Resveratrol and 2-Ethyl-6-Methyl-3-Hydroxypiridine N-Acetyl Cysteinate as Protecting Agents upon the Stress Exposure
Abstract
:1. Introduction
2. Results
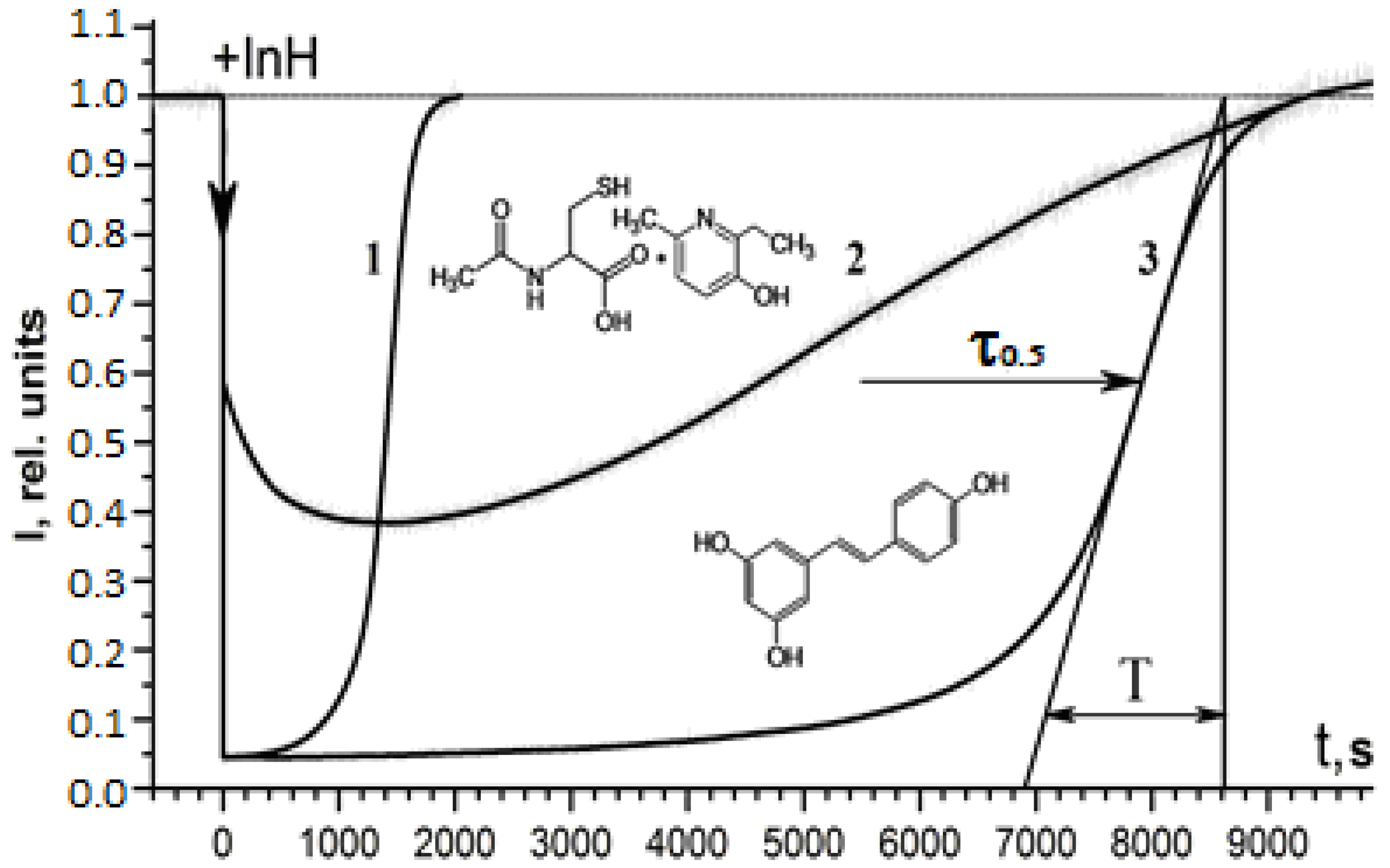
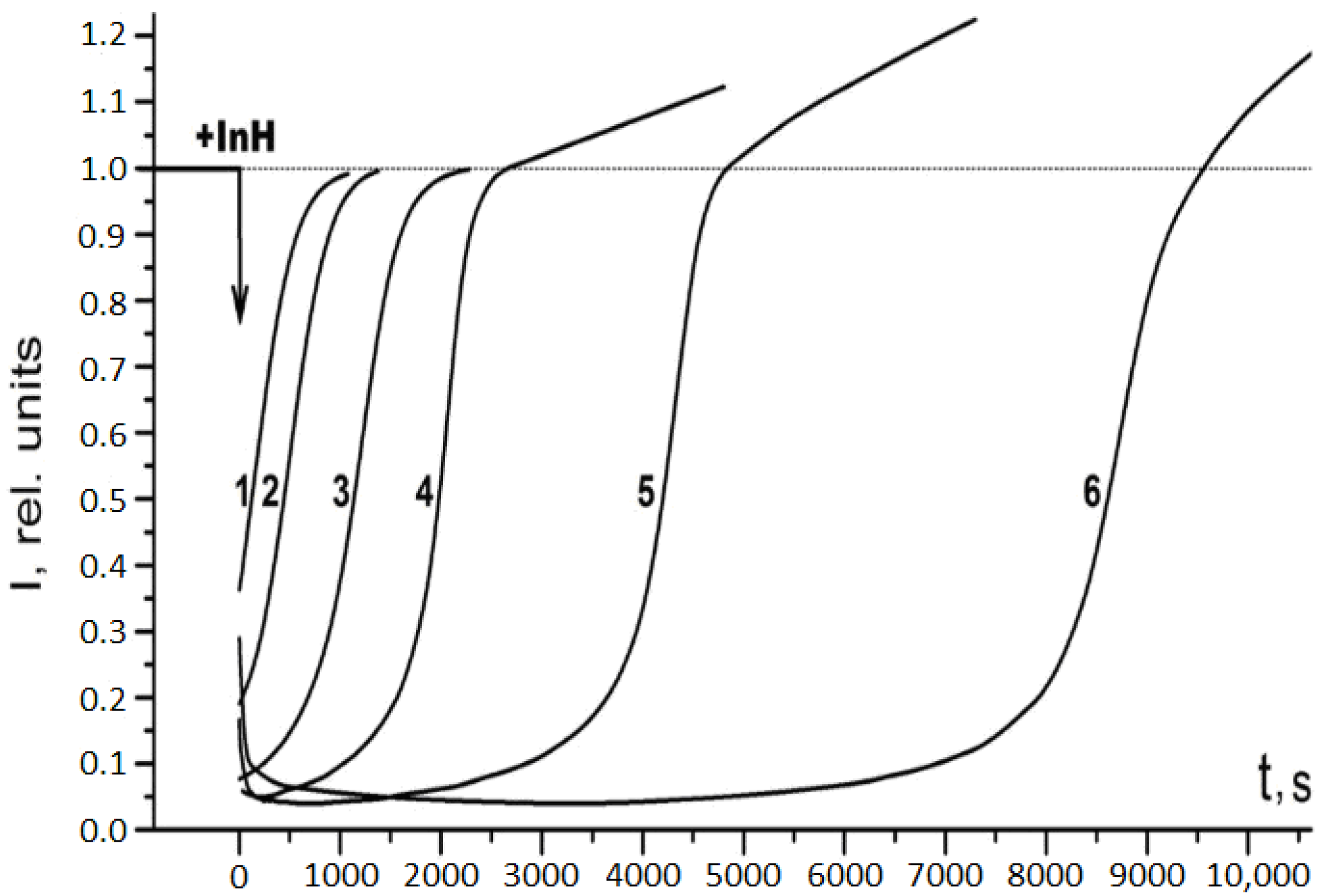
2.1. Antiradical Activity of RSV and NAC-3-HP
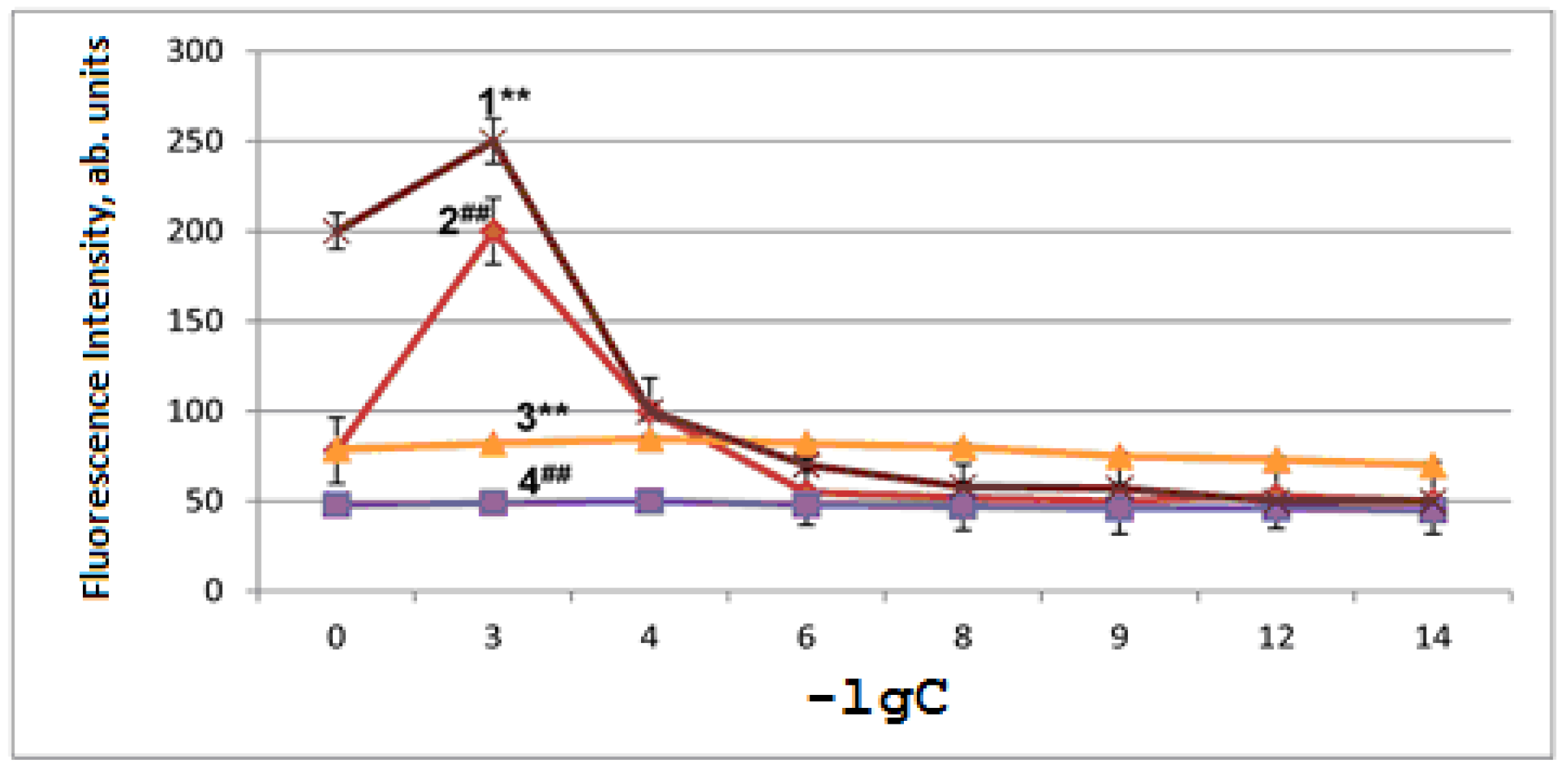
2.2. Antioxidant and Pro-Oxidant Activities of RSV and NAC-3-HP in Aging Mitochondria
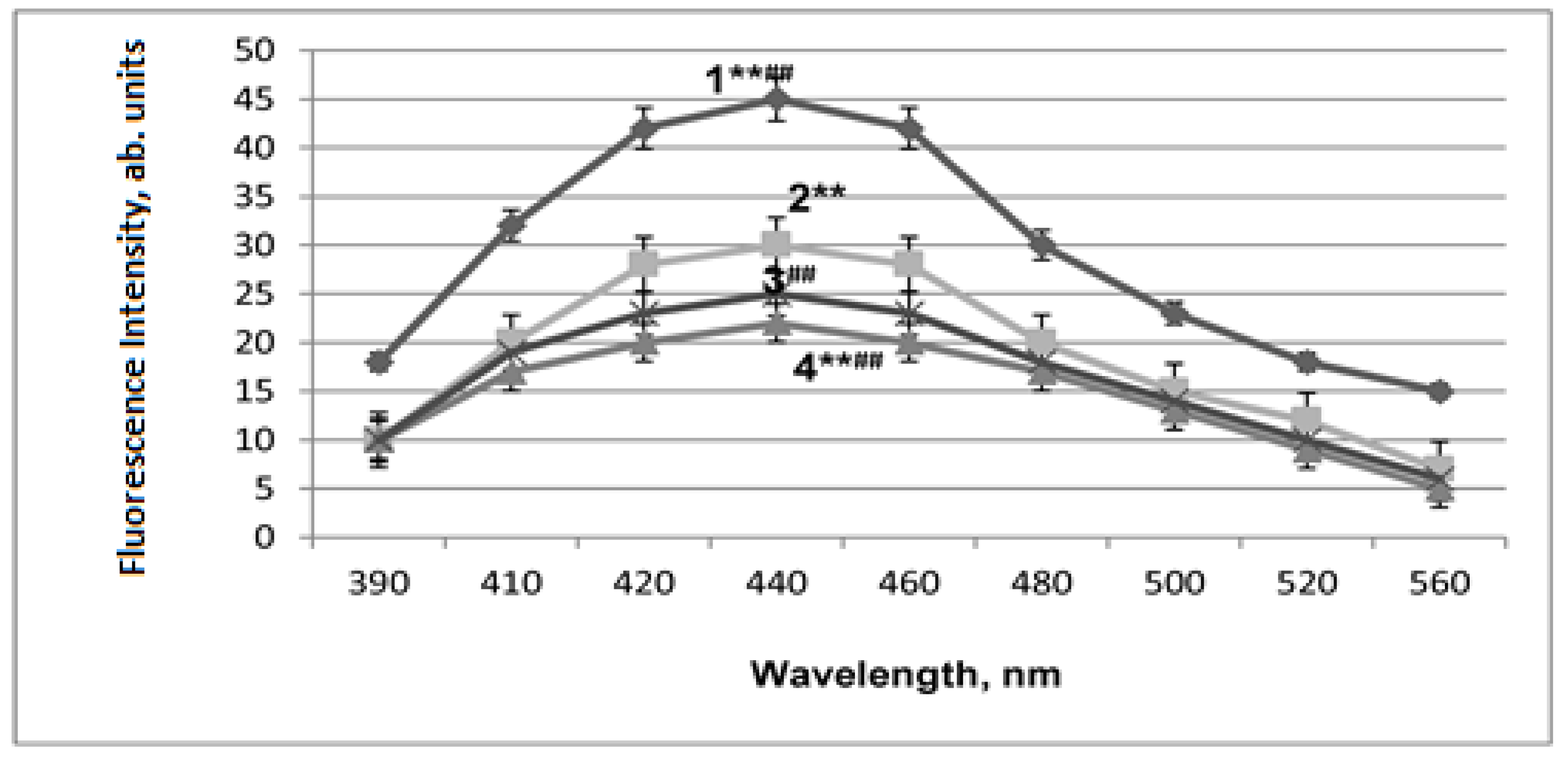
2.3. LPO Activation in Mitochondrial Membranes under Conditions of Acute Hypobaric Hypoxia and Alcohol Poisoning
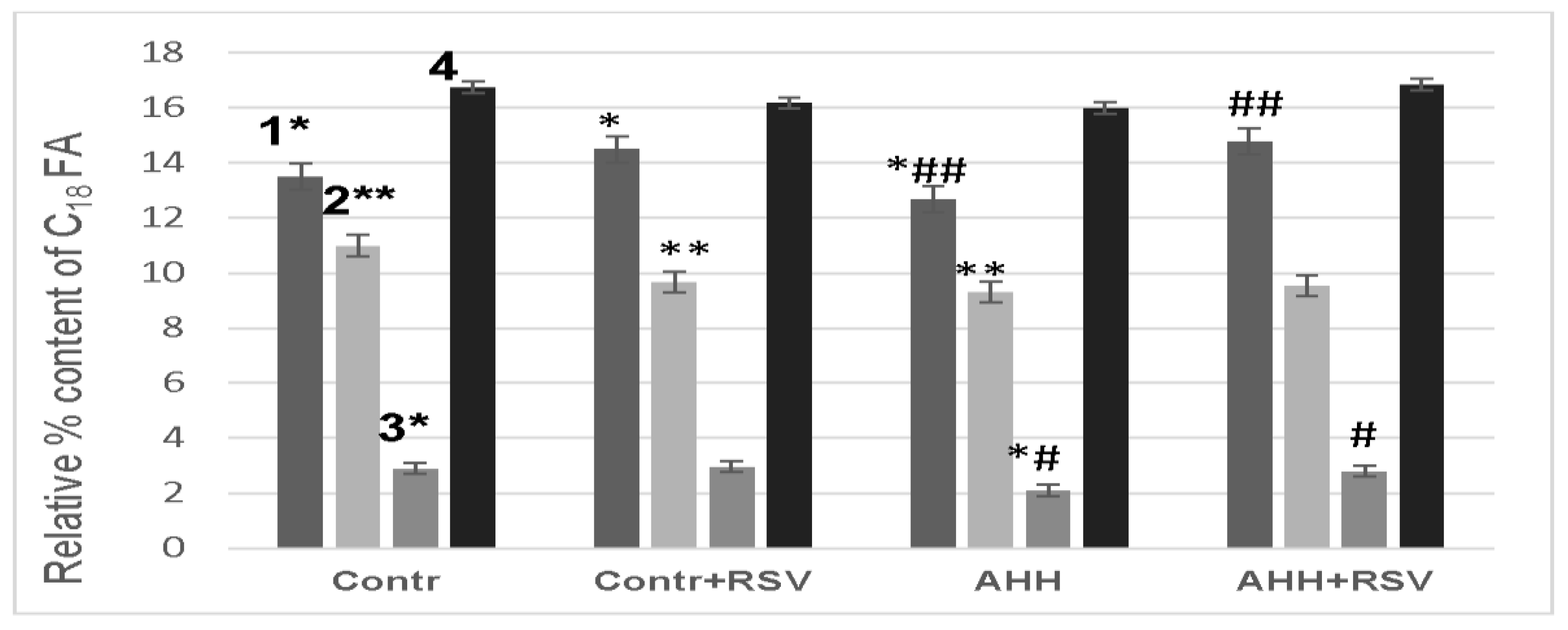
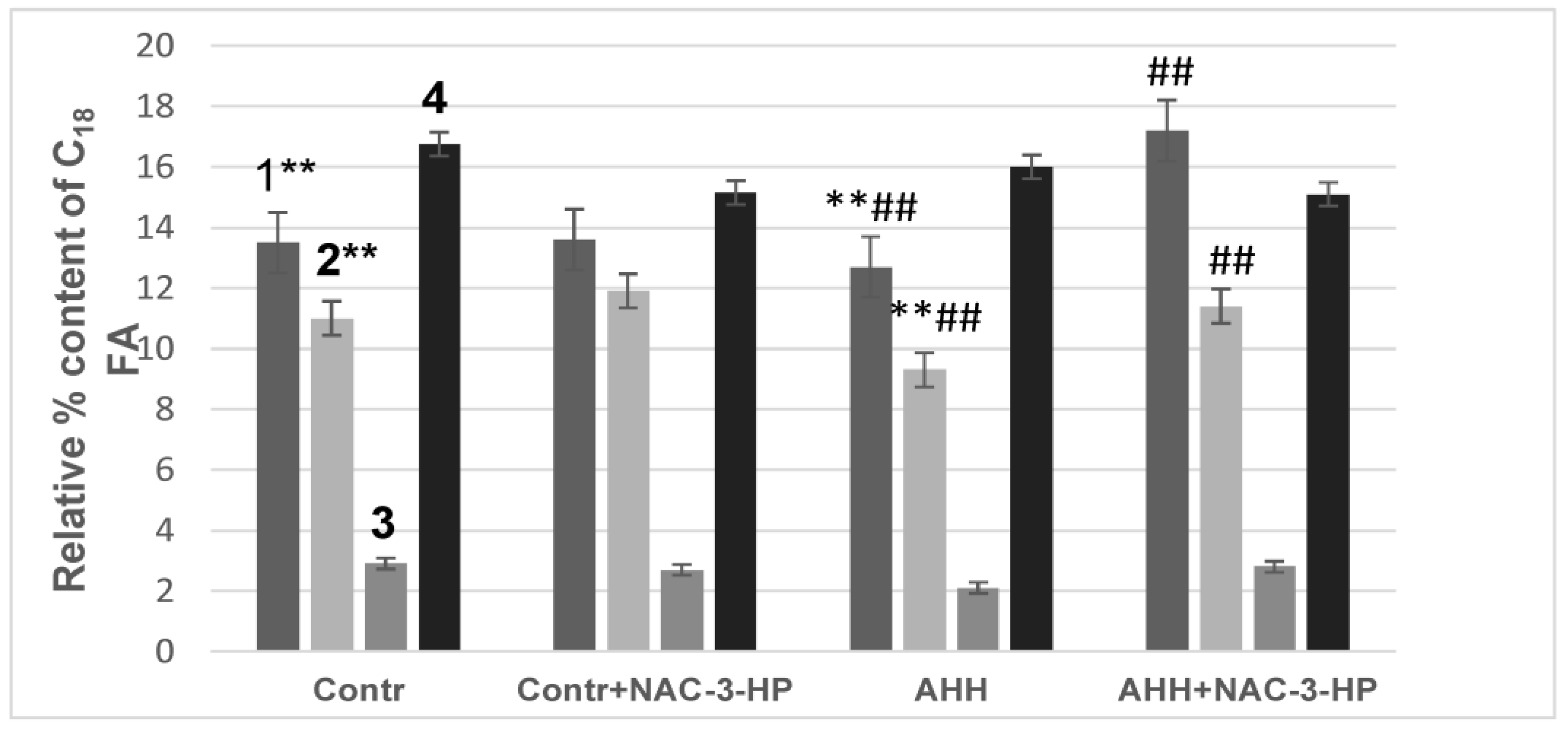
2.4. Fatty Acid Composition of Mitochondrial Membranes under Conditions of Acute Hypobaric Hypoxia
2.5. Anti-Stress Properties of RSV and NAC-3-HP
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemiluminescence Measurements
4.3. Fluorescence Measurements
4.4. Experiments with Animals and Biomaterials
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant therapy: Current status and future prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef]
- Saso, L.; Suzen, S.; Borges, F.; Csont, T. Chemistry and pharmacology of modulators of oxidative stress. Curr. Med. Chem. 2020, 27, 2038–2039. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.D.; Ferreira Freire, V.A.; Diogo, Í.L.; de Lima Santos, H.; Barbosa, L.A.; de Carvalho, L.E.D. Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: Role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants 2023, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic systems in stress: Structural and molecular genetic approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Shevtsova, E.; Kireeva, E.G.; Oxenkrug, G.F.; Sablin, S.O. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann. N. Y. Acad. Sci. 2003, 99, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.D.; Ramanathan, K.B.; McGee, J.E.; Newman, K.P.; Weber, K.T. Oxidative stress and cardiomyocyte necrosis with elevated serum troponins: Pathophysiologic mechanisms. Am. J. Med. Sci. 2011, 342, 129–134. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Lenaz, G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv. Exp. Med. Biol. 2012, 942, 93–136. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.-F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghaza, N.; Pham, T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Forte, M.; Damato, A.; Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.; Bartolo, M.; Maciag, A.; et al. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.Z.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway y to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta 2016, 1860, 727–745. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Novikov, V.E.; Kovaleva, L.A.; Losenkova, S.O.; Klimkina, E.I. Pharmacology of antioxidants based on 3-oxypyridine. Vestn. Smolensk. Gos. Med. Acad. 2004, 3, 69–77. (In Russian) [Google Scholar]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Fedorova, G.F.; Menshov, V.A.; Trofimov, A.V.; Vasil’ev, R.F. Facile chemiluminescence assay for antioxidative properties of vegetable lipids: Fundamentals and illustrative examples. Analyst 2009, 134, 2128–2134. [Google Scholar] [CrossRef]
- Belyakov, V.A.; Vasil’ev, R.F.; Fedorova, G.F. Kinetics of oxy-chemiluminescence and its use in the analysis of antioxidants. Kinet. Catal. 2004, 45, 329–336. [Google Scholar] [CrossRef]
- Rusina, I.F.; Veprintsev, T.L.; Vasil’ev, R.F. Antioxidant activity of diatomic phenols. Russ. J. Phys. Chem. B 2022, 16, 50–57. [Google Scholar] [CrossRef]
- Rusina, I.F.; Karpukhin, O.N.; Kasaikina, O.T. Chemiluminescent methods for studying inhibited oxidation. Russ. J. Phys. Chem. B 2013, 7, 463–477. [Google Scholar] [CrossRef]
- Vasil’ev, R.F.; Veprintsev, T.L.; Dolmatova, L.S.; Naumov, V.V.; Trofimov, A.V.; Tsaplev, Y.B. Kinetics of ethylbenzene oxy-chemiluminescence in the presence of antioxidants from tissues of the marine invertebrate Eupentacta fraudatrix: Estimating the concentration and reactivity of the natural antioxidants. Kinet. Catal. 2014, 55, 148–153. [Google Scholar] [CrossRef]
- Bastos, E.L.; Romoff, P.; Eckert, C.R.; Baader, W.J. Evaluation of antiradical capacity by H2O2-hemin-induced luminol chemiluminescence. J. Agric. Food Chem. 2003, 51, 7481–7488. [Google Scholar] [CrossRef]
- Zhigacheva, I.V.; Rusina, I.F.; Generozova, I.P.; Veprintsev, T.L.; Kuznetsov, Y.V. Antiradical and anti-stress properties of N-acetylcysteinate 2-ethyl-6-methyl-3-hydroxypyridine. Russ. J. Phys. Chem. B 2018, 12, 1055–1060. [Google Scholar] [CrossRef]
- Lukyanova, L.; Germanova, E.; Khmil, N.; Pavlik, L.; Mikheeva, I.; Shigaeva, M.; Mironova, G. Signaling role of mitochondrial enzymes and ultrastructure in the formation of molecular mechanisms of adaptation to hypoxia. Int. J. Mol. Sci. 2021, 22, 8636. [Google Scholar] [CrossRef]
- Efremenko, E.S. The role of NADPH (nicotinamide adenine dinucleotide phosphate reduced)-oxidase in the formation of alcohol-induced oxidative stress. Sci. Rev. Med. Sci. 2020, 2, 10–15. (In Russian) [Google Scholar] [CrossRef]
- Dani, C.; Bonatto, D.; Salvador, M.; Pereira, M.D.; Henriques, J.A.; Eleutherio, E. Antioxidant protection of resveratrol and catechin in Saccharomyces cerevisiae. J. Agric. Food Chem. 2008, 56, 4268–4272. [Google Scholar] [CrossRef]
- Gu, T.; Wang, N.; Wu, T.; Ge, Q.; Chen, L. Antioxidative stress mechanisms behind resveratrol: A multidimensional analysis. J. Food Qual. 2021, 2021, 5571733. [Google Scholar] [CrossRef]
- Danilenko, L.M.; Skachilova, S.Y.; Nadezhdin, S.V.; Timokhina, A.S.; Shcheblykina, O.V.; Kotelnikova, A.S. Pharmacological screening of substances with cardioprotective effect in the group of 3-oxypyridine derivatives. Res. Results Pharmacol. 2018, 4, 125–131. [Google Scholar] [CrossRef]
- Yasnetsov, V.V.; Karsanov, S.K.; Yasnetsov, V.V. Study of neuroprotective and antiamnestic effects of melatonin combinations with 3-hydroxypyridine derivatives. Aerospace Environ. Med. (Aviakosmicheskaya I Ehkologicheskaya Med.) 2017, 51, 30–34. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Rahimi, A.; Samimagham, H.R.; Azad, M.H.; Hooshyar, D.; Arabi, M.; KazemiJahromi, M. The efficacy of N-Acetylcysteine in severe COVID-19 patients: A structured summary of a study protocol for a randomized controlled trial. Trials 2021, 22, 271–273. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: Molecular and pharmacological aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef]
- Kuhn, G.; Pallauf, C.; Schulz, C.; Birringer, M.; Diaz-Rica, B.; de Pascual-Teresa, S.; Rimbach, G. Resveratrol modulates desaturase expression and fatty acid composition of cultured hepatocytes. Front. Nutr. 2018, 5, 106. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef]
- Belyakov, V.A.; Vasil’ev, R.F.; Fedorova, G.F. Chemiluminescence in liquid-phase oxidation of organic compounds. 1. Methods of determining quantitative characteristics. High Energy Chem. 1978, 12, 208–213. [Google Scholar]
- Fletcher, B.I.; Dillard, C.D.; Tappel, A.L. Measurement of fluorescent lipid peroxidation products in biological systems and tissues. Anal. Biochem. 1973, 52, 1–9. [Google Scholar] [CrossRef]
- Carreau, J.P.; Dubacq, J.P. Adaptation of macroscal method to the microscale for fatty acid methyl transesterification of biological lipid extracts. J. Chromatogr. A 1979, 151, 384–390. [Google Scholar] [CrossRef]
- Wang, Y.; Sunwoo, H.; Cherian, G.; Sim, J.S. Fatty acid determination in chicken egg yolk: A comparison of different methods. Poult. Sci. 2000, 79, 1168–1171. [Google Scholar] [CrossRef]
- Golovina, R.V.; Kuzmenko, T.E. Thermodynamic evaluation interaction of fatty acid methyl esters with polar and nonpolar stationary phases, based on their retention indices chromatographia. Chromatography 1977, 10, 545–546. [Google Scholar] [CrossRef]
| Antioxidant | 104 kinh (Ms)−1 | f |
|---|---|---|
| RSV 1 | 23.6 ± 0.04 | 2.1 ± 0.2 |
| NAC-3-HP 1 | 3.84 ± 0.04 | 0.78 ± 0.2 |
| Acetylcysteine 1 | 2.73 ± 0.04 | 0.32 ± 0.2 |
| Butylated hydroxytoluene 2 | 2.0 | 1.9 |
| Pyrocatechol 3 | 280 | 2.0 |
| Mexidol (2-ethyl-6-methyl-3-hydroxypyridine) 4 | 4.7 | 1.9 |
| CrC1 2 | 452 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhigacheva, I.V.; Rusina, I.F.; Krikunova, N.I.; Goloschapov, A.N.; Veprintsev, T.L.; Yablonskaya, O.I.; Trofimov, A.V. Resveratrol and 2-Ethyl-6-Methyl-3-Hydroxypiridine N-Acetyl Cysteinate as Protecting Agents upon the Stress Exposure. Int. J. Mol. Sci. 2023, 24, 13172. https://doi.org/10.3390/ijms241713172
Zhigacheva IV, Rusina IF, Krikunova NI, Goloschapov AN, Veprintsev TL, Yablonskaya OI, Trofimov AV. Resveratrol and 2-Ethyl-6-Methyl-3-Hydroxypiridine N-Acetyl Cysteinate as Protecting Agents upon the Stress Exposure. International Journal of Molecular Sciences. 2023; 24(17):13172. https://doi.org/10.3390/ijms241713172
Chicago/Turabian StyleZhigacheva, Irina V., Irina F. Rusina, Natalia I. Krikunova, Aleksandr N. Goloschapov, Timur L. Veprintsev, Olga I. Yablonskaya, and Aleksei V. Trofimov. 2023. "Resveratrol and 2-Ethyl-6-Methyl-3-Hydroxypiridine N-Acetyl Cysteinate as Protecting Agents upon the Stress Exposure" International Journal of Molecular Sciences 24, no. 17: 13172. https://doi.org/10.3390/ijms241713172
APA StyleZhigacheva, I. V., Rusina, I. F., Krikunova, N. I., Goloschapov, A. N., Veprintsev, T. L., Yablonskaya, O. I., & Trofimov, A. V. (2023). Resveratrol and 2-Ethyl-6-Methyl-3-Hydroxypiridine N-Acetyl Cysteinate as Protecting Agents upon the Stress Exposure. International Journal of Molecular Sciences, 24(17), 13172. https://doi.org/10.3390/ijms241713172







