheimer Disease. Front. Neurosci. 2021, 15, 619051. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Boziki, M.; Gavalas, E.; Zavos, C.; Deretzi, G.; Grigoriadis, N.; Tsolaki, M.; Chatzopoulos, D.; Katsinelos, P.; Tzilves, D.; et al. Increased Cerebrospinal Fluid Helicobacter Pylori Antibody in Alzheimer’s Disease. Int. J. Neurosci. 2009, 119, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Arrona-Cardoza, P.; Spillane, M.B.; Morales-Marroquin, E. Alzheimer’s disease and gut microbiota: Does trimethylamine N-oxide (TMAO) play a role? Nutr Rev. 2022, 80, 271–281. [Google Scholar] [CrossRef]
- Del Rio, D.; Zimetti, F.; Caffarra, P.; Tassotti, M.; Bernini, F.; Brighenti, F.; Zini, A.; Zanotti, I. The Gut Microbial Metabolite Trimethylamine-N-Oxide Is Present in Human Cerebrospinal Fluid. Nutrients 2017, 9, 1053. [Google Scholar] [CrossRef]
- Craciun, S.; Balskus, E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Romanenko, M.; Kholin, V.; Koliada, A.; Vaiserman, A. Nutrition, Gut Microbiota, and Alzheimer’s Disease. Front. Psychiatry 2021, 12, 712673. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [PubMed]
- Craciun, S.; Marks, J.A.; Balskus, E.P. Characterization of Choline Trimethylamine-Lyase Expands the Chemistry of Glycyl Radical Enzymes. ACS Chem. Biol. 2014, 9, 1408–1413. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018, 37, 716–723. [Google Scholar] [CrossRef]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bruheim, I.; Bjørndal, B. Krill oil reduces plasma triacylglycerol level and improves related lipoprotein particle concentration, fatty acid composition and redox status in healthy young adults—A pilot study. Lipids Health Dis. 2015, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Ağagündüz, D.; Kocaadam-Bozkurt, B.; Bozkurt, O.; Sharma, H.; Esposito, R.; Özoğul, F.; Capasso, R. Microbiota alteration and modulation in Alzheimer’s disease by gerobiotics: The gut-health axis for a good mind. Biomed. Pharmacother. 2022, 153, 113430. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Stamova, B.; Jin, L.W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef]
- Chen, C.K.; Wu, Y.T.; Chang, Y.C. Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 2017, 9, 56. [Google Scholar] [CrossRef]
- Kamer, A.R.; Pirraglia, E.; Tsui, W.; Rusinek, H.; Vallabhajosula, S.; Mosconi, L.; Yi, L.; McHugh, P.; Craig, R.G.; Svetcov, S.; et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging 2015, 36, 627–633. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability In Vitro and In Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7, 1544. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Riva, M.; Björk, P.; Swärd, K.; Mörgelin, M.; Leanderson, T.; Ivars, F. CD14 Is a Co-Receptor for TLR4 in the S100A9-Induced Pro-Inflammatory Response in Monocytes. PLoS ONE 2016, 11, e0156377. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Barichello, T.; Giridharan, V.V.; Dal-Pizzol, F. A cerebrospinal fluid biosignature for the diagnosis of Alzheimer’s disease. Braz. J. Psychiatry 2019, 41, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Zilberter, Y.; Zilberter, M. The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J. Neurosci. Res. 2017, 95, 2217–2235. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol. Nutr. Food Res. 2020, 64, 1900636. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406.e10. [Google Scholar] [CrossRef]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Dua, P.Z.Y. Microbial Sources of Amyloid and Relevance to Amyloidogenesis and AlzheimerÂ’s Disease (AD). J. Alzheimers Dis. Park. 2015, 5, 177. [Google Scholar] [CrossRef]
- Yao, Z.; Cary, B.P.; Bingman, C.A.; Wang, C.; Kreitler, D.F.; Satyshur, K.A.; Forest, K.T.; Gellman, S.H. Use of a Stereochemical Strategy To Probe the Mechanism of Phenol-Soluble Modulin α3 Toxicity. J. Am. Chem. Soc. 2019, 141, 7660–7664. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; Bianchetti, A.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2021, 164, 105314. [Google Scholar] [CrossRef]
- Shishov, V.A.; Kirovskaia, T.A.; Kudrin, V.S.; Oleskin, A.V. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikl. Biokhim. Mikrobiol. 2009, 45, 550–554. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Botvinko, I.V.; Kudrin, V.S.; Oleskin, A.V. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl. Biochem. 2000, 372, 115–117. [Google Scholar]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-Aminobutyric Acid by Lactic Acid Bacteria Isolated from a Variety of Italian Cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [PubMed]
- Niciu, M.J.; Kelmendi, B.; Sanacora, G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012, 100, 656–664. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef]
- Bleich, S.; Römer, K.; Wiltfang, J.; Kornhuber, J. Glutamate and the glutamate receptor system: A target for drug action. Int. J. Geriatr. Psychiatry 2003, 18 (Suppl. 1), S33–S40. [Google Scholar] [CrossRef]
- Martinez, M.; Frank, A.; Diez-Tejedor, E.; Hernanz, A. Amino acid concentrations in cerebrospinal fluid and serum in Alzheimer’s disease and vascular dementia. J. Neural Transm. Park. Dis. Dement. Sect. 1993, 6, 1–9. [Google Scholar] [CrossRef]
- Choi, D. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987, 7, 369–379. [Google Scholar] [CrossRef]
- Epstein, F.H.; Lipton, S.A.; Rosenberg, P.A. Excitatory Amino Acids as a Final Common Pathway for Neurologic Disorders. N. Engl. J. Med. 1994, 330, 613–622. [Google Scholar] [CrossRef]
- Mothet, J.P.; Snyder, S.H. Brain D-amino acids: A novel class of neuromodulators. Amino Acids 2012, 43, 1809–1810. [Google Scholar] [CrossRef]
- Genchi, G. An overview on D-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Esaki, N.; Yoshimura, T.; Soda, K. Reaction Mechanism of Glutamate Racemase, a Pyridoxal Phosphate-Independent Amino Acid Racemase. J. Biochem. 1992, 112, 139–142. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, H.T.; Lane, H.Y. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019, 185, 172760. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, H.T.; Chiu, C.C.; Lane, H.Y. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci. Rep. 2017, 7, 14849. [Google Scholar] [CrossRef]
- Sanchez, S.; Rodríguez-Sanoja, R.; Ramos, A.; Demain, A.L. Our microbes not only produce antibiotics, they also overproduce amino acids. J. Antibiot. 2017, 71, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Hashimoto, K.I.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum mechanosensitive channels: Towards unpuzzling “glutamate efflux” for amino acid production. Biophys. Rev. 2018, 10, 1359–1369. [Google Scholar] [CrossRef]
- Dean, S.F.; Whalen, K.L.; Spies, M.A. Biosynthesis of a Novel Glutamate Racemase Containing a Site-Specific 7-Hydroxycoumarin Amino Acid: Enzyme–Ligand Promiscuity Revealed at the Atomistic Level. ACS Cent. Sci. 2015, 1, 364–373. [Google Scholar] [CrossRef]
- Malathi, K.C.; Wachi, M.; Nagai, K. Isolation of the murI gene from Brevibacterium lactofermentum ATCC 13869 encoding d-glutamate racemase. FEMS Microbiol. Lett. 1999, 175, 193–196. [Google Scholar] [CrossRef]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Lanctôt, K.L.; Herrmann, N.; Mazzotta, P.; Khan, L.R.; Ingber, N. GABAergic Function in Alzheimer’s Disease: Evidence for Dysfunction and Potential as a Therapeutic Target for the Treatment of Behavioural and Psychological Symptoms of Dementia. Can. J. Psychiatry 2004, 49, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Puerta, E.; Ramirez, M. Treatment Options in Alzheimer’s Disease: The GABA Story. Curr. Pharm. Des. 2015, 21, 4960–4971. [Google Scholar] [CrossRef] [PubMed]
- Naddafi, F.; Mirshafiey, A. The Neglected Role of Histamine in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2013, 28, 327–336. [Google Scholar] [CrossRef]
- del Rio, R.; Noubade, R.; Saligrama, N.; Wall, E.H.; Krementsov, D.N.; Poynter, M.E.; Zachary, J.F.; Thurmond, R.L.; Teuscher, C. Histamine H4 Receptor Optimizes T Regulatory Cell Frequency and Facilitates Anti-Inflammatory Responses within the Central Nervous System. J. Immunol. 2012, 188, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Ferstl, R.; Frei, R.; Schiavi, E.; Konieczna, P.; Barcik, W.; Ziegler, M.; Lauener, R.P.; Chassard, C.; Lacroix, C.; Akdis, C.A.; et al. Histamine receptor 2 is a key influence in immune responses to intestinal histamine-secreting microbes. J. Allergy Clin. Immunol. 2014, 134, 744–746.e3. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Franco, A.; Fernández-Novoa, L.; Cacabelos, R. Blood levels of histamine, IL-1β, and TNF-α in patients with mild to moderate alzheimer disease. Mol. Chem. Neuropathol. 1996, 29, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, A.; Mussur, M.; Unzeta, M.; Lazewska, D.; Kiec-Kononowicz, K.; Fogel, W.A. The central histamine level in rat model of vascular dementia. J. Physiol. Pharmacol. 2011, 62, 549–558. [Google Scholar]
- Tohgi, H.; Abe, T.; Takahashi, S.; Kimura, M.; Takahashi, J.; Kikuchi, T. Concentrations of serotonin and its related substances in the cerebrospinal fluid in patients with Alzheimer type dementia. Neurosci. Lett. 1992, 141, 9–12. [Google Scholar] [CrossRef]
- Palmer, A.M.; Wilcock, G.K.; Esiri, M.M.; Francis, P.T.; Bowen, D.M. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain Res. 1987, 401, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.J.; Noristani, H.N.; Verkhratsky, A. The serotonergic system in ageing and Alzheimer’s disease. Prog. Neurobiol. 2012, 99, 15–41. [Google Scholar] [CrossRef]
- Lyte, M.; Freestone, P.P.E. (Eds.) . Microbial Endocrinology; Springer: New York, NY, USA, 2010. [Google Scholar]
- Cutuli, D.; Giacovazzo, G.; Decandia, D.; Coccurello, R. Alzheimer’s disease and depression in the elderly: A trajectory linking gut microbiota and serotonin signaling. Front. Psychiatry 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Hasegawa, H. Production and Peripheral Roles of 5-HTP, a Precursor of Serotonin. Int. J. Tryptophan Res. 2009, 2, IJTR.S1022. [Google Scholar] [CrossRef]
- . Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain research 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Daugé, V.; Naudon, L.; Rabot, S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Ivković, S.; Mitić, M.; Adžić, M. Tryptophan metabolites in depression: Modulation by gut microbiota. Front. Behav. Neurosci. 2022, 16, 987697. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host–Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Castorina, S.; Imbesi, R.; Szychlinska, M.A.; Scuderi, S.; Loreto, C.; Giunta, S. Changes in serotonin (5-HT) and brain-derived neurotrophic factor (BDFN) expression in frontal cortex and hippocampus of aged rat treated with high tryptophan diet. Brain Res. Bull. 2015, 119 Pt A, 12–18. [Google Scholar] [CrossRef]
- Noristani, H.N.; Verkhratsky, A.; Rodríguez, J.J. High tryptophan diet reduces CA1 intraneuronal β-amyloid in the triple transgenic mouse model of Alzheimer’s disease. Aging Cell 2012, 11, 810–822. [Google Scholar] [CrossRef]
- Álvarez, J.; Fernández Real, J.M.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; Saenz de Pipaon, M.; Sanz, Y. Microbiota intestinal y salud. Gastroenterol. Hepatol. 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Bidesi, N.S.R.; Vang Andersen, I.; Windhorst, A.D.; Shalgunov, V.; Herth, M.M. The role of neuroimaging in Parkinson’s disease. J. Neurochem. 2021, 159, 660–689. [Google Scholar] [CrossRef]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Carey, G.; Görmezoğlu, M.; Jong, J.J.A.; Hofman, P.A.M.; Backes, W.H.; Dujardin, K.; Leentjens, A.F.G. Neuroimaging of Anxiety in Parkinson’s Disease: A Systematic Review. Mov. Disord. 2021, 36, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Dirkx, M.F.; Bologna, M. The pathophysiology of Parkinson’s disease tremor. J. Neurol. Sci. 2022, 435, 120196. [Google Scholar] [CrossRef]
- Dowling, L.R.; Strazzari, M.R.; Keely, S.; Kaiko, G.E. Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. J. Allergy Clin. Immunol. 2022, 150, 513–522. [Google Scholar] [CrossRef]
- Metta, V.; Leta, V.; Mrudula, K.R.; Prashanth, L.K.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; Chaudhuri, K.R. Gastrointestinal dysfunction in Parkinson’s disease: Molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J. Neurol. 2022, 269, 1154–1163. [Google Scholar] [CrossRef]
- Warnecke, T.; Schäfer, K.H.; Claus, I.; Del Tredici, K.; Jost, W.H. Gastrointestinal involvement in Parkinson’s disease: Pathophysiology, diagnosis, and management. NPJ Park. Dis. 2022, 8, 31. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef]
- Lai, F.; Jiang, R.; Xie, W.; Liu, X.; Tang, Y.; Xiao, H.; Gao, J.; Jia, Y.; Bai, Q. Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) Mouse Model of Parkinson’s Disease. Neurochem. Res. 2018, 43, 1986–1999. [Google Scholar] [CrossRef]
- Baiano, C.; Barone, P.; Trojano, L.; Santangelo, G. Prevalence and Clinical Aspects of Mild Cognitive Impairment in Parkinson’s Disease: A Meta-Analysis. Mov. Disord. 2020, 35, 45–54. [Google Scholar] [CrossRef]
- Gaig, C.; Compta, Y.; Heidbreder, A.; Marti, M.J.; Titulaer, M.J.; Crijnen, Y.; Högl, B.; Lewerenz, J.; Erro, M.E.; Garcia-Monco, J.C.; et al. Frequency and Characterization of Movement Disorders in Anti-IgLON5 Disease. Neurology 2021, 97, 1367–1381. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Chen, D.; Xu, X.; Li, W.; Li, K.; He, J.; Su, W.; Luo, Q. The alteration of intestinal mucosal α-synuclein expression and mucosal microbiota in Parkinson’s disease. Appl. Microbiol. Biotechnol. 2023, 107, 1917–1929. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Yin, S.; Wan, F.; Hu, J.; Kou, L.; Sun, Y.; Wu, J.; Zhou, Q.; Huang, J.; et al. Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson’s Disease. Front. Immunol. 2021, 12, 719807. [Google Scholar] [CrossRef]
- Hasuike, Y.; Endo, T.; Koroyasu, M.; Matsui, M.; Mori, C.; Yamadera, M.; Fujimura, H.; Sakoda, S. Bile acid abnormality induced by intestinal dysbiosis might explain lipid metabolism in Parkinson’s disease. Med. Hypotheses 2020, 134, 109436. [Google Scholar] [CrossRef]
- Matheoud, D.; Cannon, T.; Voisin, A.; Penttinen, A.M.; Ramet, L.; Fahmy, A.M.; Ducrot, C.; Laplante, A.; Bourque, M.J.; Zhu, L.; et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1−/− mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef]
- Schneider, M.M.; Gautam, S.; Herling, T.W.; Andrzejewska, E.; Krainer, G.; Miller, A.M.; Trinkaus, V.A.; Peter, Q.A.E.; Ruggeri, F.S.; Vendruscolo, M.; et al. The Hsc70 disaggregation machinery removes monomer units directly from α-synuclein fibril ends. Nat. Commun. 2021, 12, 5999. [Google Scholar] [CrossRef]
- Del Chierico, F.; Grassini, P.; Quagliariello, A.; Torti, M.; Russo, A.; Reddel, S.; Stocchi, F. The impact of intestinal microbiota on weight loss in Parkinson’s disease patients: A pilot study. Future Microbiol. 2020, 15, 1393–1404. [Google Scholar] [CrossRef]
- Pietrucci, D.; Teofani, A.; Unida, V.; Cerroni, R.; Biocca, S.; Stefani, A.; Desideri, A. Can Gut Microbiota Be a Good Predictor for Parkinson’s Disease? A Machine Learning Approach. Brain Sci. 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Kulkarni, S.; Pasricha, P.J.; Lee, G.; Pomper, M.G.; et al. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Persillet, M.; Zhang, L.; Zhang, Y.; Xiuping, S.; Li, X.; Ran, G.; Breger, L.S.; Dovero, S.; Porras, G.; et al. Evaluation of blood flow as a route for propagation in experimental synucleinopathy. Neurobiol. Dis. 2021, 150, 105255. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Dodiya, H.B.; Engen, P.A.; Forsyth, C.B.; Huschens, A.M.; Shaikh, M.; Voigt, R.M.; Naqib, A.; Green, S.J.; Kordower, J.H.; et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut 2019, 68, 829–843. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, F.; Ning, J.; Peng, R.; Shang, J.; Liu, H.; Shang, M.; Bao, X.Q.; Zhang, D. Novel compound FLZ alleviates rotenone-induced PD mouse model by suppressing TLR4/MyD88/NF-κB pathway through microbiota–gut–brain axis. Acta Pharm. Sin. B 2021, 11, 2859–2879. [Google Scholar] [CrossRef]
- Abg Abd Wahab, D.Y.; Gau, C.H.; Zakaria, R.; Muthu Karuppan, M.K.; A-rahbi, B.S.; Abdullah, Z.; Alrafiah, A.; Abdullah, J.M.; Muthuraju, S. Review on Cross Talk between Neurotransmitters and Neuroinflammation in Striatum and Cerebellum in the Mediation of Motor Behaviour. Biomed. Res. Int. 2019, 2019, 1767203. [Google Scholar] [CrossRef]
- Thompson, A.I.; Lees, C.W. Genetics of ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Tatton-Brown, K.; Murray, A.; Hanks, S.; Douglas, J.; Armstrong, R.; Banka, S.; Bird, L.M.; Clericuzio, C.L.; Cormier-Daire, V.; Cushing, T.; et al. Weaver syndrome and EZH2 mutations: Clarifying the clinical phenotype. Am. J. Med. Genet. A 2013, 161, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Fuhrmann, R.; Scharnweber, T.; Franke, R.P. Stimulation of monocytes and macrophages: Possible influence of surface roughness. Clin. Hemorheol. Microcirc. 2008, 39, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.N.; Esteves, A.R.; Empadinhas, N.; Cardoso, S.M. Parkinson’s Disease: A Multisystem Disorder. Neurosci. Bull. 2023, 39, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Cuvelier, E.; Bonte, M.A.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M.C. Glycosphingolipids and neuroinflammation in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 59. [Google Scholar] [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J. Neuroinflammation 2022, 19, 135. [Google Scholar] [CrossRef]
- Jennings, D.; Huntwork-Rodriguez, S.; Henry, A.G.; Sasaki, J.C.; Meisner, R.; Diaz, D.; Solanoy, H.; Wang, X.; Negrou, E.; Bondar, V.V.; et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson’s disease. Sci. Transl. Med. 2022, 14, eabj2658. [Google Scholar] [CrossRef]
- Barazzuol, L.; Giamogante, F.; Brini, M.; Calì, T. PINK1/Parkin Mediated Mitophagy, Ca2+ Signalling, and ER–Mitochondria Contacts in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1772. [Google Scholar] [CrossRef]
- Panicker, N.; Ge, P.; Dawson, V.L.; Dawson, T.M. The cell biology of Parkinson’s disease. J. Cell Biol. 2021, 220, e202012095. [Google Scholar] [CrossRef]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; He, X.; Zhou, F. Parkinson’s disease-associated DJ-1 mutations increase abnormal phosphorylation of tau protein through Akt/GSK-3β pathways. J. Mol. Neurosci. 2013, 51, 911–918. [Google Scholar] [CrossRef]
- Kumar, A.; Mukherjee, D.; Satpati, P. Mutations in Parkinson’s Disease Associated Protein DJ-1 Alter the Energetics of DJ-1 Dimerization. J. Chem. Inf. Model. 2019, 59, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lei, L.; Song, Y.; Liu, M.; Lu, S.; Lou, D.; Shi, Y.; Wang, Z.; He, D. Mutation of hop-1 and pink-1 attenuates vulnerability of neurotoxicity in C. elegans: The role of mitochondria-associated membrane proteins in Parkinsonism. Exp. Neurol. 2018, 309, 67–78. [Google Scholar] [CrossRef]
- Rostami, J.; Mothes, T.; Kolahdouzan, M.; Eriksson, O.; Moslem, M.; Bergström, J.; Ingelsson, M.; O’Callaghan, P.; Healy, L.M.; Falk, A.; et al. Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J. Neuroinflammation 2021, 18, 124. [Google Scholar] [CrossRef]
- . Mossine, V.V.; Waters, J.K.; Sun, G.Y.; Gu, Z.; Mawhinney, T.P. Microglia Signaling Pathway Reporters Unveiled Manganese Activation of the Interferon/STAT1 Pathway and Its Mitigation by Flavonoids. Mol. Neurobiol. 2023, 60(8), 4679–4692. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; An, Y.; Xu, L.; Wang, Y.; Wang, C.; Li, P.; Li, M.; Yan, D.; Wang, M.; Zhong, G.; et al. The protective role of microbiota in the prevention of MPTP/P-induced Parkinson’s disease by resveratrol. Food Funct. 2023, 14, 4647–4661. [Google Scholar] [CrossRef]
- Su, C.F.; Jiang, L.; Zhang, X.W.; Iyaswamy, A.; Li, M. Resveratrol in Rodent Models of Parkinson’s Disease: A Systematic Review of Experimental Studies. Front. Pharmacol. 2021, 12, 644219. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Gao, Q.; Ping, D.; Wang, Y.; Wu, W.; Lin, X.; Fang, Y.; Zhang, J.; Shao, A. The Role of Exosomal microRNAs and Oxidative Stress in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 3232869. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Arab, H.H.; Safar, M.M.; Shahin, N.N. Targeting ROS-Dependent AKT/GSK-3β/NF-κB and DJ-1/Nrf2 Pathways by Dapagliflozin Attenuates Neuronal Injury and Motor Dysfunction in Rotenone-Induced Parkinson’s Disease Rat Model. ACS Chem. Neurosci. 2021, 12, 689–703. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Yang, Y.; Li, J.; Tu, H. Mitochondrial Sirtuins in Parkinson’s Disease. Neurochem. Res. 2022, 47, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, Ü.; Bora Tokçaer, A.; Atay, L. Dopamine transporter SPECT imaging in Parkinson’s disease and parkinsonian disorders. Turk. J. Med. Sci. 2021, 51, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clinica Chimica Acta 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef] [PubMed]
- Salis, C.; Papageorgiou, L.; Papakonstantinou, E.; Hagidimitriou, M.; Vlachakis, D. Olive Oil Polyphenols in Neurodegenerative Pathologies. Adv. Exp. Med. Biol. 2020, 1195, 77–91. [Google Scholar]
- Singh SSen Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-κB-Mediated Neuroinflammation in Parkinson’s Disease and Potential Therapeutic Effect of Polyphenols. Neurotox. Res. 2020, 37, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Lin, C.H. Gut microenvironmental changes as a potential trigger in Parkinson’s disease through the gut–brain axis. J. Biomed. Sci. 2022, 29, 54. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Ye, Y.; Yan, X.; Cheng, Y.; Zhao, L.; Chen, F.; Ling, Z. Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease. Front. Immunol. 2022, 13, 937555. [Google Scholar] [CrossRef]
- Hirayama, M.; Ohno, K. Parkinson’s Disease and Gut Microbiota. Ann. Nutr. Metab. 2021, 77 (Suppl. 2), 28–35. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Gotoh, M.; Miyamoto, Y.; Ikeshima-Kataoka, H. Astrocytic Neuroimmunological Roles Interacting with Microglial Cells in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 1599. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef]
- Park, J.; Kim, C.H. Regulation of common neurological disorders by gut microbial metabolites. Exp. Mol. Med. 2021, 53, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.T.; Wu, Y.W.; Liou, J.M.; Wu, M.S.; Kuo, C.H.; Lin, C.H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids With Gut Microbiota and Clinical Severity in Patients With Parkinson Disease. Neurology 2022, 98, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Demirkan, A.; Twa, G.; Cohen, G.; Dean, M.N.; Standaert, D.G.; Sampson, T.R.; Payami, H. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 2022, 13, 6958. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Huang, P.; Li, B.; Du, J.; He, Y.; Su, B.; Xu, L.M.; Wang, L.; et al. Gut metagenomics-derived genes as potential biomarkers of Parkinson’s disease. Brain 2020, 143, 2474–2489. [Google Scholar] [CrossRef]
- Castillo-Álvarez, F.; Marzo-Sola, M.E. Role of the gut microbiota in the development of various neurological diseases. Neurologia 2022, 37, 492–498. [Google Scholar] [CrossRef]
- O’Keeffe, G.W.; Sullivan, A.M. Evidence for dopaminergic axonal degeneration as an early pathological process in Parkinson’s disease. Park. Relat. Disord. 2018, 56, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gordián-Vélez, W.J.; Chouhan, D.; España, R.A.; Chen, H.I.; Burdick, J.A.; Duda, J.E.; Cullen, D.K. Restoring lost nigrostriatal fibers in Parkinson’s disease based on clinically-inspired design criteria. Brain Res. Bull. 2021, 175, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Zhao, C.; Pang, S.; Lu, J.; Chan, P. α-Synuclein aggregation causes muscle atrophy through neuromuscular junction degeneration. J. Cachexia Sarcopenia Muscle 2023, 14, 226–242. [Google Scholar] [CrossRef]
- Dopkins, N.; Nagarkatti, P.S.; Nagarkatti, M. The role of gut microbiome and associated metabolome in the regulation of neuroinflammation in multiple sclerosis and its implications in attenuating chronic inflammation in other inflammatory and autoimmune disorders. Immunology 2018, 154, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Oskarsson, B.; Gendron, T.F.; Staff, N.P. Amyotrophic Lateral Sclerosis: An. Update for 2018. Mayo Clin. Proc. 2018, 93, 1617–1628. [Google Scholar] [CrossRef]
- Niedermeyer, S.; Murn, M.; Choi, P.J. Respiratory Failure in Amyotrophic Lateral Sclerosis. Chest 2019, 155, 401–408. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut Microbiota in Multiple Sclerosis: Possible Influence of Immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef]
- Ntranos, A.; Park, H.J.; Wentling, M.; Tolstikov, V.; Amatruda, M.; Inbar, B.; Kim-Schulze, S.; Frazier, C.; Button, J.; Kiebish, M.A.; et al. Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain 2022, 145, 569–583. [Google Scholar] [CrossRef]
- Cox, L.M.; Calcagno, N.; Gauthier, C.; Madore, C.; Butovsky, O.; Weiner, H.L. The microbiota restrains neurodegenerative microglia in a model of amyotrophic lateral sclerosis. Microbiome 2022, 10, 47. [Google Scholar] [CrossRef]
- Zeng, Q.; Shen, J.; Chen, K.; Zhou, J.; Liao, Q.; Lu, K.; Yuan, J.; Bi, F. The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci. Rep. 2020, 10, 12998. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Martin, S.; Battistini, C.; Sun, J. A Gut Feeling in Amyotrophic Lateral Sclerosis: Microbiome of Mice and Men. Front. Cell. Infect. Microbiol. 2022, 12, 839526. [Google Scholar] [CrossRef]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef]
- Di Gioia, D.; Bozzi Cionci, N.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggìa, F.; Lucenti, M.A.; Bersano, E.; Cantello, R.; et al. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med. 2020, 18, 153. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.J.; Park, N.Y.; Dubey, S.K.; Kim, T.; Ruan, K.; Lim, S.B.; Park, S.H.; Ha, S.; Kovlyagina, I.; et al. Multi-omic analysis of selectively vulnerable motor neuron subtypes implicates altered lipid metabolism in ALS. Nat. Neurosci. 2021, 24, 1673–1685. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef]
- Liu, Z.; Waters, J.; Rui, B. Metabolomics as a promising tool for improving understanding of multiple sclerosis: A review of recent advances. Biomed. J. 2022, 45, 594–606. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Haidar, M.; Kooij, G.; Hendriks, J.J.A. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv. Drug Deliv. Rev. 2020, 159, 198–213. [Google Scholar] [CrossRef]
- Bhargava, P.; Anthony, D.C. Metabolomics in multiple sclerosis disease course and progression. Mult. Scler. J. 2020, 26, 591–598. [Google Scholar] [CrossRef]
- Signoriello, E.; Iardino, P.; Casertano, S.; De Lucia, D.; Pucciarelli, A.; Puoti, G.; Chiosi, E.; Lus, G. 12-months prospective Pentraxin-3 and metabolomic evaluation in multiple sclerosis patients treated with glatiramer acetate. J. Neuroimmunol. 2020, 348, 577385. [Google Scholar] [CrossRef]
- Yang, J.; Hamade, M.; Wu, Q.; Wang, Q.; Axtell, R.; Giri, S.; Mao-Draayer, Y. Current and Future Biomarkers in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 5877. [Google Scholar] [CrossRef]
- Kalyan, M.; Tousif, A.H.; Sonali, S.; Vichitra, C.; Sunanda, T.; Praveenraj, S.S.; Ray, B.; Gorantla, V.R.; Rungratanawanich, W.; Mahalakshmi, A.M.; et al. Role of Endogenous Lipopolysaccharides in Neurological Disorders. Cells 2022, 11, 4038. [Google Scholar] [CrossRef]
- Rival, M.; Galoppin, M.; Thouvenot, E. Biological Markers in Early Multiple Sclerosis: The Paved Way for Radiologically Isolated Syndrome. Front. Immunol. 2022, 13, 866092. [Google Scholar] [CrossRef]
- Sandi, D.; Fricska-Nagy, Z.; Bencsik, K.; Vécsei, L. Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy—Kynurenines Are Important Players. Molecules 2021, 26, 3423. [Google Scholar] [CrossRef]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and Modified Mediterranean Diet as a Tool to Counteract Neuroinflammation in Multiple Sclerosis: Nutritional Suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Franco, N.F.; Ng, M.L.; Pai, S.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Current Evidence for a Role of the Kynurenine Pathway of Tryptophan Metabolism in Multiple Sclerosis. Front. Immunol. 2016, 7, 246. [Google Scholar] [CrossRef]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: A compensatory mechanism or a driving force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef]
- Rajda, C.; Galla, Z.; Polyák, H.; Maróti, Z.; Babarczy, K.; Pukoli, D.; Vécsei, L. Cerebrospinal Fluid Neurofilament Light Chain Is Associated with Kynurenine Pathway Metabolite Changes in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 2665. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, K.; Shao, T.; Hou, L.; Zhang, S.; Ye, W.; Josephson, L.; Meyer, J.H.; Zhang, M.R.; Vasdev, N.; et al. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm. Sin. B 2021, 11, 373–393. [Google Scholar] [CrossRef]
- Lee, J.M.; Tan, V.; Lovejoy, D.; Braidy, N.; Rowe, D.B.; Brew, B.J.; Guillemin, G.J. Involvement of quinolinic acid in the neuropathogenesis of amyotrophic lateral sclerosis. Neuropharmacology 2017, 112, 346–364. [Google Scholar] [CrossRef]
- Lamb, Y.N. Ocrelizumab: A Review in Multiple Sclerosis. Drugs 2022, 82, 323–334. [Google Scholar] [CrossRef]
- Banati, R.B.; Newcombe, J.; Gunn, R.N.; Cagnin, A.; Turkheimer, F.; Heppner, F.; Price, G.; Wegner, F.; Giovannoni, G.; Miller, D.H.; et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis. Brain 2000, 123, 2321–2337. [Google Scholar] [CrossRef]
- Parravicini, C.; Lecca, D.; Marangon, D.; Coppolino, G.T.; Daniele, S.; Bonfanti, E.; Fumafalli, M.; Raveglia, L.; Martini, C.; Gianazza, E.; et al. Development of the first in vivo GPR17 ligand through an iterative drug discovery pipeline: A novel disease-modifying strategy for multiple sclerosis. PLoS ONE 2020, 15, e0231483. [Google Scholar] [CrossRef]
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in multiple sclerosis. WIREs Mech. Dis. 2023, 15, e1583. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef]
- Bargiela, D.; Chinnery, P.F. Mitochondria in neuroinflammation—Multiple sclerosis (MS), leber hereditary optic neuropathy (LHON) and LHON-MS. Neurosci. Lett. 2019, 710, 132932. [Google Scholar] [CrossRef]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Tomino, Y.; Lu, K.C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef]
- Laudani, S.; Torrisi, S.A.; Alboni, S.; Bastiaanssen, T.F.S.; Benatti, C.; Rivi, V.; Moloney, R.D.; Fuochi, V.; Furneri, P.M.; Drago, F.; et al. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav. Immun. 2023, 107, 385–396. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, I.H.; Hyun, J.S.; Kong, B.S.; Kim, H.J.; Park, S.J. Metabolomic profiling of CSF in multiple sclerosis and neuromyelitis optica spectrum disorder by nuclear magnetic resonance. PLoS ONE 2017, 12, e0181758. [Google Scholar] [CrossRef]
- Luo, S.; Ma, C.; Zhu, M.Q.; Ju, W.N.; Yang, Y.; Wang, X. Application of Iron Oxide Nanoparticles in the Diagnosis and Treatment of Neurodegenerative Diseases With Emphasis on Alzheimer’s Disease. Front. Cell Neurosci. 2020, 14, 21. [Google Scholar] [CrossRef]
- Rowin, J.; Xia, Y.; Jung, B.; Sun, J. Gut inflammation and dysbiosis in human motor neuron disease. Physiol. Rep. 2017, 5, e13443. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Ogbu, D.; Zhang, Y.; Claud, K.; Xia, Y.; Sun, J. Target Metabolites to Slow Down Progression of Amyotrophic Lateral Sclerosis in Mice. Metabolites 2022, 12, 1253. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef]
- Hong, D.; Zhang, C.; Wu, W.; Lu, X.; Zhang, L. Modulation of the gut–brain axis via the gut microbiota: A new era in treatment of amyotrophic lateral sclerosis. Front. Neurol. 2023, 14, 1133546. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Tian, J.; Fan, D. Differentiating Slowly Progressive Subtype of Lower Limb Onset ALS From Typical ALS Depends on the Time of Disease Progression and Phenotype. Front. Neurol. 2022, 13, 872500. [Google Scholar] [CrossRef] [PubMed]
- Druml, W. Intestinaler Crosstalk. Med. Klin. Intensivmed. Notfmed. 2018, 113, 470–477. [Google Scholar] [CrossRef]
- Niccolai, E.; Di Pilato, V.; Nannini, G.; Baldi, S.; Russo, E.; Zucchi, E.; Martinelli, I.; Menicatti, M.; Bartolucci, G.; Mandrioli, J.; et al. The Gut Microbiota-Immunity Axis in ALS: A Role in Deciphering Disease Heterogeneity? Biomedicines 2021, 9, 753. [Google Scholar] [CrossRef]
- Kang, I.; Bucala, R. The immunobiology of MIF: Function, genetics and prospects for precision medicine. Nat. Rev. Rheumatol. 2019, 15, 427–437. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Zhou, J.; Huang, J.; Zhou, L.; Luo, J.; Wan, Y.Y.; Long, H.; Zhu, B. EZH2 Inhibitor GSK126 Suppresses Antitumor Immunity by Driving Production of Myeloid-Derived Suppressor Cells. Cancer Res. 2019, 79, 2009–2020. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Martins, A.C.; Avila, D.S.; Gritsenko, V.A.; Skalny, A.V.; Santamaria, A.; Lee, E.; Bowman, A.B.; Aschner, M. Gut Microbiota as a Potential Player in Mn-Induced Neurotoxicity. Biomolecules 2021, 11, 1292. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 247054702210763. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Zhang, J.; Dong, J.; Ma, J.; Zhang, Y.; Jin, K.; Lu, J. Effect of prebiotics, probiotics, synbiotics on depression: Results from a meta-analysis. BMC Psychiatry 2023, 23, 477. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef]
- Sun, Y.; Baptista, L.C.; Roberts, L.M.; Jumbo-Lucioni, P.; McMahon, L.L.; Buford, T.W.; Buford, T.W.; Carter, C.S. The Gut Microbiome as a Therapeutic Target for Cognitive Impairment. J. Gerontol. Ser. A 2020, 75, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Park, G.S.; Jeong, D.Y.; Lee, Y.H.; Im, S.; Song, W.H.; Kang, J. Multidisciplinary and Comparative Investigations of Potential Psychobiotic Effects of Lactobacillus Strains Isolated From Newborns and Their Impact on Gut Microbiota and Ileal Transcriptome in a Healthy Murine Model. Front. Cell. Infect. Microbiol. 2019, 9, 269. [Google Scholar] [CrossRef]
- Liao, J.F.; Cheng, Y.F.; You, S.T.; Kuo, W.C.; Huang, C.W.; Chiou, J.J.; Hsu, C.C.; Hsieh-Li, H.H.; Wang, S.; Tsai, Y.C. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav. Immun. 2020, 90, 26–46. [Google Scholar] [CrossRef]
- Matheson, J.A.T.; Holsinger, R.M.D. The Role of Fecal Microbiota Transplantation in the Treatment of Neurodegenerative Diseases: A Review. Int. J. Mol. Sci. 2023, 24, 1001. [Google Scholar] [CrossRef]
- Sampaio, K.B.; Fusco, V.; Alves, J.L.d.B.; de Souza, E.L. Probiotics: Concepts, evolution, and applications. In Probiotics for Human Nutrition in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–24. [Google Scholar]
- Adıgüzel, E.; Çiçek, B.; Ünal, G.; Aydın, M.F.; Barlak-Keti, D. Probiotics and prebiotics alleviate behavioral deficits, inflammatory response, and gut dysbiosis in prenatal VPA-induced rodent model of autism. Physiol. Behav. 2022, 256, 113961. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Eid, E.E.M.; Hussin, S.; Alshawsh, M.A. Probiotics supplementation in patients with colorectal cancer: A systematic review of randomized controlled trials. Nutr. Rev. 2021, 80, 22–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jeon, S.H.; Ju, I.G.; Gee, M.S.; Do, J.; Oh, M.S.; Lee, J.K. Transplantation of gut microbiota derived from Alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav. Immun. 2021, 98, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.; Gottel, N.; Zhang, X.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
- Wang, M.; Cao, J.; Gong, C.; Amakye, W.K.; Yao, M.; Ren, J. Exploring the microbiota-Alzheimer’s disease linkage using short-term antibiotic treatment followed by fecal microbiota transplantation. Brain Behav. Immun. 2021, 96, 227–238. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Wei, D.; Song, Z.; Chen, D.; et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Elangovan, S.; Borody, T.J.; Holsinger, R.M.D. Fecal Microbiota Transplantation Reduces Pathology and Improves Cognition in a Mouse Model of Alzheimer’s Disease. Cells 2022, 12, 119. [Google Scholar] [CrossRef]
- Hazan, S. Rapid improvement in Alzheimer’s disease symptoms following fecal microbiota transplantation: A case report. J. Int. Med. Res. 2020, 48, 030006052092593. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, J.H.; Shin, J.; Kim, J.S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: A case report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tapia, E.; Almeida-Toledano, L.; Sebastiani, G.; Serra-Delgado, M.; García-Algar, Ó.; Andreu-Fernández, V. Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 2351. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.T.; Xu, X.W.; Jin, C.Y.; Yuan, X.Y.; Wang, X.G. The Influence of the Gut Microbiota on Alzheimer’s Disease: A Narrative Review. J. Integr. Neurosci. 2023, 22, 38. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Xin, Y.; Diling, C.; Tianlu, C.; Jun, Z.; Xiaocui, T.; Yinrui, G.; Ou, S.; Tianming, D.; Guoyan, H. Oligosaccharides from Morinda officinalis Slow the Progress of Aging Mice by Regulating the Key Microbiota-Metabolite Pairs. Evid. Based Complement. Altern. Med. 2019, 2019, 9306834. [Google Scholar] [CrossRef]
- Deng, S.; Lu, H.; Chi, H.; Wang, Y.; Li, X.; Ye, H. Neuroprotective Effects of OMO within the Hippocampus and Cortex in a D-Galactose and A β25–35-Induced Rat Model of Alzheimer’s Disease. Evid. Based Complement. Altern. Med. 2020, 2020, 1067541. [Google Scholar] [CrossRef]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic Effect of Fructooligosaccharides from Morinda officinalis on Alzheimer’s Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front. Aging Neurosci. 2017, 9, 403. [Google Scholar] [CrossRef]
- LaRue, B.; Hogg, E.; Sagare, A.; Jovanovic, S.; Maness, L.; Maurer, C.; Deane, R.; Zlokovic, B. Method for measurement of the blood–brain barrier permeability in the perfused mouse brain: Application to amyloid-β peptide in wild type and Alzheimer’s Tg2576 mice. J. Neurosci. Methods 2004, 138, 233–242. [Google Scholar] [CrossRef]
- Bourasset, F.; Ouellet, M.; Tremblay, C.; Julien, C.; Do, T.M.; Oddo, S.; Laferla, F.; Calon, F. Reduction of the cerebrovascular volume in a transgenic mouse model of Alzheimer’s disease. Neuropharmacology 2009, 56, 808–813. [Google Scholar] [CrossRef]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef]
- Mallikarjuna, N.; Praveen, K.; Yellamma, K. Role of Lactobacillus plantarum MTCC1325 in membrane-bound transport ATPases system in Alzheimer’s disease-induced rat brain. BioImpacts 2016, 6, 203–209. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05. [Google Scholar] [CrossRef] [PubMed]
- Athari Nik Azm, S.; Djazayeri, A.; Safa, M.; Azami, K.; Ahmadvand, B.; Sabbaghziarani, F.; Sharifzadeh, M.; Vafa, M. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1–42) injected rats. Appl. Physiol. Nutr. Metab. 2018, 43, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Asl, Z.; Sepehri, G.; Salami, M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav. Brain Res. 2019, 376, 112183. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, W.; Gao, H.; Che, N.; Xu, M.; Yang, L.; Zhang, Y.; Ye, M. Fecal Microbiota Transplantation Exerts a Protective Role in MPTP-Induced Parkinson’s Disease via the TLR4/PI3K/AKT/NF-κB Pathway Stimulated by α-Synuclein. Neurochem. Res. 2021, 46, 3050–3058. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Jia, X.B.; Sun, M.F.; Zhu, Y.L.; Qiao, C.M.; Zhang, B.P.; Zhao, L.P.; Yang, Q.; Cui, C.; Chen, X.; et al. Neuroprotection of Fasting Mimicking Diet on MPTP-Induced Parkinson’s Disease Mice via Gut Microbiota and Metabolites. Neurotherapeutics 2019, 16, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, T.; Chen, X.; Zhao, Z.; Chen, Z. Gut microbiota relieves inflammation in the substantia nigra of chronic Parkinson’s disease by protecting the function of dopamine neurons. Exp. Ther. Med. 2021, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, H.; Luo, Q.; He, J.; Li, M.; Chen, H.; Tangm, W.; Nie, Y.; Zhou, Y. Fecal microbiota transplantation to treat Parkinson’s disease with constipation. Medicine 2019, 98, e16163. [Google Scholar] [CrossRef]
- Segal, A.; Zlotnik, Y.; Moyal-Atias, K.; Abuhasira, R.; Ifergane, G. Fecal microbiota transplant as a potential treatment for Parkinson’s disease—A case series. Clin. Neurol. Neurosurg. 2021, 207, 106791. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.y.; Yao, X.h.; Xu, L.j.; Zhou, Y.q.; Zhang, L.p.; Liu, Y.; Pei, S.F.; Zhou, C.L. Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb. Cell Fact. 2021, 20, 98. [Google Scholar] [CrossRef]
- Xue, L.J.; Yang, X.Z.; Tong, Q.; Shen, P.; Ma, S.J.; Wu, S.N.; Zheng, J.L.; Wang, H.G. Fecal microbiota transplantation therapy for Parkinson’s disease: A preliminary study. Medicine 2020, 99, e22035. [Google Scholar] [CrossRef]
- DuPont, H.L.; Suescun, J.; Jiang, Z.D.; Brown, E.L.; Essigmann, H.T.; Alexander, A.S.; Dupont, A.W.; Iqbal, T.; Utay, N.; Newmark, M.; et al. Fecal microbiota transplantation in Parkinson’s disease—A randomized repeat-dose, placebo-controlled clinical pilot study. Front. Neurol. 2023, 14, 1104759. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-β1–42-induced rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Rezende, R.M.; Moreira, T.G.; Tankou, S.K.; Cox, L.M.; Wu, M.; Song, A.; Dhang, F.H.; Wei, Z.; Costamagna, G.; et al. Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia muciniphila. Cell Host Microbe 2019, 26, 779–794.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, S.; Hu, L.; Yin, X.; Mai, Y.; Jiang, C.; Peng, X.; Cao, X.; Huang, Z.; Zhou, H.; et al. Protection of Fecal Microbiota Transplantation in a Mouse Model of Multiple Sclerosis. Mediat. Inflamm. 2020, 2020, 2058272. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Wen, X.; Mu, J.; Sun, M.; Song, X.; Lui, B.; Chen, J.; Fan, X. The Efficacy of Fecal Microbiota Transplantation in Experimental Autoimmune Encephalomyelitis: Transcriptome and Gut Microbiota Profiling. J. Immunol. Res. 2021, 2021, 4400428. [Google Scholar] [CrossRef]
- Borody, T.J.; Campbell, J. Fecal microbiota transplantation: Current status and future directions. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 653–655. [Google Scholar] [CrossRef]
- Makkawi, S.; Camara-Lemarroy, C.; Metz, L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol. Neuroimmunol. Neuroinflammation 2018, 5, e459. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, V.; Ali, S.I.; Dupont, A.W. S2314 Fecal Microbiota Transplantation Associated With Disease Stabilization in a Patient With Multiple Sclerosis. Am. J. Gastroenterol. 2020, 115, S1224. [Google Scholar] [CrossRef]
- Engen, P.A.; Zaferiou, A.; Rasmussen, H.; Naqib, A.; Green, S.J.; Fogg, L.F.; Forsyth, C.B.; Raeisi, S.; Hamaker, B.; Keshavarzian, A. Single-Arm, Non-randomized, Time Series, Single-Subject Study of Fecal Microbiota Transplantation in Multiple Sclerosis. Front. Neurol. 2020, 11, 978. [Google Scholar] [CrossRef]
- Al, K.F.; Craven, L.J.; Gibbons, S.; Parvathy, S.N.; Wing, A.C.; Graf, C.; Parham, K.A.; Kerfoot, S.M.; Wilcox, H.; Burton, J.P.; et al. Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 205521732210866. [Google Scholar] [CrossRef]
- Mandrioli, J.; Amedei, A.; Cammarota, G.; Niccolai, E.; Zucchi, E.; D’Amico, R.; Ricci, F.; Quaranta, G.; Spanu, T.; Masucci, L. FETR-ALS Study Protocol: A Randomized Clinical Trial of Fecal Microbiota Transplantation in Amyotrophic Lateral Sclerosis. Front. Neurol. 2019, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wen, Q.; Cui, B.; Li, Q.; Zhang, F. Washed microbiota transplantation stopped the deterioration of amyotrophic lateral sclerosis: The first case report and narrative review. J. Biomed. Res. 2023, 37, 69. [Google Scholar] [CrossRef] [PubMed]
- Fleck, A.K.; Schuppan, D.; Wiendl, H.; Klotz, L. Gut–CNS-Axis as Possibility to Modulate Inflammatory Disease Activity—Implications for Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 1526. [Google Scholar] [CrossRef] [PubMed]
- Maes, E.; Sadovskaya, I.; Lévêque, M.; Elass-Rochard, E.; Payré, B.; Grard, T.; Théodorou, V.; Guérardel, Y.; Mercier-Bonin, M. Structure and biological activities of a hexosamine-rich cell wall polysaccharide isolated from the probiotic Lactobacillus farciminis. Glycoconj. J. 2019, 36, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Yokote, H.; Miyake, S.; Croxford, J.L.; Oki, S.; Mizusawa, H.; Yamamura, T. NKT Cell-Dependent Amelioration of a Mouse Model of Multiple Sclerosis by Altering Gut Flora. Am. J. Pathol. 2008, 173, 1714–1723. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Rynda, A.; Ascón, M.A.; Yang, X.; Kochetkova, I.; Riccardi, C.; Callis, G.; Trunkle, T.; Pascual, D.W. IL-13 Production by Regulatory T Cells Protects against Experimental Autoimmune Encephalomyelitis Independently of Autoantigen. J. Immunol. 2008, 181, 954–968. [Google Scholar] [CrossRef]
- Jun, S.; Ochoa-Repáraz, J.; Zlotkowska, D.; Hoyt, T.; Pascual, D.W. Bystander-mediated stimulation of proteolipid protein-specific regulatory T (Treg) cells confers protection against experimental autoimmune encephalomyelitis (EAE) via TGF-β. J. Neuroimmunol. 2012, 245, 39–47. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef]
- Ezendam, J.; De Klerk, A.; Gremmer, E.R.; Van Loveren, H. Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents. Clin. Exp. Immunol. 2008, 154, 424–431. [Google Scholar] [CrossRef]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A Novel Probiotic Mixture Exerts a Therapeutic Effect on Experimental Autoimmune Encephalomyelitis Mediated by IL-10 Producing Regulatory T Cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef]
- Kwon, H.K.; Kim, G.C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.E.; Nam, J.H.; Im, S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Prasad, A.A. Probiotics Treatment Improves Hippocampal Dependent Cognition in a Rodent Model of Parkinson’s Disease. Microorganisms 2020, 8, 1661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.g.; Wu, S.; Yi, J.; Xia, Y.; Jin, D.; Zhou, J.; Sun, J. Target Intestinal Microbiota to Alleviate Disease Progression in Amyotrophic Lateral Sclerosis. Clin. Ther. 2017, 39, 322–336. [Google Scholar] [CrossRef]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uceda, S.; Echeverry-Alzate, V.; Reiriz-Rojas, M.; Martínez-Miguel, E.; Pérez-Curiel, A.; Gómez-Senent, S.; Beltrán-Velasco, A.I. Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach—A Comprehensive Narrative Review. Int. J. Mol. Sci. 2023, 24, 13294. https://doi.org/10.3390/ijms241713294
Uceda S, Echeverry-Alzate V, Reiriz-Rojas M, Martínez-Miguel E, Pérez-Curiel A, Gómez-Senent S, Beltrán-Velasco AI. Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach—A Comprehensive Narrative Review. International Journal of Molecular Sciences. 2023; 24(17):13294. https://doi.org/10.3390/ijms241713294
Chicago/Turabian StyleUceda, Sara, Víctor Echeverry-Alzate, Manuel Reiriz-Rojas, Esther Martínez-Miguel, Ana Pérez-Curiel, Silvia Gómez-Senent, and Ana Isabel Beltrán-Velasco. 2023. "Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach—A Comprehensive Narrative Review" International Journal of Molecular Sciences 24, no. 17: 13294. https://doi.org/10.3390/ijms241713294
APA StyleUceda, S., Echeverry-Alzate, V., Reiriz-Rojas, M., Martínez-Miguel, E., Pérez-Curiel, A., Gómez-Senent, S., & Beltrán-Velasco, A. I. (2023). Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach—A Comprehensive Narrative Review. International Journal of Molecular Sciences, 24(17), 13294. https://doi.org/10.3390/ijms241713294







