Genetic Variation in miR-27a Is Associated with Fluoropyrimidine-Associated Toxicity in Patients with Dihydropyrimidine Dehydrogenase Variants after Genotype-Guided Dose Reduction
Abstract
:1. Introduction
2. Results
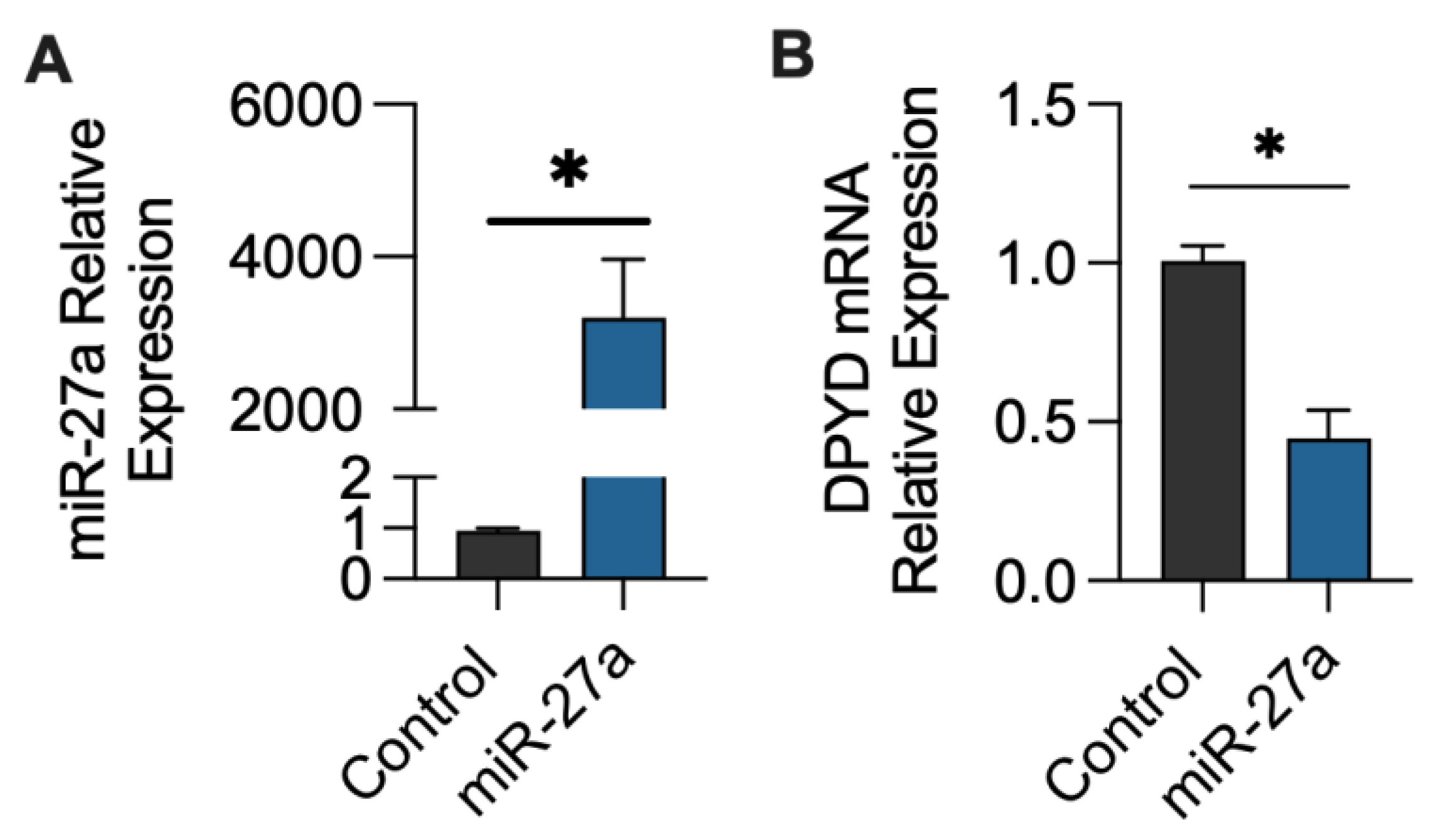
2.1. miR-27a-3p Regulation of DPYD Expression
2.2. Study Population
2.3. Association of miR-27a SNVs and Fluoropyrimidine-Associated Toxicity in the Total Patient Population
2.4. Association of miR-27a SNVs and Fluoropyrimidine-Associated Toxicity in Patients’ Wildtype for DPYD
2.5. Association of miR-27a SNVs and Fluoropyrimidine-Associated Toxicity in DPYD Variant Carriers
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. miR-27a mimic Transfection
4.3. Real-Time RT-PCR
4.4. Study Design
4.5. Genotyping and Sequencing of miR-27a
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wigle, T.J.; Tsvetkova, E.V.; Welch, S.A.; Kim, R.B. Fluorouracil-Based Chemotherapy: Mini Review and Case Report. Pharmaceutics 2019, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, U.; Henricks, L.M.; Offer, S.M.; Barbarino, J.; Schellens, J.H.M.; Swen, J.J.; Klein, T.E.; McLeod, H.L.; Caudle, K.E.; Diasio, R.B.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Kitai, H.; Suzuki, H.I. Network Regulation of microRNA Biogenesis and Target Interaction. Cells 2023, 12, 306. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2017, 9, 852. [Google Scholar] [CrossRef]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Hezova, R.; Kovarikova, A.; Bienertova-Vasku, J.; Sachlova, M.; Redova, M.; Vasku, A.; Svoboda, M.; Radova, L.; Kiss, I.; Vyzula, R.; et al. Evaluation of SNPs in miR-196-a2, miR-27a and miR-146a as risk factors of colorectal cancer. World J. Gastroenterol. 2012, 18, 2827–2831. [Google Scholar] [CrossRef]
- Kupcinskas, J.; Bruzaite, I.; Juzenas, S.; Gyvyte, U.; Jonaitis, L.; Kiudelis, G.; Skieceviciene, J.; Leja, M.; Pauzas, H.; Tamelis, A.; et al. Lack of association between miR-27a, miR-146a, miR-196a-2, miR-492 and miR-608 gene polymorphisms and colorectal cancer. Sci. Rep. 2014, 4, 5993. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Wang, Y.; Liu, X.; Xuan, Y.; Hu, S. Association between miR-27a genetic variants and susceptibility to colorectal cancer. Diagn. Pathol. 2014, 9, 146. [Google Scholar] [CrossRef]
- Bian, Q.; Chen, J.J.; Gu, J.P.; Xu, J. Association between pre-miR-27a functional polymorphism and risk of colorectal cancer in north Chinese Han population. Onco Targets Ther. 2015, 8, 3003–3007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lin, D.H.; Xu, J.P.; Chen, W.X.; Zheng, S.J.; Song, L. Genotype GG of rs895819 Functional Polymorphism Within miR-27a Might Increase Genetic Susceptibility to Colorectal Cancer in Han Chinese Population. J. Clin. Lab. Anal. 2016, 30, 351–355. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, J.; Fang, Y.; Chen, Q.; Li, H. Association between a functional variant in microRNA-27a and susceptibility to colorectal cancer in a Chinese Han population. Genet. Mol. Res. 2014, 13, 7420–7427. [Google Scholar] [CrossRef]
- Feng, Y.; Duan, F.; Song, C.; Zhao, X.; Dai, L.; Cui, S. Systematic evaluation of cancer risk associated with rs2292832 in miR-149 and rs895819 in miR-27a: A comprehensive and updated meta-analysis. Oncotarget 2016, 7, 22368–22384. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dear, K.; Huang, L.; Liu, L.; Shi, Y.; Nie, S.; Liu, Y.; Lu, Y.; Xiang, H. Association between microRNA-27a rs895819 polymorphism and risk of colorectal cancer: A meta-analysis. Cancer Genet. 2016, 209, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fang, W.; Wu, X.; Bian, S.; Chen, G.; Lu, L.; Weng, Y. Distinct effects of rs895819 on risk of different cancers: An update meta-analysis. Oncotarget 2017, 8, 75336–75349. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, Y.; Gong, Y.; Gu, D.; Chen, J. Association of microRNA-27a rs895819 polymorphism with the risk of cancer: An updated meta-analysis. Gene 2020, 728, 144185. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Hao, X.; Tian, W.; Zhou, B. Association of miR-27a polymorphism with the risk of digestive system cancers. Pathol. Res. Pract. 2020, 216, 153115. [Google Scholar] [CrossRef]
- Alidoust, M.; Hamzehzadeh, L.; Rivandi, M.; Pasdar, A. Polymorphisms in non-coding RNAs and risk of colorectal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 132, 100–110. [Google Scholar] [CrossRef]
- Pan, X.M.; Xiao, X.; Qin, H.J.; Zhang, Z.; Li, Z.H.; Gao, L.B.; Jia, J. MicroRNA variants and colorectal cancer risk: A meta-analysis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, T.T.; Ren, Y. miR-27a rs895819 polymorphism and risk of cancer in Chinese population: A meta-analysis. J. Evid. Based Med. 2015, 8, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.Q.; Zhang, X.M.; Chen, B.; Yang, X.D.; Wu, H.R.; Gong, W. MicroRNA gene polymorphisms and the risk of colorectal cancer. Oncol. Lett. 2017, 13, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Zhang, T.; Peng, B.; Yu, L.; Jiang, D.K. Association between microRNA polymorphisms and cancer risk based on the findings of 66 case-control studies. PLoS ONE 2013, 8, e79584. [Google Scholar] [CrossRef]
- Shankaran, Z.S.; Walter, C.E.J.; Prakash, N.; Ramachandiran, K.; Priya Doss, C.G.; Johnson, T. Investigating the role of microRNA-27a gene polymorphisms and its interactive effect with risk factors in gastrointestinal cancers. Heliyon 2020, 6, e03565. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, G.H.; Lee, K.S.; Lee, K.H.; Suh, J.S.; Eisenhut, M.; van der Vliet, H.J.; Kronbichler, A.; Stubbs, B.; Solmi, M.; et al. Genetic variations in MicroRNA genes and cancer risk: A field synopsis and meta-analysis. Eur. J. Clin. Investig. 2020, 50, e13203. [Google Scholar] [CrossRef] [PubMed]
- Deac, A.L.; Burz, C.C.; Militaru, C.; Bocșan, I.C.; Pop, R.M.; Achimaș-Cadariu, P.; Buzoianu, A.D. Role of microRNAs in fluoropyrimidine-related toxicity: What we know. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Offer, S.M.; Butterfield, G.L.; Jerde, C.R.; Fossum, C.C.; Wegner, N.J.; Diasio, R.B. microRNAs miR-27a and miR-27b directly regulate liver dihydropyrimidine dehydrogenase expression through two conserved binding sites. Mol. Cancer Ther. 2014, 13, 742–751. [Google Scholar] [CrossRef]
- Hirota, T.; Date, Y.; Nishibatake, Y.; Takane, H.; Fukuoka, Y.; Taniguchi, Y.; Burioka, N.; Shimizu, E.; Nakamura, H.; Otsubo, K.; et al. Dihydropyrimidine dehydrogenase (DPD) expression is negatively regulated by certain microRNAs in human lung tissues. Lung Cancer 2012, 77, 16–23. [Google Scholar] [CrossRef]
- Falvella, F.S.; Cheli, S.; Martinetti, A.; Mazzali, C.; Iacovelli, R.; Maggi, C.; Gariboldi, M.; Pierotti, M.A.; Di Bartolomeo, M.; Sottotetti, E.; et al. DPD and UGT1A1 deficiency in colorectal cancer patients receiving triplet chemotherapy with fluoropyrimidines, oxaliplatin and irinotecan. Br. J. Clin. Pharmacol. 2015, 80, 581–588. [Google Scholar] [CrossRef]
- Amstutz, U.; Offer, S.M.; Sistonen, J.; Joerger, M.; Diasio, R.B.; Largiadèr, C.R. Polymorphisms in MIR27A Associated with Early-Onset Toxicity in Fluoropyrimidine-Based Chemotherapy. Clin. Cancer Res. 2015, 21, 2038–2044. [Google Scholar] [CrossRef]
- Meulendijks, D.; Henricks, L.M.; Amstutz, U.; Froehlich, T.K.; Largiadèr, C.R.; Beijnen, J.H.; de Boer, A.; Deenen, M.J.; Cats, A.; Schellens, J.H. Rs895819 in MIR27A improves the predictive value of DPYD variants to identify patients at risk of severe fluoropyrimidine-associated toxicity. Int. J. Cancer 2016, 138, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Bai, H.; Hu, H. rs11671784 G/A variation in miR-27a decreases chemo-sensitivity of bladder cancer by decreasing miR-27a and increasing the target RUNX-1 expression. Biochem. Biophys. Res. Commun. 2015, 458, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jie, Z.; Ye, S.; Li, Z.; Han, Z.; Wu, J.; Yang, C.; Jiang, Y. Genetic variations in miR-27a gene decrease mature miR-27a level and reduce gastric cancer susceptibility. Oncogene 2014, 33, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yan, G.; Hao, H.; Yang, B. rs11671784 G/A and rs895819 A/G polymorphisms inversely affect gastric cancer susceptibility and miR-27a expression in a Chinese population. Med. Sci. Monit. 2014, 20, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, W.; Ning, M.; Zhou, X.; Wan, X.; Mi, Q.; Yang, X.; Zhang, D.; Zhang, Y.; Jiang, B.; et al. A functional SNP rs895819 on pre-miR-27a is associated with bipolar disorder by targeting NCAM1. Commun. Biol. 2022, 5, 309. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xu, H.; Ma, D.; Ma, R.; Wu, J.; Yu, X.; Feng, J.; Liu, Q. Pre-miR-27a rs895819 polymorphism and risk of diffuse large B-cell lymphoma. J. Clin. Lab. Anal. 2020, 34, e23088. [Google Scholar] [CrossRef]
- Takuse, Y.; Watanabe, M.; Inoue, N.; Ozaki, R.; Ohtsu, H.; Saeki, M.; Katsumata, Y.; Hidaka, Y.; Iwatani, Y. Association of IL-10-Regulating MicroRNAs in Peripheral Blood Mononuclear Cells with the Pathogenesis of Autoimmune Thyroid Disease. Immunol. Investig. 2017, 46, 590–602. [Google Scholar] [CrossRef]
- Radanova, M.; Levkova, M.; Mihaylova, G.; Manev, R.; Maneva, M.; Hadgiev, R.; Conev, N.; Donev, I. Single Nucleotide Polymorphisms in microRNA Genes and Colorectal Cancer Risk and Prognosis. Biomedicines 2022, 10, 156. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y.; Wang, Y.; Du, Z.; Zhang, C.; Gao, J. Association of microRNA gene polymorphisms with recurrent spontaneous abortion: An updated meta-analysis. Exp. Ther. Med. 2023, 25, 179. [Google Scholar] [CrossRef]
- Gholami, M.; Asgarbeik, S.; Razi, F.; Esfahani, E.N.; Zoughi, M.; Vahidi, A.; Larijani, B.; Amoli, M.M. Association of microRNA gene polymorphisms with Type 2 diabetes mellitus: A systematic review and meta-analysis. J. Res. Med. Sci. 2020, 25, 56. [Google Scholar] [CrossRef]
- Medwid, S.; Li, M.M.J.; Knauer, M.J.; Lin, K.; Mansell, S.E.; Schmerk, C.L.; Zhu, C.; Griffin, K.E.; Yousif, M.D.; Dresser, G.K.; et al. Fexofenadine and Rosuvastatin Pharmacokinetics in Mice with Targeted Disruption of Organic Anion Transporting Polypeptide 2B1. Drug Metab. Dispos. 2019, 47, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Wigle, T.J.; Povitz, B.L.; Medwid, S.; Teft, W.A.; Legan, R.M.; Lenehan, J.; Nevison, S.; Panuganty, V.; Keller, D.; Mailloux, J.; et al. Impact of pretreatment dihydropyrimidine dehydrogenase genotype-guided fluoropyrimidine dosing on chemotherapy associated adverse events. Clin. Transl. Sci. 2021, 14, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Burwinkel, B. A bias in genotyping the miR-27a rs895819 and rs11671784 variants. Breast Cancer Res. Treat. 2012, 134, 899–901. [Google Scholar] [CrossRef] [PubMed]
| Total Population (N = 225) | Patients with DPYD Variants 1 (N = 45) | Patients with No DPYD Variants 1 (N = 180) | |
|---|---|---|---|
| Age (range) | 62 (33–89) | 63 (34–86) | 62 (33–89) |
| Sex, N (%) | |||
| Male | 107 (48) | 21 (47) | 86 (48) |
| Female | 118 (52) | 24 (53) | 94 (52) |
| Tumor Site, N (%) | |||
| Colorectal | 119 (53) | 23 (51) | 96 (53) |
| Gastric and esophagus | 35 16) | 7 (16) | 28 (16) |
| Pancreas | 24 (11) | 6 (13) | 18 (10) |
| Breast | 12 (5) | 3 (7) | 9 (5) |
| Head and neck | 9 (4) | 2 (4) | 7 (4) |
| Anal | 8 (4) | 1 (2) | 7 (4) |
| Other 2 | 18 (8) | 3 (7) | 15 (8) |
| DPYD Genotype, N (%) | |||
| Wildtype | 180 (80) | 0 (0) | 180 (100) |
| c.2846A>T | 18 (8) | 18 (40) | 0 (0) |
| c.1905+1G>A | 9 (4) | 9 (20) | 0 (0) |
| c.1679T>G | 0 (0) | 0 (0) | 0 (0) |
| c.1236G>A | 18 (8) | 18 (40) | 0 (0) |
| miR-27a rs895819, N (%) | |||
| A/A | 100 (44) | 20 (44) | 80 (44) |
| A/G | 88 (39) | 19 (42) | 69 (38) |
| G/G | 37 (16) | 6 (13) | 31 (17) |
| miR-27a rs11671784, N (%) | |||
| C/C | 218 (97) | 44 (98) | 174 (97) |
| C/T | 7 (3) | 1 (2) | 6 (3) |
| T/T | 0 (0) | 0 (0) | 0 (0) |
| Total Population (N = 225) | Patients with DPYD Variants 1 (N = 45) | Patients with No DPYD Variants 1 (N = 180) | |
|---|---|---|---|
| Regimen, N (%) | |||
| Capecitabine monotherapy 2 | 39 (17) | 10 (22) | 29 (16) |
| Capecitabine with radiation | 36 (16) | 8 (18) | 28 (16) |
| Capecitabine with oxaliplatin | 21 (9) | 2 (4) | 19 (11) |
| Capecitabine with other agents 3 | 16 (7) | 3 (7) | 13 (7) |
| FOLFOX 2 | 42 (19) | 7 (16) | 35 (19) |
| FOLFIRI/FOLFIRINOX | 22 (10) | 7 (16) | 15 (8) |
| 5-FU with cisplatin–carboplatin | 26 (12) | 4 (9) | 22 (12) |
| 5-FU with other agents 4 | 23 (10) | 4 (9) | 19 (11) |
| BSA, mean (SD) 5 | 1.88 (0.25) | 1.85 (0.23) | 1.90 (0.26) |
| Initial dose intensity, mean (SD) 6 | 82 (21) | 52 (18) | 90 (12) |
| Average dose intensity, mean (SD) | 80 (18) | 54 (14) | 87 (13) |
| Treatment cycles, median (range) 7 | 5 (1–24) | 5 (1–20) | 4 (1–24) |
| Genotype | Grade ≥ 3 Toxicity 1 during Total Treatment Period N, (%) | OR (95% CI) (Adjusted) | p-Value | |
|---|---|---|---|---|
| miR-27a rs895819 | ||||
| Total Population | A/A | 25 (25) | 1.0 (reference) | |
| A/G | 38 (43) | 2.38 (1.26 to 4.57) | 0.0079 | |
| G/G | 12 (32) | 1.49 (0.63 to 3.43) | 0.3588 | |
| DPYD Wildtype | A/A | 22 (28) | 1.0 (reference) | |
| A/G | 30 (43) | 1.99 (1.00 to 3.99) | 0.0507 | |
| G/G | 11 (35) | 1.44 (0.58 to 3.49) | 0.4278 | |
| DPYD Variant Carriers 2 | A/A | 3 (15) | 1.0 (reference) | |
| A/G | 8 (42) | 8.10 (1.16 to 86.21) | 0.0497 | |
| G/G | 1 (17) | 3.87 (0.12 to 90.67) | 0.3879 | |
| miR-27a rs11671784 | ||||
| Total Population | C/C | 74 (34) | 1.0 (reference) | |
| C/T | 1 (14) | 0.33 (0.02 to 2.06) | 0.3155 | |
| DPYD Wildtype | C/C | 63 (36) | - | - |
| C/T | 0 (0) | |||
| DPYD Variant Carriers 2 | C/C | 11 (25) | - | - |
| C/T | 1 (100) |
| Genotype | Grade ≥ 3 Toxicity 1 during Cycles 1 and 2 N, (%) | OR (95% CI) (Adjusted) | p-Value | |
|---|---|---|---|---|
| miR-27a rs895819 | ||||
| Total Population | A/A | 18 (18) | 1.00 (reference) | |
| A/G | 28 (32) | 2.29 (1.14 to 4.71) | 0.0219 | |
| G/G | 7 (19) | 1.05 (0.37 to 2.75) | 0.9206 | |
| DPYD Wildtype | A/A | 15 (19) | 1.00 (reference) | |
| A/G | 24 (35) | 2.30 (1.09 to 4.97) | 0.0310 | |
| G/G | 7 (23) | 1.21 (0.41 to 3.27) | 0.7209 | |
| DPYD Variant Carrier 2 | A/A | 3 15) | - | - |
| A/G | 4 (21) | |||
| G/G | 0 (0) | |||
| miR-27a rs11671784 | ||||
| Total Population | C/C | 52 (24) | 1.00 (reference) | |
| C/T | 1 (14) | 0.56 (0.03 to 3.54) | 0.6029 | |
| DPYD Wildtype | C/C | 46 (26) | - | - |
| C/T | 0 (0) | |||
| DPYD Variant Carrier 2 | C/C | 6 (14) | - | - |
| C/T | 1 (100) |
| miR-27a rs895819 | Grade ≥ 3 Adverse Events 1 during Total Treatment Period | Discontinued Treatment 3 | Death 4 | |||||
|---|---|---|---|---|---|---|---|---|
| GI | MS | Cardiac | HFS | Other 2 | ||||
| Total Population | A/A | 8 (8) | 10 (10) | 1 (1) | 2 (2) | 11 (11) | 16 (16) | 0 (0) |
| A/G | 13 (15) | 19 (22) | 3 (3) | 4 (5) | 7 (8) | 20 (23) | 1 (1) | |
| G/G | 3 (8) | 5 (14) | 1 (3) | 0 (0) | 6 (16) | 6 (16) | 0 (0) | |
| DPYD Wildtype | A/A | 7 (9) | 7 (9) | 1 (1) | 2 (3) | 10 (13) | 12 (15) | 0 (0) |
| A/G | 9 (13) | 16 (23) | 3 (3) | 2 (3) | 6 (9) | 14 (20) | 1 (1) | |
| G/G | 2 (6) | 5 (16) | 1 (3) | 0 (0) | 6 (19) | 6 (19) | 0 (0) | |
| DPYD Variant Carriers 5 | A/A | 1 (5) | 3 (15) | 0 (0) | 0 (0) | 1 (5) | 4 (20) | 0 (0) |
| A/G | 4 (21) | 3 (16) | 0 (0) | 2 (11) | 1 (5) | 6 (32) | 0 (0) | |
| G/G | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| miR-27a rs895819 | Grade ≥ 3 Adverse Events 1 during Total Treatment Period | Discontinued Treatment 3 | Death 4 | |||||
|---|---|---|---|---|---|---|---|---|
| GI | MS | Cardiac | HFS | Other 2 | ||||
| Total Population | A/A | 8 (8) | 6 (6) | 1 (1) | 1 (1) | 8 (8) | 9 (9) | 0 (0) |
| A/G | 11 (13) | 16 (18) | 2 (2) | 3 (3) | 4 (5) | 11 (13) | 0 (0) | |
| G/G | 2 (5) | 4 (11) | 1 (3) | 0 (0) | 2 (5) | 4 (11) | 0 (0) | |
| DPYD Wildtype | A/A | 7 (8) | 3 (4) | 1 (1) | 1 (1) | 7 (9) | 5 (6) | 0 (0) |
| A/G | 9 (13) | 14 (20) | 2 (3) | 2 (3) | 3 (4) | 10 (14) | 0 (0) | |
| G/G | 2 (6) | 4 (13) | 1 (3) | 0 (0) | 2 (6) | 4 (13) | 0 (0) | |
| DPYD Variant Carriers 5 | A/A | 1 (5) | 3 (15) | 0 (0) | 0 (0) | 1 (5) | 4 (20) | 0 (0) |
| A/G | 2 (11) | 2 (11) | 0 (0) | 1 (5) | 1 (5) | 1 (5) | 0 (0) | |
| G/G | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medwid, S.; Wigle, T.J.; Ross, C.; Kim, R.B. Genetic Variation in miR-27a Is Associated with Fluoropyrimidine-Associated Toxicity in Patients with Dihydropyrimidine Dehydrogenase Variants after Genotype-Guided Dose Reduction. Int. J. Mol. Sci. 2023, 24, 13284. https://doi.org/10.3390/ijms241713284
Medwid S, Wigle TJ, Ross C, Kim RB. Genetic Variation in miR-27a Is Associated with Fluoropyrimidine-Associated Toxicity in Patients with Dihydropyrimidine Dehydrogenase Variants after Genotype-Guided Dose Reduction. International Journal of Molecular Sciences. 2023; 24(17):13284. https://doi.org/10.3390/ijms241713284
Chicago/Turabian StyleMedwid, Samantha, Theodore J. Wigle, Cameron Ross, and Richard B. Kim. 2023. "Genetic Variation in miR-27a Is Associated with Fluoropyrimidine-Associated Toxicity in Patients with Dihydropyrimidine Dehydrogenase Variants after Genotype-Guided Dose Reduction" International Journal of Molecular Sciences 24, no. 17: 13284. https://doi.org/10.3390/ijms241713284
APA StyleMedwid, S., Wigle, T. J., Ross, C., & Kim, R. B. (2023). Genetic Variation in miR-27a Is Associated with Fluoropyrimidine-Associated Toxicity in Patients with Dihydropyrimidine Dehydrogenase Variants after Genotype-Guided Dose Reduction. International Journal of Molecular Sciences, 24(17), 13284. https://doi.org/10.3390/ijms241713284






