Identification of Potential Gene Targets for Suppressing Oviposition in Holotrichia parallela Using Comparative Transcriptome Analysis
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Diets on Reproduction
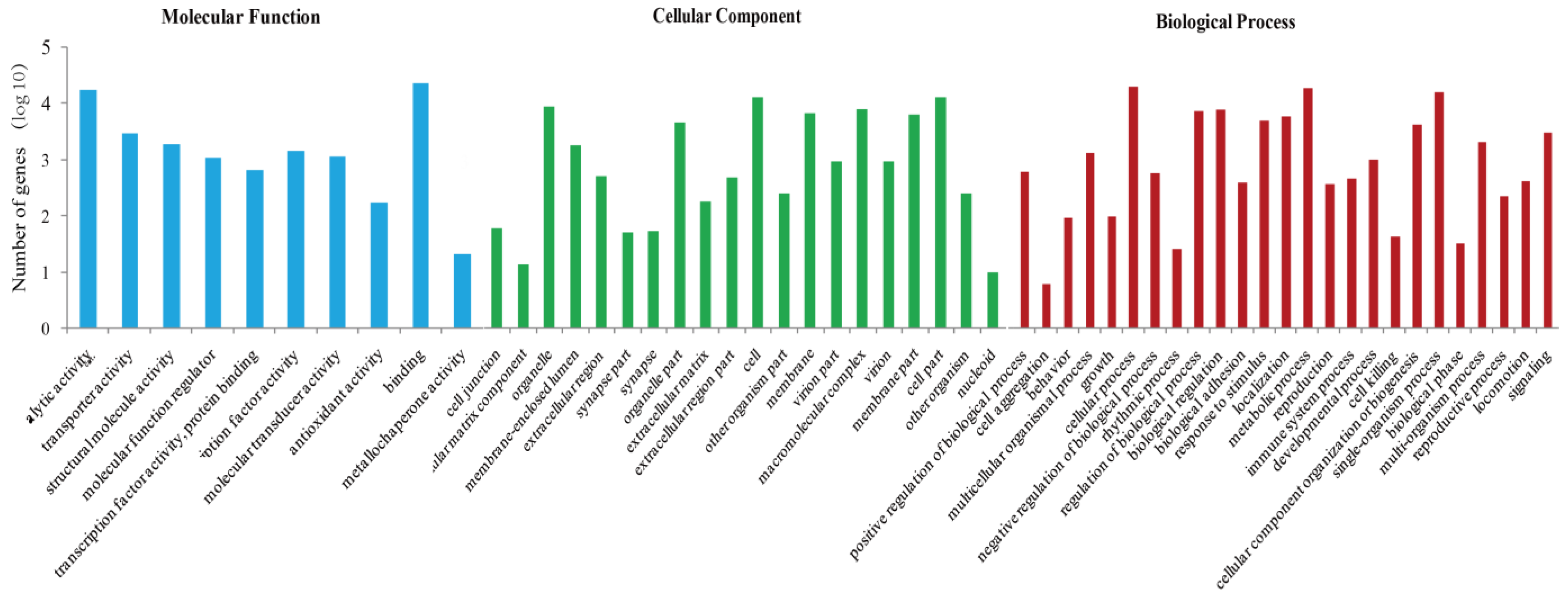
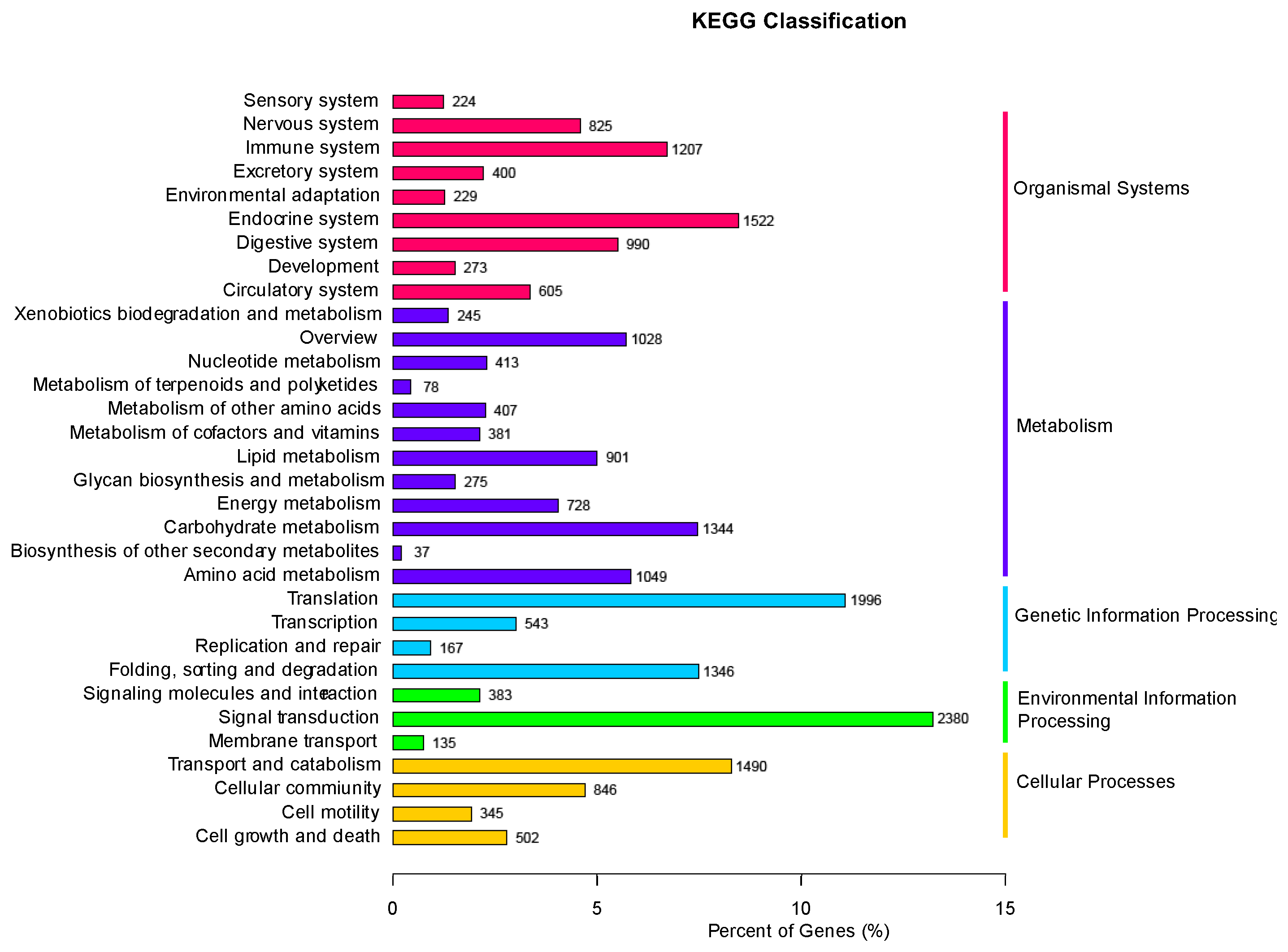
2.2. Sequencing and Analysis of Reproductive Tissues at the Transcriptome Level
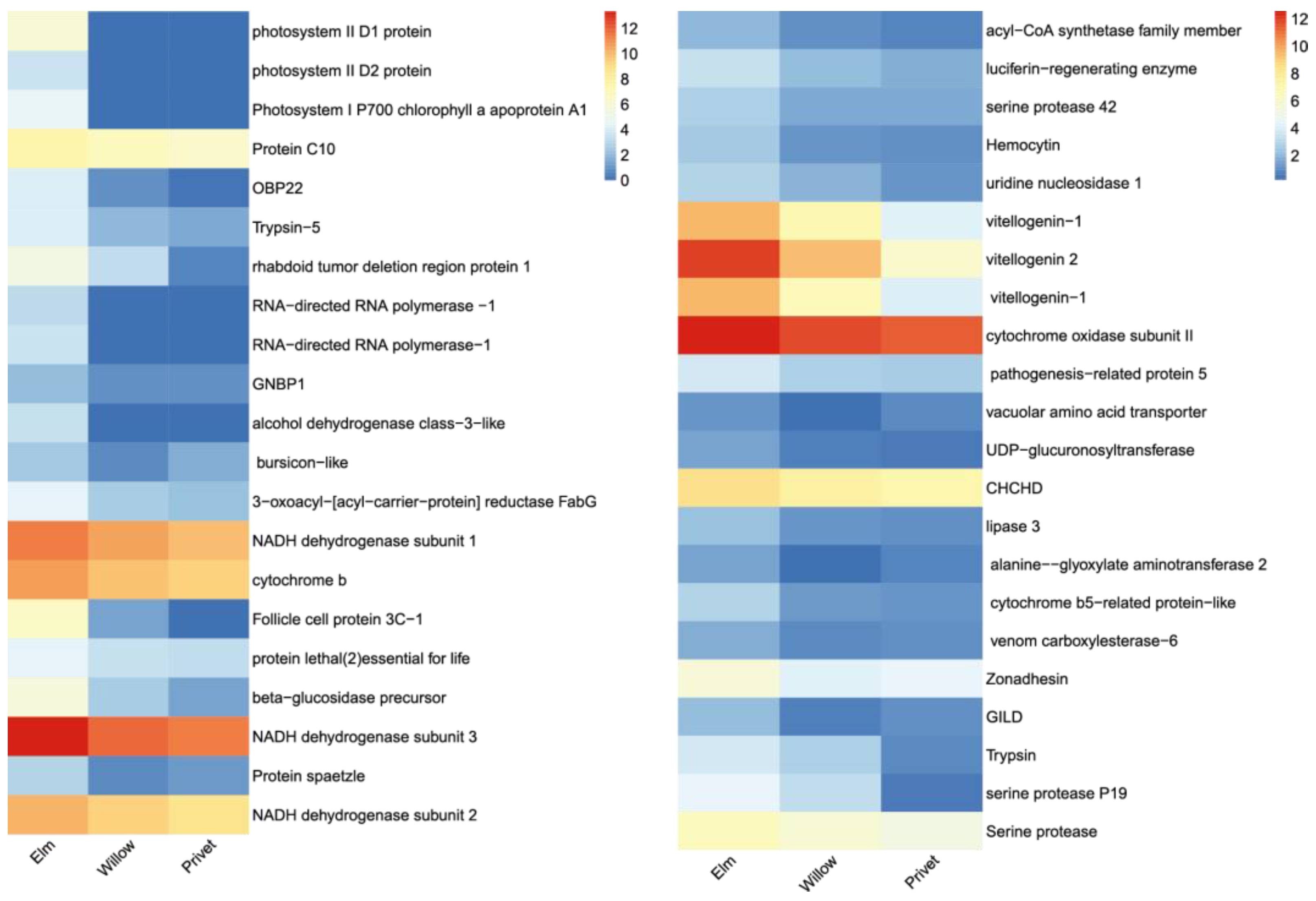
2.3. Differentially Expressed Genes in Response to Different Hosts
2.4. Oviposition-Related Genes
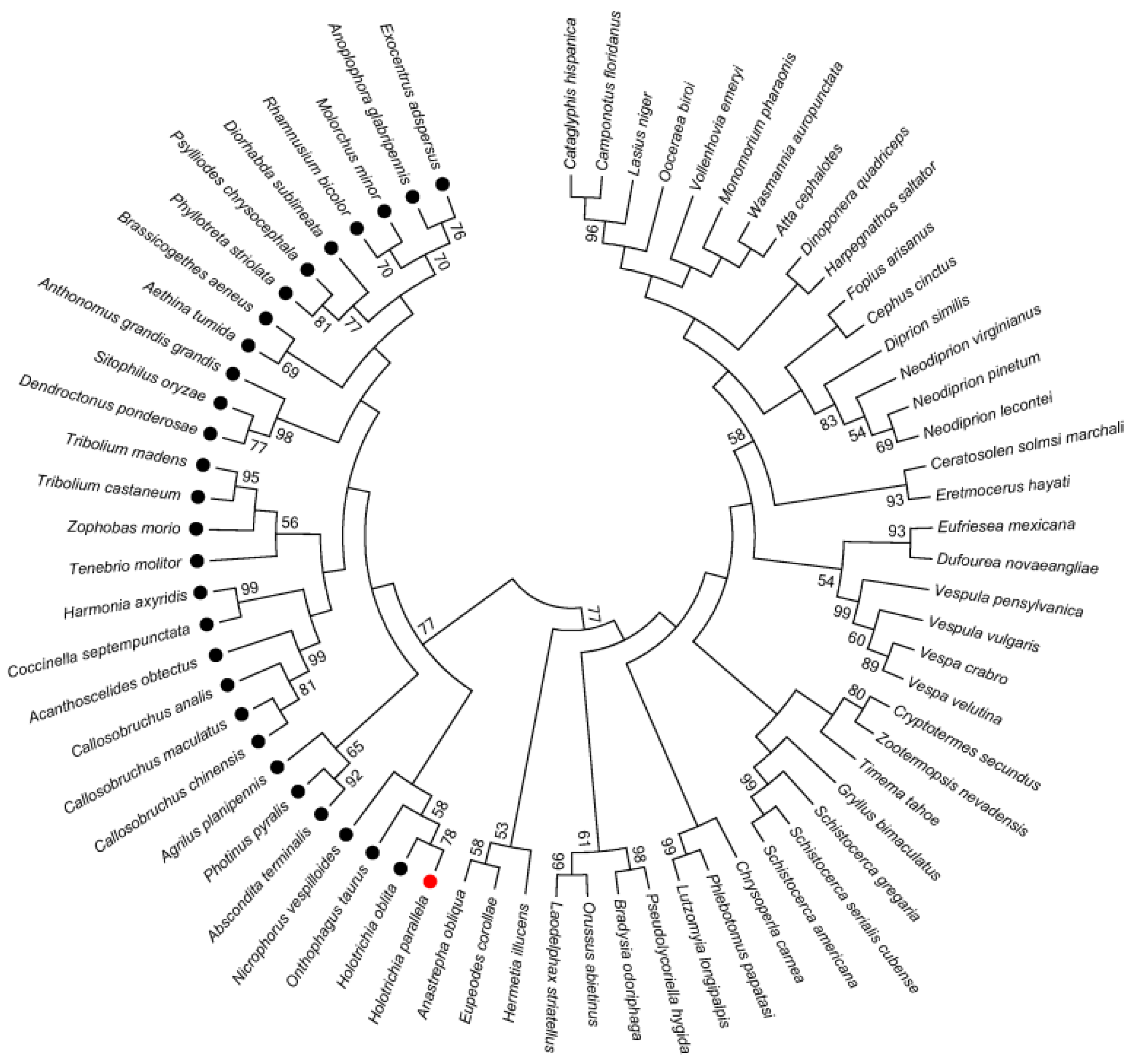
2.5. Phylogenetic Analysis
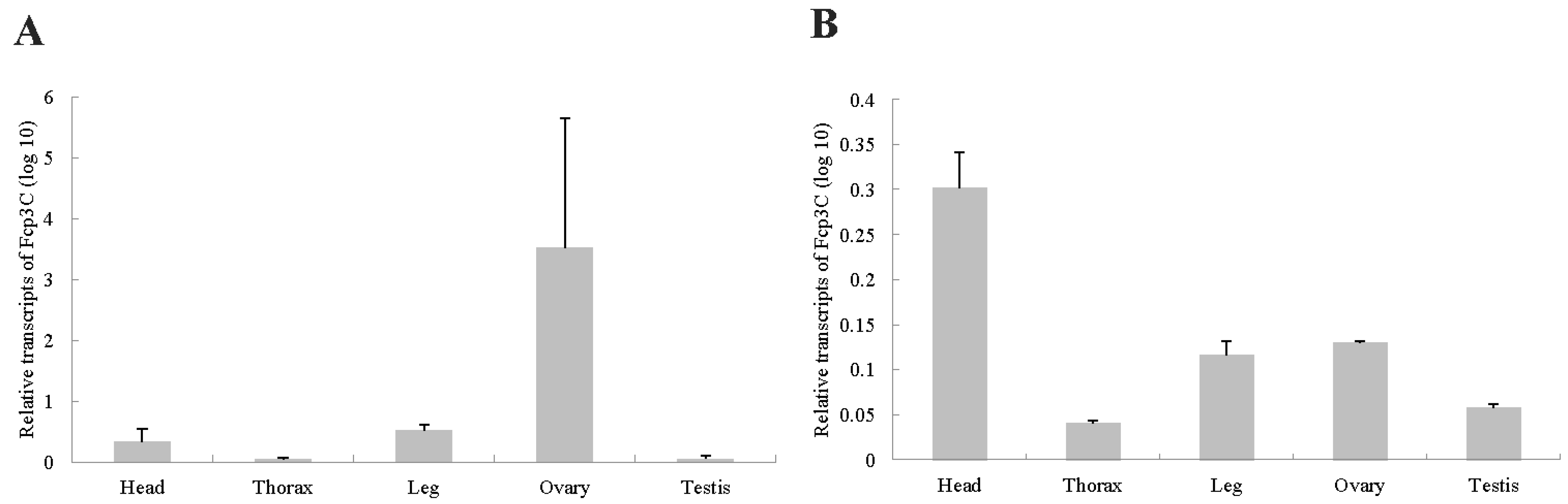
2.6. Gene Expression Analysis
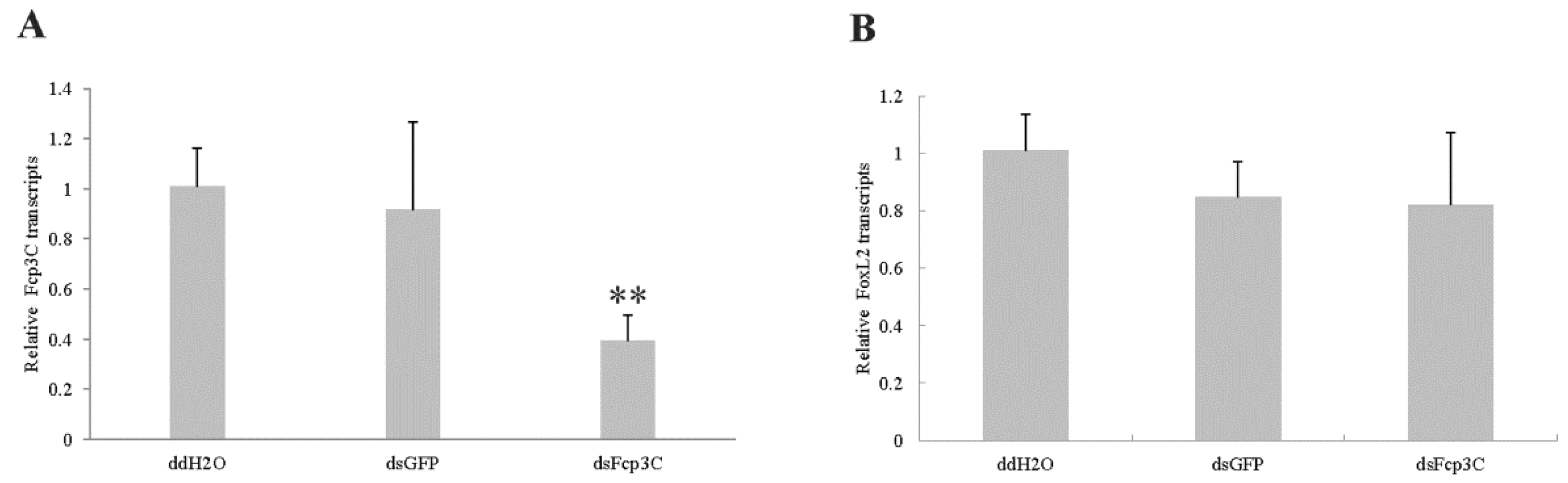
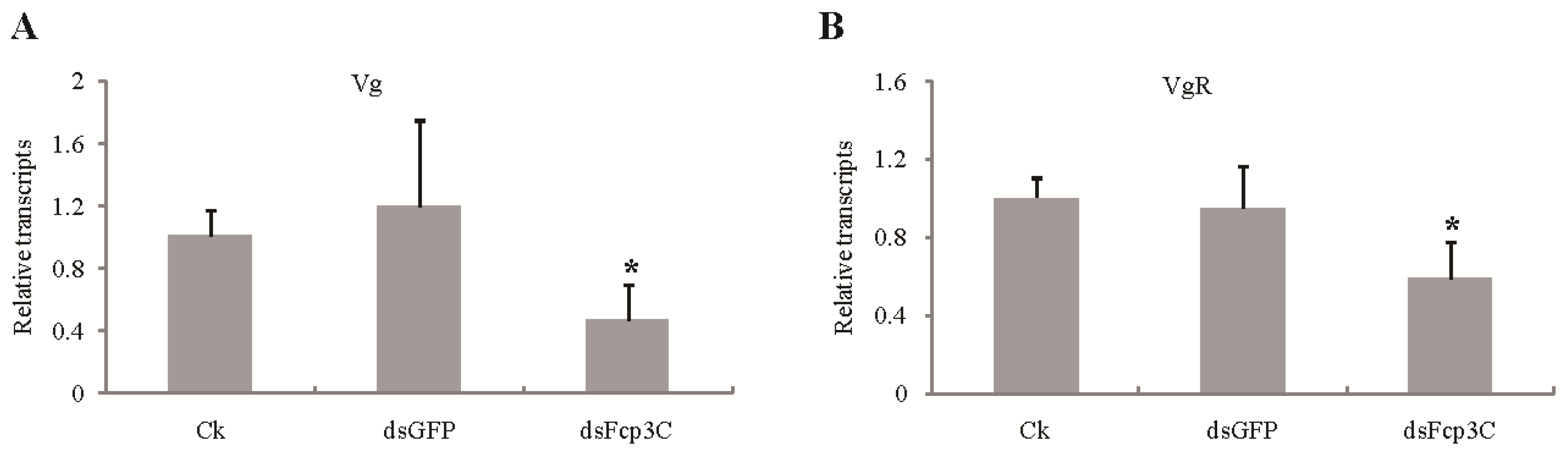
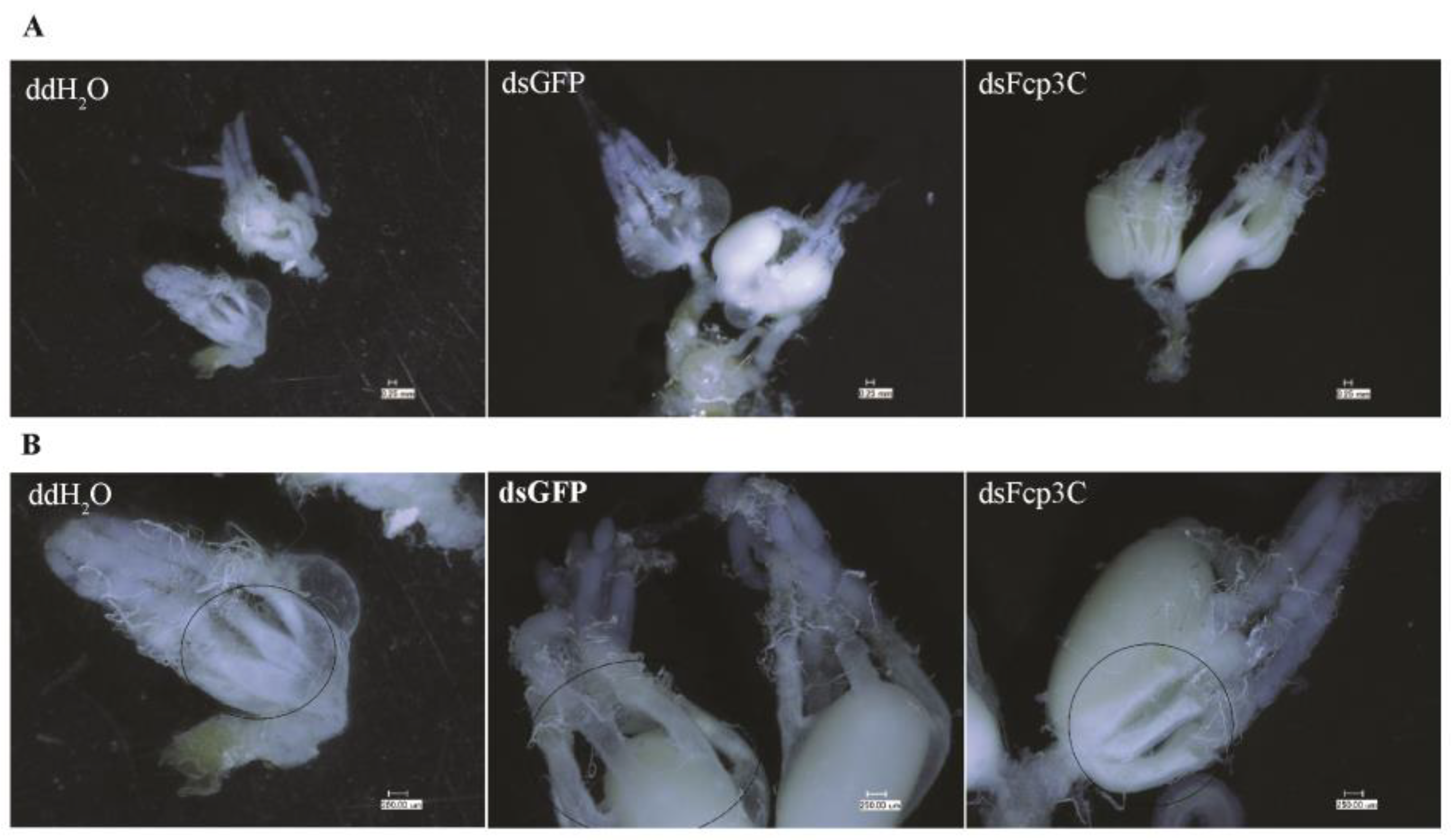
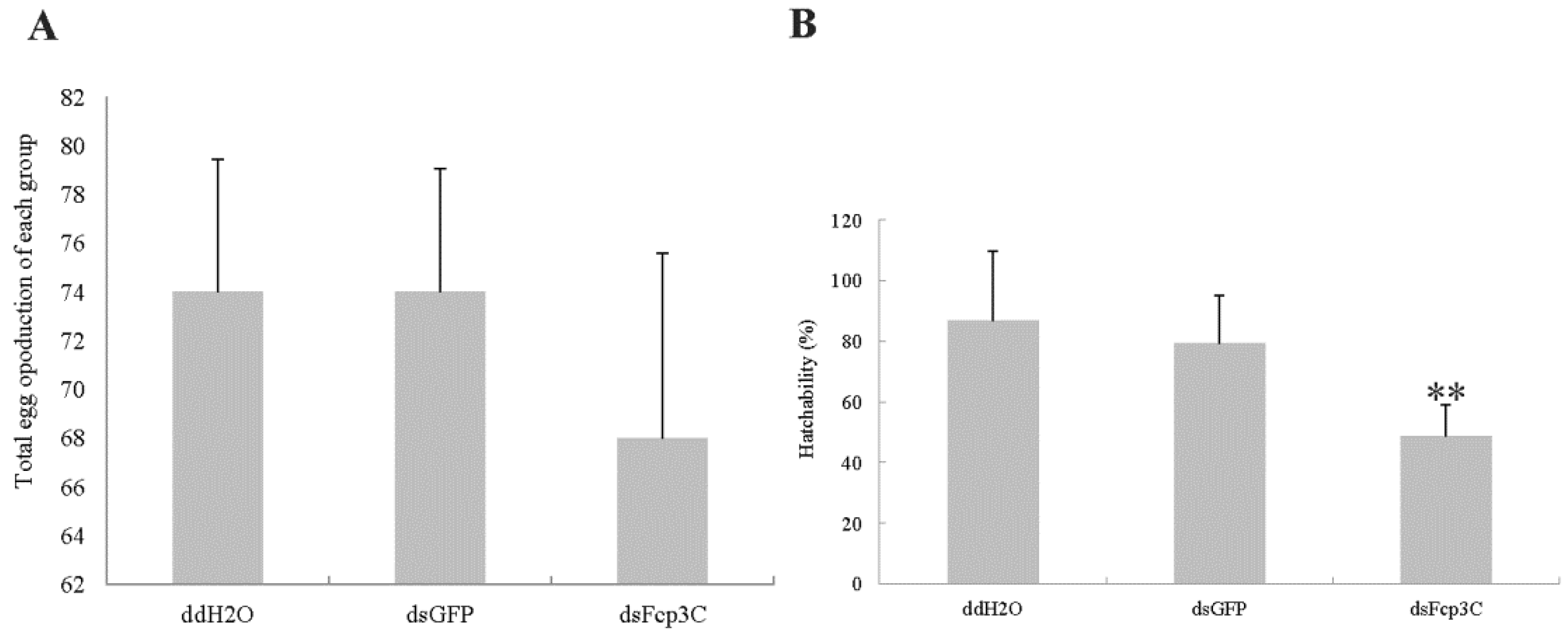
2.7. Effect of Fcp3C on Oviposition
3. Discussion
4. Materials and Methods
4.1. Tissue Sampling
4.2. RNA Extraction and Sequencing
4.3. Transcriptome Assembly and Analysis
4.4. Differential Gene Expression Analysis
4.5. Synthesis of dsRNA and RNA Interference
4.6. Gene Expression Analysis
4.7. Microscopic Observation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.M.; Ju, Q.; Qu, M.J.; Zhao, Z.Q.; Dong, S.L.; Han, Z.J.; Yu, S.L. EAG and behavioral responses of the large black chafer, Holotrichia parallela (Coleoptera: Scarabaeidae) to its sex pheromone. Acta Entomol. Sin. 2009, 52, 121–125. [Google Scholar]
- Zhang, H.F.; Teng, X.H.; Luo, Q.W.; Sheng, Z.Y.; Guo, X.R.; Wang, G.P.; Li, W.Z.; Yuan, G.H. Flight and walking performance of dark black chafer beetle Holotrichia parallela (Coleoptera: Scarabaeidae) in the presence of known hosts and attractive nonhost plants. J. Insect Sci. 2019, 19, 14. [Google Scholar] [CrossRef]
- Li, W.Z.; Yang, L.; Shen, X.W.; Yuan, Y.H.; Yuan, G.H.; Luo, M.H.; Guo, X.R. Prescription screening and field evaluation of broad spectrum attractants of scarab beetles from Ricinus communis. Chin. J. Eco 2013, 21, 480–486. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Smagghe, G.; Zotti, M.; Retnakaran, A. Targeting female reproduction in insects with biorational insecticides for pest management: A critical review with suggestions for future research. Curr. Opin. Insect Sci. 2019, 31, 65–69. [Google Scholar] [CrossRef]
- Attardo, G.M.; Hansen, I.A.; Raikhel, A.S. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem. Mol. Biol. 2005, 35, 661–675. [Google Scholar] [CrossRef]
- Clifton, M.E.; Noriega, F.G. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. J. Insect Physiol. 2011, 57, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Schillewaert, S.; Vantaux, A.; Van den Ende, W.; Wenseleers, T. The effect of host plants on genotype variability in fitness and honeydew composition of Aphis fabae. Insect Sci. 2017, 24, 781–788. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Zhu, X.L.; Zhao, D.; Qi, G.H.; Guo, W.; Li, R.J. Analysis of nutritional components in plant leaves affecting the feeding, longevity and fecundity of Holotrichia parallela (Coleoptera: Scarabaeoidea) adults. Acta Entomol. Sin. 2019, 62, 1205–1211. [Google Scholar] [CrossRef]
- Shobana, K.; Murugan, K.; Naresh Kumar, A. Influence of host plants on feeding, growth and reproduction of Papiliopolytes(The common mormon). J. Insect Physiol. 2010, 56, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Duan, A.; Wang, L.; Wang, S.; Han, R.; Miao, J. Effects of feeding 17 different plants on the reproductive capacity of Holotrichia parallela. J. Shanxi Agrl. Sci. 2015, 61, 25–27. [Google Scholar]
- Feng, X.J.; Liu, F.S.; Xi, G.C.; Liu, Y.T.; Li, J.Y.; Liu, C.Q.; Wang, Q.L. Influences of diet plants on ovary development and fecundity of Holotrichia parallela. Plant Protect. 2015, 41, 52–55+83. [Google Scholar]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.K.; Yang, S.; Wang, S.; Wang, J.; Zhang, X.X.; Liu, Y.; Xi, J.H. Identification of candidate chemosensory receptors in the antennal transcriptome of the large black chafer Holotrichia parallela Motschulsky (Coleoptera: Scarabaeidae). Comp. Biochem. Physiol. D 2018, 28, 63–71. [Google Scholar] [CrossRef]
- Shu, C.L.; Tan, S.Q.; Yin, J.; Soberón, M.; Bravo, A.; Liu, C.Q.; Geng, L.L.; Song, F.P.; Li, K.B.; Zhang, J. Assembling of Holotrichia parallela (dark black chafer) midgut tissue transcriptome and identification of midgut proteins that bind to Cry8Ea toxin from Bacillus thuringiensis. Appl. Microbiol. Biot. 2015, 99, 7209–7218. [Google Scholar] [CrossRef] [PubMed]
- Li, E.T.; Qin, J.H.; Feng, H.L.; Li, J.Q.; Li, X.F.; Nyamwasa, I.; Cao, Y.Z.; Ruan, W.B.; Li, K.B.; Yin, J. Immune-related genes of the larval Holotrichia parallela in response to entomopathogenic nematodes Heterorhabditisbeicherriana LF. BMC Genom. 2021, 22, 192. [Google Scholar] [CrossRef]
- Li, E.T.; Wu, H.J.; Wang, Z.M.; Li, K.B.; Zhang, S.; Cao, Y.Z.; Yin, J. PI3K/Akt/CncC signaling pathway mediates the response to EPN-Bt infection in Holotrichia parallela larvae. Pest Manag. Sci. 2023, 79, 1660–1673. [Google Scholar] [CrossRef]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Zheng, R.W.; Yao, L.; Peng, J.; Chen, Z.H.; Yang, F.; Chen, S.X.; Tang, Q.F. Comparative transcriptome analysis reveals key candidate genes mediating ovarian development in Spodoptera frugiperda fed on two host plants. Front. Physiol. 2022, 13, 1056540. [Google Scholar] [CrossRef]
- Guo, S.K.; Zhao, Z.H.; Liu, L.J.; Li, Z.H.; Shen, J. Comparative transcriptome analyses uncover key candidate genes mediating flight capacity in Bactrocera dorsalis (Hendel) and Bactroceracorrecta (Bezzi) (Diptera: Tephritidae). Int. J. Mol. Sci. 2018, 19, 396. [Google Scholar] [CrossRef]
- Xue, Y.X.; Muhammad, S.; Yang, J.L.; Wang, X.; Zhao, N.; Qin, B.X.; Qiu, Y.F.; Du, Z.M.; Ulhassan, Z.; Zhou, W.J.; et al. Comparative transcriptome-wide identification and differential expression of genes and lncRNAs in rice near-isogenic line (KW-Bph36-NIL) in response to BPH feeding. Front. Plant Sci. 2023, 13, 1095602. [Google Scholar] [CrossRef]
- Papaj, D.R. Ovarian dynamics and host use. Annu. Rev. Entomol. 2000, 45, 423–448. [Google Scholar] [CrossRef]
- Sun, R.H.; Li, A.H.; Wang, T.; Zhang, Y.; Song, G.C. Effects of different foods on adult longevity and reproduction of two scarab beetles. J. Environ. Entomol. 2010, 32, 544–548. [Google Scholar] [CrossRef]
- Li, H.L.; Wang, X.Y.; Zheng, X.L.; Lu, W. Research progress on oviposition-related genes in insects. J. Insect Sci. 2020, 20, 36. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Buck, N.A. A role for storage proteins in autogenous reproduction in Aedes atropalpus. J. Insect Physiol. 1996, 42, 961–966. [Google Scholar] [CrossRef]
- Seehuus, S.C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Tang, B.Z.; Hou, Y.M.; Xie, Y.X. Molecular cloning and expression of the vitellogenin gene and its correlation with ovarian development in an invasive pest Octodontanipae on two host plants. Bull. Entomol. Res. 2016, 106, 642–650. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Li, G.C.; Wang, Z.Q.; Guo, Y.R.; Liu, N.Y. Combined transcriptomic, proteomic and genomic analysis identifies reproductive-related proteins and potential modulators of female behaviors in Spodoptera litura. Genomics 2021, 113, 1876–1894. [Google Scholar] [CrossRef]
- Sun, Y.L.; Huang, L.Q.; Pelosi, P.; Wang, C.Z. Expression in antennae and reproductive organs suggests a dual role of an odorant-binding protein in two sibling Helicoverpa species. PLoS ONE 2012, 7, e30040. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Dong, X.L.; Liu, J.X.; Hu, M.Y.; Zhong, G.H.; Geng, P.; Yi, X. Molecular cloning, expression and molecular modeling of chemosensory protein from Spodoptera litura and its binding properties with Rhodojaponin III. PLoS ONE 2012, 7, e47611. [Google Scholar] [CrossRef]
- Li, S.; Picimbon, J.F.; Ji, S.; Kan, Y.C.; Chuanling, Q.; Zhou, J.J.; Pelosi, P. Multiple functions of an odorant-binding protein in the mosquito Aedes aegypti. Biochem. Biophys. Res. Commun. 2008, 372, 464–468. [Google Scholar] [CrossRef]
- Du, J.; Zhu, H.X.; Cao, C.L.; Ma, Y. Expression of Macrobrachiumrosenbergii lipopolysaccharide-and β-1, 3-glucan-binding protein (LGBP) in Saccharomyces cerevisiae and evaluation of its immune function. Fish Shellfish Immunol. 2019, 84, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.X.; Xiu, Y.J.; Yuan, M.J.; Bi, J.X.; Hou, L.B.; Gu, W.; Meng, Q.G. Spiroplasmaeriocheiris invasion into Macrobrachiumrosenbergii hemocytes is mediated by pathogen enolase and host lipopolysaccharide and β-1, 3-Glucan Binding Protein. Front. Immunol. 2019, 10, 1852. [Google Scholar] [CrossRef]
- Li, S.S.; Hao, Z.P.; Xu, H.H.; Gao, Y.; Zhang, M.Y.; Liang, J.; Dang, X.L. Silencing β-1, 3-glucan binding protein enhances the susceptibility of Plutellaxylostella to entomopathogenic fungus Isaria cicadae. Pest Manag. Sci. 2022, 78, 3117–3127. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Klett, E.L.; De Paula, I.F.; Ramos, I.B.; Coleman, R.A.; Gondim, K.C. Long-chain acyl-CoA synthetase 2 knockdown leads to decreased fatty acid oxidation in fat body and reduced reproductive capacity in the insect Rhodnius prolixus. BBA Mol. Cell Biol. Lipids 2016, 1861, 650–662. [Google Scholar] [CrossRef]
- Xu, X.; Bi, H.L.; Wang, Y.H.; Li, X.W.; Xu, J.; Liu, Z.L.; He, L.; Li, K.; Huang, Y.P. Disruption of the ovarian serine protease (Osp) gene causes female sterility in Bombyx mori and Spodoptera litura. Pest Manag. Sci. 2020, 76, 1245–1255. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.H.; Liu, Z.L.; Wang, Y.Q.; He, L.; Li, K.; Huang, Y.P. Disruption of egg-specific protein causes female sterility in Bombyx mori. Insect Sci. 2022, 29, 128–138. [Google Scholar] [CrossRef]
- Ye, Y.X.; Pan, P.L.; Xu, J.Y.; Shen, Z.F.; Kang, D.; Lu, J.B.; Hu, Q.L.; Huang, H.J.; Lou, Y.H.; Zhou, N.M.; et al. Forkhead box transcription factor L2 activates Fcp3C to regulate insect chorion formation. Open Biol. 2017, 7, 170061. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.; Waring, G.L.; Popodi, E.; Minoo, P. Characterization and sequence of follicle cell genes selectively expressed during vitelline membrane formation in Drosophila. Dev. Biol. 1987, 124, 441–450. [Google Scholar] [CrossRef]
- Marchal, E.; Hult, E.F.; Huang, J.; Tobe, S.S. Sequencing and validation of housekeeping genes for quantitative real-time PCR during the gonadotrophic cycle of Diploptera punctata. BMC Res. Notes 2013, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, A.; Belles, X.; Piulachs, M.D. Chorion formation in panoistic ovaries requires windei and trimethylation of histone 3 lysine 9. Exp. Cell Res. 2014, 320, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Yoon, J.S.; Mogilicherla, K.; Gurusamy, D.; Chen, X.; Cheredy, S.C.R.R.; Palli, S.R. Double-stranded RNA binding protein, Staufen, is required for the initiation of RNAi in coleopteran insects. Proc. Natl. Acad. Sci. USA 2018, 115, 8334–8339. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Shukla, J.N.; Gong, Z.J.; Mogilicherla, K.; Palli, S.R. RNA interference in the Colorado potato beetle, Leptinotarsa decemlineata: Identification of key contributors. Insect Biochem. Mol. Biol. 2016, 78, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, L.; Li, S.; Zhang, J. Bacteria-mediated RNA interference for management of Plagiodera versicolora (Coleoptera: Chrysomelidae). Insects 2019, 10, 415. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106–R117. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Lü, J.; Liu, Z.; Guo, W.; Guo, M.; Chen, S.; Li, H.; Yang, C.; Zhang, Y.; Pan, H. Feeding delivery of dsHvSnf7 is a promising method for management of the pest Henosepilachnavigintioctopunctata (Coleoptera: Coccinellidae). Insects 2019, 31, 34. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.X.; Huang, L.Q. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The genome sequence archive family: Toward explosive data growth and diverse data types. Genom. Proteom. Bioinf. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- CNCB-NGDC Members and Partners. Database resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mean Number of Eggs Laid Per Female | Oviposition Duration (d) | Adult Longevity (d) | Survival Rate (%) |
|---|---|---|---|---|
| Elm | 67.4 ± 0.240 a | 59.3 ± 2.027 a | 66 ± 0.000 a | 90.0 ± 5.773 a |
| Willow | 21.1 ± 0.623 b | 46.7 ± 1.333 b | 66 ± 0.000 a | 70.0 ± 5.773 b |
| Purpus privet | 8.6 ± 0.607 c | 39.3 ± 4.667 c | 66 ± 0.000 a | 33.3 ± 3.333 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Zhang, J.; Li, Y.; Li, H.; Zhang, Z.; Qin, Y.; Jiang, Y.; Duan, Y.; Li, T.; Miao, J.; et al. Identification of Potential Gene Targets for Suppressing Oviposition in Holotrichia parallela Using Comparative Transcriptome Analysis. Int. J. Mol. Sci. 2023, 24, 13138. https://doi.org/10.3390/ijms241713138
Gong Z, Zhang J, Li Y, Li H, Zhang Z, Qin Y, Jiang Y, Duan Y, Li T, Miao J, et al. Identification of Potential Gene Targets for Suppressing Oviposition in Holotrichia parallela Using Comparative Transcriptome Analysis. International Journal of Molecular Sciences. 2023; 24(17):13138. https://doi.org/10.3390/ijms241713138
Chicago/Turabian StyleGong, Zhongjun, Jing Zhang, Yanmin Li, Huiling Li, Ziqi Zhang, Yifan Qin, Yueli Jiang, Yun Duan, Tong Li, Jin Miao, and et al. 2023. "Identification of Potential Gene Targets for Suppressing Oviposition in Holotrichia parallela Using Comparative Transcriptome Analysis" International Journal of Molecular Sciences 24, no. 17: 13138. https://doi.org/10.3390/ijms241713138
APA StyleGong, Z., Zhang, J., Li, Y., Li, H., Zhang, Z., Qin, Y., Jiang, Y., Duan, Y., Li, T., Miao, J., & Wu, Y. (2023). Identification of Potential Gene Targets for Suppressing Oviposition in Holotrichia parallela Using Comparative Transcriptome Analysis. International Journal of Molecular Sciences, 24(17), 13138. https://doi.org/10.3390/ijms241713138





