Hepatocellular Carcinoma: Past and Present Challenges and Progress in Molecular Classification and Precision Oncology
Abstract
:1. Introduction
2. Origins and Basic Developments in Precision Oncology
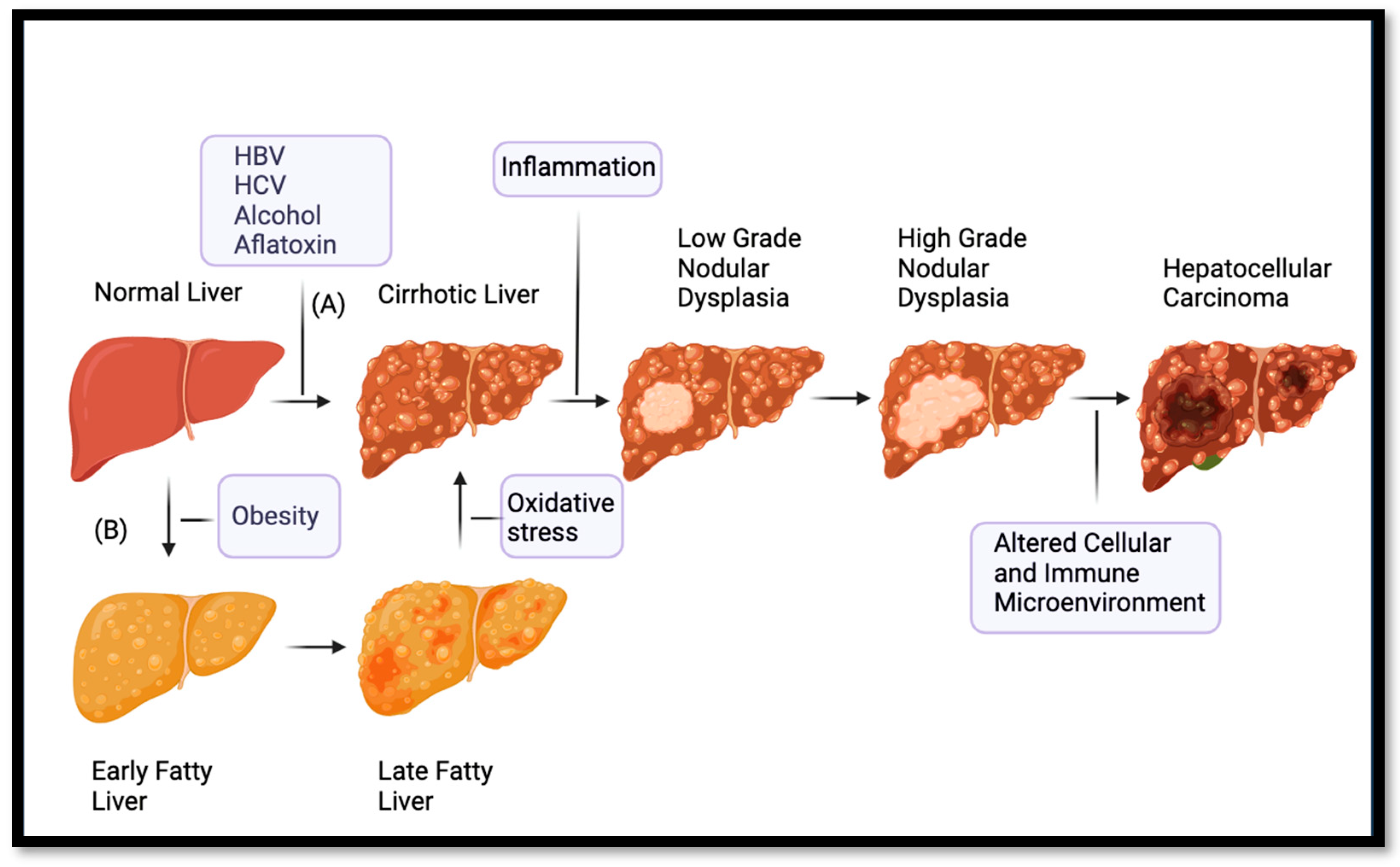
3. Pathogenesis of Hepatocellular Carcinoma
4. Molecular Subclassification of HCC
4.1. Lee Classification
4.2. Boyault Classification
4.3. Chiang Classification
4.4. Hoshida Classification
4.5. iCluster Classification
5. Early Attempts at Precision Therapy
5.1. Advances in Precision Therapy for Hepatocellular Carcinoma
5.1.1. Multi-Kinase Inhibition
5.1.2. Immune Checkpoint Inhibition
5.2. Matching Molecular Subclassification and Biomarkers with Systemic Therapy
6. Review Limitations
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sim, H.W.; Knox, J. Hepatocellular Carcinoma in the Era of Immunotherapy. Curr. Probl. Cancer 2018, 42, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, R.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study. JAMA Oncol. 2015, 3, 1683–1691. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Hartke, J.; Johnson, M.; Ghabril, M. The Diagnosis and Treatment of Hepatocellular Carcinoma. Semin. Diagn. Pathol. 2017, 34, 153–159. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2014, 10, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. J. Hepatol. 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. J. Hepatol. 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Yegin, E.G.; Oymaci, E.; Karatay, E.; Coker, A. Progress in surgical and nonsurgical approaches for hepatocellular carcinoma treatment. Hepatob. Pancreat. Dis. 2016, 15, 234–256. [Google Scholar] [CrossRef]
- Delis, S.; Dervenis, C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J. Gastroenterol. 2008, 14, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Maximin, S.; Meier, J.; Pokharel, S.; Bhargava, P. Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging Diagnosis, Response Assessment, and Treatment. Curr. Probl. Diag. Radiol. 2015, 44, 479–486. [Google Scholar] [CrossRef]
- Podlasek, A.; Abdulla, M.; Broering, D.; Bzeizi, K. Recent advances in locoregional therapy of hepatocellular carcinoma. Cancers 2023, 15, 3347. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Jiang, L.; Chen, Y. Recent Advances in Systemic Therapy for Hepatocellular Carcinoma. Biomark. Res. 2022, 10, 3. [Google Scholar] [CrossRef]
- Faivre, S.; Rimassa, L.; Finn, R.S. Molecular therapies for HCC: Looking outside the box. J. Hepatol. 2020, 72, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision Oncology: Who, How, What, When, and When Not? In American Society of Clinical Oncology Educational Book, Proceedings of the American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA, 2–6 June 2017; American Society of Clinical Oncology: Alexandria, VA, USA, 2017; Volume 37, pp. 160–169. [Google Scholar]
- Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Gathmann, I.; Kantarjian, H.; Gattermann, N.; Deininger, M.W.; Silver, R.T.; Goldman, J.M.; Stone, R.M.; et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Eng. J. Med. 2006, 355, 2408–2417. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Talpaz, M. Imatinib mesylate: Clinical results in Philadelphia chromosome-positive leukemias. Semin. Oncol. 2001, 28 (Suppl. 17), 9–18. [Google Scholar] [CrossRef]
- Prasad, V.; Fojo, T.; Brada, M. Precision Oncology: Origins, Optimism, and Potential. Lancet Oncol. 2016, 17, e81–e86. [Google Scholar] [CrossRef]
- Nadal, E.; Olavarria, E. Imainib mesylate (Gleevec/Glivec) a molecular-targeted therapy for chronic myeloid leukemia and other malignancies. Int. J. Clin. Pract. 2004, 58, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Dahlman, K.B.; Knol, J.D.; Gilbert, J.; Puzanov, I.; Means-Powell, J.A.; Balko, J.M.; Lovly, C.M.; Keedy, V.L.; Reddy, N.M.; et al. Enabling a genetically informed approach to cancer medicine: A retrospective evaluation of the impact of comprehensive tumor profiling using a targeted next-generation sequencing panel. J. Clin. Oncol. 2014, 32 (Suppl. 15), 11089. [Google Scholar] [CrossRef]
- Lassen, U.N.; Makaroff, L.E.; Stenzinger, A.; Italiano, A.; Vassal, G.; Garcia-Foncillas, J.; Avouac, B. Precision Oncology: A Clinical and Patient Perspective. Future Oncol. 2021, 17, 3995–4009. [Google Scholar] [CrossRef]
- Hyman, D.M.; Taylor, B.S.; Baselga, J. Implementing genome-driven oncology. Cell 2017, 168, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Totoki, Y.; Tatsuno, K.; Covington, K.R.; Ueda, H.; Creighton, C.J.; Kato, M.; Tsuji, S.; Donehower, L.A.; Slagle, B.L.; Nakamura, H.; et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 2014, 46, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Di Tommaso, L.; Nakano, M.; Destro, A.; Torzilli, G.; Donadon, M.; Maggioni, M.; Bosari, S.; Bulfamante, G.; Matsuda, M.; et al. Morphophenotypic changes in human multistep hepatocarcinogenesis with translational implications. J. Hepatol. 2016, 64, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Rebouissou, S.; Nault, J. Advances in Molecular Classification and Precision Oncology in Hepatocellular Carcinoma. J. Hepatol. 2020, 72, 215–229. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef]
- Hernandez–Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006, 387, 349–360. [Google Scholar] [CrossRef]
- Hoshida, Y.; Villanueva, A.; Kobayashi, M.; Peix, J.; Chiang, D.Y.; Camargo, A.; Gupta, S.; Moore, J.; Wrobel, M.J.; Lerner, J.; et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Eng. J. Med. 2008, 359, 1995–2004. [Google Scholar] [CrossRef]
- Meador, C.B.; Micheel, C.M.; Levy, M.A.; Lovly, C.M.; Horn, L.; Warner, J.L.; Johnson, D.B.; Zhao, Z.; Anderson, I.A.; Sosman, J.A.; et al. Beyond histology: Translating tumor genotypes into clinically effective targeted therapies. Clin. Cancer Res. 2014, 20, 2264–2275. [Google Scholar] [CrossRef]
- Xu, L.; He, M.; Dai, Z.; Yu, J.; Wang, J.; Li, X.; Jiang, B.; Ke, Z.; Su, T.; Peng, Z.; et al. Genomic and Transcriptional Heterogeneity of Multifocal Hepatocellular Carcinoma. Ann. Oncol. 2019, 30, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.C.; de Lima, M.A.F.D.; de Faria, P.A.S.; Vargas, F.R. TP53 germline and somatic mutations in a patient with fibrolamellar hepatocellular carcinoma. Fam. Cancer. 2018, 17, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Malkin, D.; Li, F.P.; Strong, L.C.; Fraumeni, J.F.; Nelson, C.E.; Kim, D.H.; Kassel, J.; Gryka, M.A.; Bischoff, F.Z.; Tainsky, M.A.; et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990, 250, 1233–1238. [Google Scholar] [CrossRef]
- Pinyol, R.; Tovar, V.; Llovet, J.M. TERT promoter mutations: Gatekeeper and driver of hepatocellular carcinoma. J. Hepatol. 2014, 61, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Wurmbach, E.; Chen, Y.-B.; Khitrov, G.; Zhang, W.; Roayaie, S.; Schwartz, M.; Fiel, I.; Thung, S.; Mazzaferro, V.; Bruix, J.; et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 2007, 45, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Toffanin, S.; Lachenmayer, A.; Villanueva, A.; Minguez, B.; Llovet, J. Molecular Classification and Novel Targets in Hepatocellular Carcinoma: Recent Advancements. Semin. Liver Dis. 2010, 30, 035–051. [Google Scholar] [CrossRef] [PubMed]
- Adrian, A.; Balasundaram, M.; Carlsen, R.; Chuah, E.; Clarke, A.; Dhalla, N.; Holt, R.A.; Jones, S.J.M.; Lee, D.; Ma, Y.; et al. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S.; Letouzé, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome Sequencing of Hepatocellular carcinoma Identifies new Mutational Signatures and Potential Therapeutic Targets. Nat. Genet. 2015, 5, 505–511. [Google Scholar] [CrossRef]
- Hytiroglou, P.; Kotoula, V.; Thung, S.W.; Tsokos, M.; Fiel, M.I.; Papadimitriou, C. Telomerase Activity in Precancerous Hepatic Nodules. Cancer 1998, 82, 1831–1838. [Google Scholar] [CrossRef]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef]
- Tomoda, R.; Seto, M.; Tsumuki, H.; Iida, K.; Yamazaki, T.; Sonoda, J.; Matsumine, A.; Uchida, A. Telomerase activity and human telomerase reverse transcriptase mRNA expression are correlated with clinical aggressiveness in soft tissue tumors. Cancer 2002, 95, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Paterlini-Bréchot, P.; Saigo, K.; Murakami, Y.; Chami, M.; Gozuacik, D.; Mugnier, C.; Lagorce, D.; Bréchot, C. Hepatitis B virus-related insertional mutagenesis occurs frequently in human liver cancers and recurrently targets human telomerase gene. Oncogene 2003, 22, 3911–3916. [Google Scholar] [CrossRef] [PubMed]
- Neuveut, C.; Wei, Y.; Buendia, M.A. Mechanisms of HBV-related hepatocarcinogenesis. J. Hepatol. 2010, 52, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.-F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Lee, J.S.; Chu, I.S.; Heo, J.; Calvisi, D.F.; Sun, Z.; Roskams, T.; Durnez, A.; Demetris, A.J.; Thorgeirsson, S.S. Classification and Prediction of Survival in Hepatocellular Carcinoma by Gene Expression Profiling. Hepatology 2004, 40, 667–676. [Google Scholar] [CrossRef]
- Lee, J.-S.; Heo, J.; Libbrecht, L.; Chu, I.-S.; Kaposi-Novak, P.; Calvisi, D.F.; Mikaelyan, A.; Roberts, L.R.; Demetris, A.J.; Sun, Z.; et al. A Novel Prognostic Subtype of Human Hepatocellular Carcinoma Derived from Hepatic Progenitor Cells. Nat. Med. 2006, 12, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Boyault, S.; Rickman, D.S.; De Reyniès, A.; Balabaud, C.; Rebouissou, S.; Jeannot, E.; Hérault, A.; Saric, J.; Belghiti, J.; Franco, D.; et al. Transcriptome Classification of HCC Is Related to Gene Alterations and to New Therapeutic Targets. Hepatology 2007, 45, 42–52. [Google Scholar] [CrossRef]
- Chiang, D.Y.; Villanueva, A.; Hoshida, Y.; Peix, J.; Newell, P.; Minguez, B.; LeBlanc, A.C.; Donovan, D.J.; Thung, S.N.; Solé, M.; et al. Focal Gains of VEGFA and Molecular Classification of Hepatocellular Carcinoma. Cancer Res. 2008, 68, 6779–6788. [Google Scholar] [CrossRef]
- Hoshida, Y.; Nijman, S.M.B.; Kobayashi, M.; Chan, J.A.; Brunet, J.-P.; Chiang, D.Y.; Villanueva, A.; Newell, P.; Ikeda, K.; Hashimoto, M.; et al. Integrative Transcriptome Analysis Reveals Common Molecular Subclasses of Human Hepatocellular Carcinoma. Cancer Res. 2009, 69, 7385–7392. [Google Scholar] [CrossRef]
- Nault, J.; Galle, P.R.; Marquardt, J.U. The role of molecular enrichment on future therapies in hepatocellular carcinoma. J. Hepatol. 2018, 69, 237–247. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Alsinet, C.; Savic, R.; Cabellos, L.; Toffanin, S.; Hoshida, Y.; Villanueva, A.; Minguez, B.; Newell, P.; Tsai, H.-W.; et al. Wnt-Pathway Activation in Two Molecular Classes of Hepatocellular Carcinoma and Experimental Modulation by Sorafenib. Clin. Cancer Res. 2012, 18, 4997–5007. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.; Llovet, J.M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Ancukiewicz, M.; Supko, J.G.; Sahani, D.V.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Abrams, T.A.; McCleary, N.J.; Bhargava, P.; Muzikansky, A.; et al. Efficacy, Safety, Pharmacokinetics, and Biomarkers of Cediranib Monotherapy in Advanced Hepatocellular Carcinoma: A Phase II Study. Clin. Cancer Res. 2013, 19, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.T.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.-H.; Heo, J.; Xu, J.; et al. Brivanib Versus Sorafenib As First-Line Therapy in Patients With Unresectable, Advanced Hepatocellular Carcinoma: Results From the Randomized Phase III BRISK-FL Study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Decaens, T.; Raoul, J.-L.; Boucher, E.; Kudo, M.; Chang, C.; Kang, Y.-K.; Assenat, E.; Lim, H.-Y.; Boige, V.; et al. Brivanib in Patients With Advanced Hepatocellular Carcinoma Who Were Intolerant to Sorafenib or for Whom Sorafenib Failed: Results From the Randomized Phase III BRISK-PS Study. J. Clin. Oncol. 2018, 31, 3509–3516. [Google Scholar] [CrossRef]
- Tang, B.; Zhu, J.; Zhao, Z.; Lu, C.; Liu, S.; Fang, S.; Zheng, L.; Zhang, N.; Chen, M.; Xu, M.; et al. Diagnosis and Prognosis Models for Hepatocellular Carcinoma Patient’s Management Based on Tumor Mutation Burden. J. Adv. Res. 2021, 33, 153–165. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.-K.; Lim, H.Y.; Poon, R.T.P.; Blanc, J.-F.; Vogel, A.; Chen, C.-L.; et al. Effect of Everolimus on Survival in Advanced Hepatocellular Carcinoma After Failure of Sorafenib: The EVOLVE-1 Randomized Clinical Trial. JAMA 2014, 312, 57. [Google Scholar] [CrossRef]
- Lim, H.Y.; Heo, J.; Choi, H.J.; Lin, C.-Y.; Yoon, J.-H.; Hsu, C.; Rau, K.-M.; Poon, R.T.; Yeo, W.; Park, J.-W.; et al. A Phase II Study of the Efficacy and Safety of the Combination Therapy of the MEK Inhibitor Refametinib (BAY 86-9766) Plus Sorafenib for Asian Patients with Unresectable Hepatocellular Carcinoma. Clin. Cancer Res. 2014, 20, 5976–5985. [Google Scholar] [CrossRef]
- Lim, H.Y.; Heo, J.; Choi, H.J.; Lin, C.-Y.; Yoon, J.-H.; Hsu, C.; Rau, K.-M.; Poon, R.T.; Yeo, W.; Park, J.-W.; et al. Phase II Studies with Refametinib or Refametinib plus Sorafenib in Patients with RAS-Mutated Hepatocellular Carcinoma. Clin. Cancer Res. 2018, 24, 4650–4661. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Cheng, A.-L.; Vogel, A.; Tovoli, F.; Ueshima, K.; Aikata, H.; et al. Overall Survival and Objective Response in Advanced Unresectable Hepatocellular Carcinoma: A Subanalysis of the REFLECT Study. J. Hepatol. 2023, 78, 133–141. [Google Scholar] [CrossRef]
- Briggs, A.; Daniele, B.; Dick, K.; Evans, T.R.J.; Galle, P.R.; Hubner, R.A.; Lopez, C.; Siebert, U.; Tremblay, G. Covariate-Adjusted Analysis of the Phase 3 REFLECT Study of Lenvatinib versus Sorafenib in the Treatment of Unresectable Hepatocellular Carcinoma. Br. J. Cancer 2020, 122, 1754–1759. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ryoo, B.-Y.; Merle, P.; Park, J.-W.; Bolondi, L.; Chan, S.L.; Lim, H.Y.; Baron, A.D.; Parnis, F.; Knox, J.; et al. Second-Line Cabozantinib after Sorafenib Treatment for Advanced Hepatocellular Carcinoma: A Subgroup Analysis of the Phase 3 CELESTIAL Trial. ESMO Open 2020, 5, e000714. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus Atezolizumab versus Sorafenib for Advanced Hepatocellular Carcinoma (COSMIC-312): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Eng. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.-Y.; Yen, C.-J.; Poon, R.; Pastorelli, D.; Blanc, J.-F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.F.; et al. Ramucirumab versus Placebo as Second-Line Treatment in Patients with Advanced Hepatocellular Carcinoma Following First-Line Therapy with Sorafenib (REACH): A Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Zhu, A.X.; Nipp, R.D.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Blanc, J.-F.; Okusaka, T.; Chau, I.; Cella, D.; Girvan, A.; et al. Ramucirumab in the Second-Line for Patients with Hepatocellular Carcinoma and Elevated Alpha-Fetoprotein: Patient-Reported Outcomes across Two Randomised Clinical Trials. ESMO Open 2020, 5, e000797. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in Patients with Advanced Hepatocellular Carcinoma (CheckMate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib (KEYNOTE-224): A Non-Randomised, Open-Label Phase 2 Trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Eng. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving Therapeutic Landscape of Advanced Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervas-Stubss, S.; Melero, I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Smyth, M.J.; Ngiow, S.F.; Ribas, A.; Teng, M.W.L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016, 13, 143–158. [Google Scholar] [CrossRef]
- Llovet, J.M.; Hernandez-Gea, V. Hepatocellular carcinoma: Reasons for phase III failure and novel perspectives on trial design. Clin. Cancer Res. 2014, 20, 2072–2079. [Google Scholar] [CrossRef]
- Galle, P.R.; Dufour, J.; Peck-Radosavljevic, M.; Trojan, J.; Vogel, A. Systemic Therapy of Advanced Hepatocellular Carcinoma. Future Oncol. 2021, 17, 1237–1251. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pena, C.E.A.; Lathia, C.D.; Shan, M.; Meinhardt, G.; Bruix, J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2012, 18, 2290–2300. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Llovet, J.M. Translating ‘–Omics’ Results into Precision Medicine for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular Therapies and Precision Medicine for Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
| Classification System | Subclass(es) | |
|---|---|---|
| Major Subclasses | Proliferative | Nonproliferative |
| Lee 2004 | Cluster A | Cluster B |
| Boyault 2006 | G1, G2, G3 | G4, G5, G6 |
| Chiang 2008 | Proliferation | CTNNB1, Polysomy 7, Interferon |
| Hoshida 2009 | S1, S2 | S3 |
| TCGARN 2017 | iCluster1, iCluster2 | iCluster3 |
| Clinical Features | Poor survival, high vascular invasion, women, Asian, high AFP, HBV, normal body weight, poorly differentiated hepatocyte | Improved survival, low vascular invasion, alcohol, HCV, lower AFP, well-differentiated hepatocyte, smaller tumor size |
| Mutations | CDK4, CNNB1, CKS2, PTMA/ProT, SET, MAPK3, CCNB1, CCNA2, AXIN1, PIK3CA, TP53, AKT, MYC, TGF-β, TERT, MYBL2 PLK1, MK167 | CTNNB1, CDH1, TCF1 CDKN2A silencing by hypermethylation; Relatively high TERT promoter mutation TP53 |
| Therapy | Line of Treatment | Target (s) | Trial Name (National Clinical Trial Number) | Primary Endpoint (s) | Year |
|---|---|---|---|---|---|
| Sorafenib | First | VEGFA, KDR/VEGFR-2, FLT4/VEGFR-3 | SHARP (NCT00105443) | Overall Survival Time to Symptomatic Progression | 2007 |
| Regorafenib | Second | VEGFR1-3, TIE2, PDGFR-β, GFGR1, BRAF, RET, KIT | RESOURCE (NCT01774344) | Overall Survival | 2017 |
| Nivolumab | Second | PD1 | CheckMate 040 (NCT01658878) | Safety Tolerability Overall Response Rate | 2017 |
| Lenvatinib | First | VEGFR1-3, FGFR1-4, PDFR-α, RET, KIT | REFLECT (NCT01761266) | Overall Survival | 2018 |
| Pembrolizumab | Second | PD1 | KEYNOTE 224 (NCT02702414) KEYNOTE 240 (NCT02702401) | Objective Response Rate Overall Survival Progression Free Survival | 2018 |
| Cabozantinib | Second | AXL, FLT-3, KIT, MER, MET, RET, ROS1, TIE-2, TRKB, TYRO3, VEGFR1-4 | CELESTIAL (NCT01908426) | Overall Survival | 2019 |
| Ramucirumab | Second | VEGR2 | REACH (NCT01140347) | Overall Survival | 2019 |
| Atezolizumab–Bevacizumab | First | PD1-VEGF | IMbrave 150 (NCT03434379) | Overall Survival Progression Free Survival | 2020 |
| Nivolumab–Ipilimumab | Second | PD1-CTLA | CheckMate 040 (NCT01658878) | Safety Tolerability Overall Response Rate | 2020 |
| Darvalumab–Tremilimuab | First | PDL1-CTLA4 | HIMALAYA (NCT03298451) | Overall Survival | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coffin, P.; He, A. Hepatocellular Carcinoma: Past and Present Challenges and Progress in Molecular Classification and Precision Oncology. Int. J. Mol. Sci. 2023, 24, 13274. https://doi.org/10.3390/ijms241713274
Coffin P, He A. Hepatocellular Carcinoma: Past and Present Challenges and Progress in Molecular Classification and Precision Oncology. International Journal of Molecular Sciences. 2023; 24(17):13274. https://doi.org/10.3390/ijms241713274
Chicago/Turabian StyleCoffin, Philip, and Aiwu He. 2023. "Hepatocellular Carcinoma: Past and Present Challenges and Progress in Molecular Classification and Precision Oncology" International Journal of Molecular Sciences 24, no. 17: 13274. https://doi.org/10.3390/ijms241713274
APA StyleCoffin, P., & He, A. (2023). Hepatocellular Carcinoma: Past and Present Challenges and Progress in Molecular Classification and Precision Oncology. International Journal of Molecular Sciences, 24(17), 13274. https://doi.org/10.3390/ijms241713274





