Performance of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Targeting the RNA Polymerase Gene for the Direct Detection of SARS-CoV2 in Nasopharyngeal Swabs
Abstract
:1. Introduction
2. Results and Discussion
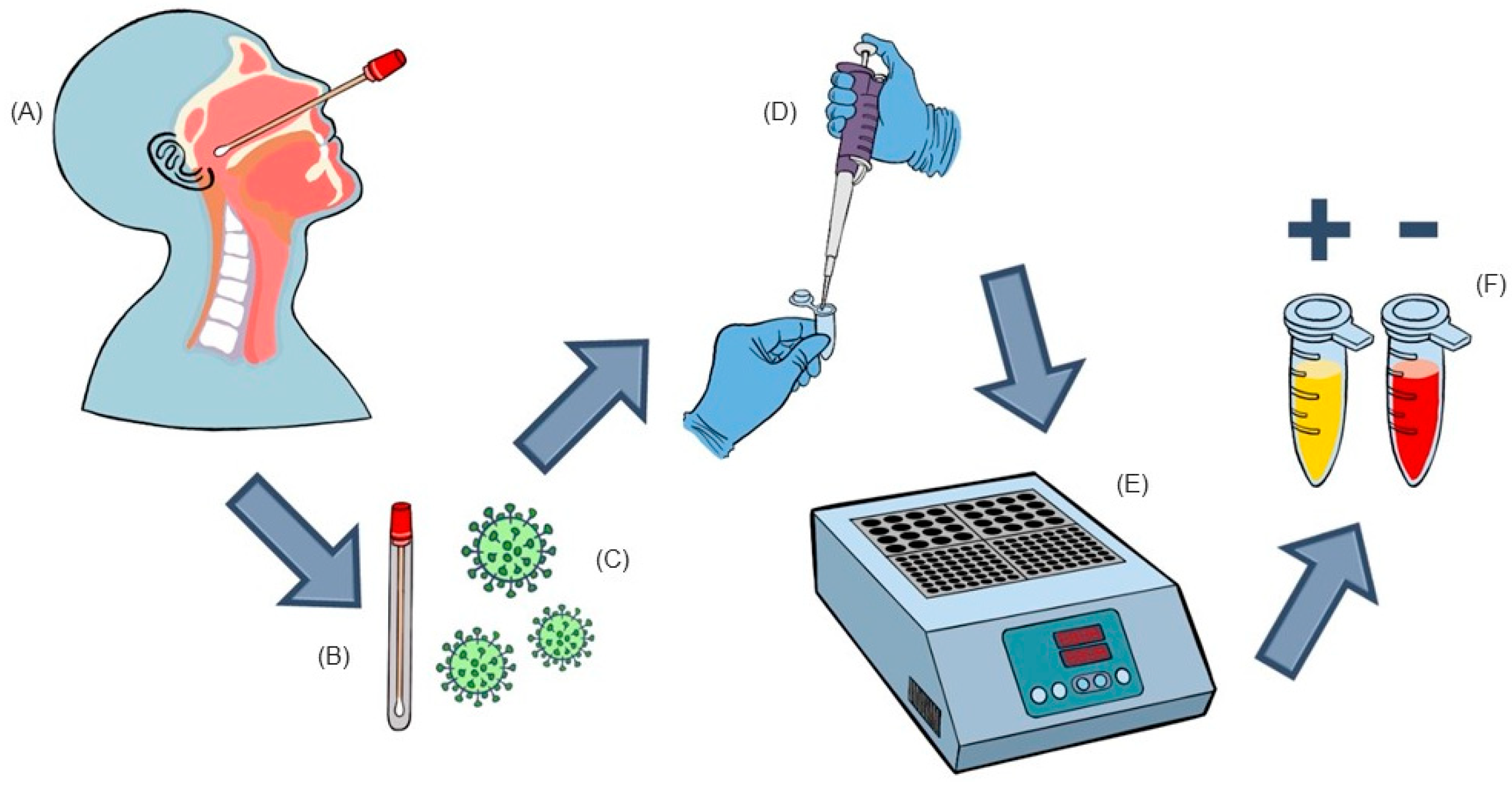
3. Materials and Methods
3.1. Nasopharyngeal Samples
3.2. Primers for RT-LAMP
3.3. RT-LAMP
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovanetti, M.; Slavov, S.N.; Fonseca, V.; Wilkinson, E.; Tegally, H.; Patané, J.S.L.; Viala, V.L.; San, E.J.; Rodrigues, E.S.; Santos, E.V.; et al. Genomic epidemiology of the SARS-CoV-2 epidemic in Brazil. Nat. Microbiol. 2022, 7, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- OPAS. Histórico da pandemia de COVID-19—OPAS/OMS|Organização Pan-Americana da Saúde. 2020. Available online: https://www.paho.org/pt/covid19/historico-da-pandemia-covid-19 (accessed on 3 December 2022).
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-Mediated Isothermal Amplification (LAMP): The Better Sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef]
- Smyrlaki, I.; Ekman, M.; Lentini, A.; Rufino de Sousa, N.; Papanicolaou, N.; Vondracek, M.; Aarum, J.; Safari, H.; Muradrasoli, S.; Rothfuchs, A.G.; et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat. Commun. 2020, 11, 4812. [Google Scholar] [CrossRef]
- Alvarez, C.U.; Lam, Q.; Baldwin, D.A.; Chernoff, J. Low saliva pH can yield false positives results in simple RT-LAMP-based SARS-CoV-2 diagnostic tests. PLoS ONE 2021, 16, e0250202. [Google Scholar]
- Zhang, C.; Zheng, T.; Wang, H.; Chen, W.; Huang, X.; Liang, J.; Qiu, L.; Han, D.; Tan, W. Rapid One-Pot Detection of SARS-CoV-2 Based on a Lateral Flow Assay in Clinical Samples. Anal. Chem. 2021, 93, 3325–3330. [Google Scholar] [CrossRef]
- Baek, Y.H.; Um, J.; Antigua, K.J.C.; Park, J.-H.; Kim, Y.; Oh, S.; Kim, Y.-I.; Choi, W.-S.; Kim, S.G.; Jeong, J.J.; et al. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 998–1007. [Google Scholar] [CrossRef]
- Haq, F.; Sharif, S.; Khurshid, A.; Ikram, A.; Shabbir, I.; Salman, M.; Ahad, A.; Rana, M.S.; Raja, A.; Badar, N.; et al. Reverse transcriptase loop-mediated isothermal amplification (RT-LAMP)-based diagnosis: A potential alternative to quantitative real-time PCR based detection of the novel SARS-CoV-2 virus. Saudi J. Biol. Sci. 2021, 28, 942–947. [Google Scholar] [CrossRef]
- Jang, W.S.; Lim, D.H.; Yoon, J.; Kim, A.; Lim, M.; Nam, J.; Yanagihara, R.; Ryu, S.-W.; Jung, B.K.; Ryoo, N.-H.; et al. Development of a multiplex Loop-Mediated Isothermal Amplification (LAMP) assay for on-site diagnosis of SARS-CoV-2. PLoS ONE 2021, 16, e0248042. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Pan, W.; Arasthfer, A.; Fang, W.; Ling, L.; Fang, H.; Daneshnia, F.; Yu, J.; Liao, W.; Pei, H.; et al. Development and validation of a rapid, single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Orihara, Y.; Kawamura, R.; Imai, K.; Sakai, J.; Tarumoto, N.; Matsuoka, M.; Takeuchi, S.; Maesaki, S.; Maeda, T. Evaluation of rapid diagnosis of novel coronavirus disease (COVID-19) using loop-mediated isothermal amplification. J. Clin. Virol. 2020, 129, 104446. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Wang, X.; Yang, J.; Zhang, L.; Deng, Q.; Zhang, X.; Wang, Z.; Hou, T.; Li, S. A novel One-pot rapid diagnostic technology for COVID-19. Anal. Chim. Acta 2021, 1154, 338310. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wu, X.; Wan, Z.; Li, Y.; Jin, X.; Zhang, C. A novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 2826. [Google Scholar] [CrossRef] [PubMed]
- Manzano, J.R.; Malpartida-Cardenas, K.; Moser, N.; Pennisi, I.; Cavuto, M.; Miglietta, L.; Moniri, A.; Penn, R.; Satta, G.; Randell, P.; et al. Handheld point-of-care system for rapid detection of SARS-CoV-2 extracted RNA in under 20 min. ACS Cent. Sci. 2021, 7, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Mostafa, A.; Berger, J.; Aydin, M.Y.; Sun, F.; de Ramirez, S.A.S.; Valera, E.; Cunningham, B.T.; King, W.P.; Bashir, R.; et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 22727–22735. [Google Scholar] [CrossRef] [PubMed]
- Wikramaratna, P.S.; Paton, R.S.; Ghafari, M.; Lourenço, J. Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR. Eurosurveillance 2020, 25, 2000568. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, V.; Negro, A.; Pirotti, T.; Trenti, T. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13706. [Google Scholar] [CrossRef] [PubMed]
- Khandker, S.S.; Nik Hashim, N.H.H.; Deris, Z.Z.; Shueb, R.H.; Islam, M.A. Diagnostic accuracy of rapid antigen test kits for detecting SARS-CoV-2: A systematic review and meta-analysis of 17,171 suspected COVID-19 patients. J. Clin. Med. 2021, 10, 3493. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Best, N.; McAuley, J.; Porter, J.L.; Seemann, T.; Schultz, M.B.; Sait, M.; Orlando, N.; Mercoulia, K.; Ballard, S.A.; et al. Validation of a single-step, single-tube reverse transcription loop-mediated isothermal amplification assay for rapid detection of SARS-CoV-2 RNA. J. Med. Microbiol. 2020, 69, 1169. [Google Scholar] [CrossRef] [PubMed]
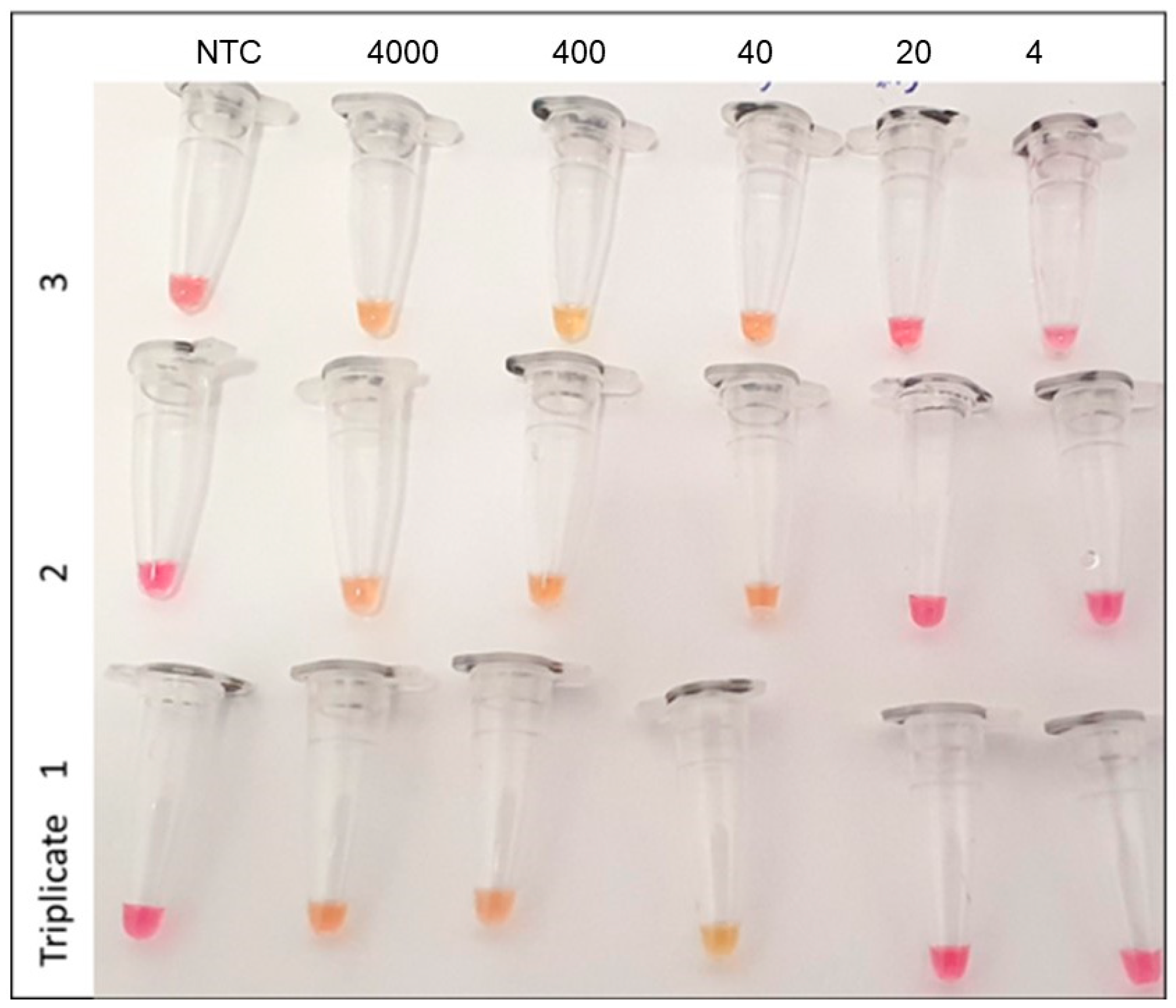
| LAMP Positive | LAMP Negative | |
|---|---|---|
| RT-PCR Positive | 39 | 4 |
| RT-PCR Negative | 0 | 72 |
| Label | Length | Tm | 5’dG | 3’dG | GC Rate | Sequence |
|---|---|---|---|---|---|---|
| F3 | 21 | 55.33 | −4.9 | −4.18 | 0.38 | ACTGACTTAACAAAGCCTTAC |
| B3 | 20 | 56.19 | −3.53 | −4.55 | 0.4 | ACTAGTGGTCCAAAACTTGT |
| FIP | 50 | GGTGGTATGTCTGATCCCAATATTT-GGGATTTGTTAAAATATGACTTCAC | ||||
| BIP | 45 | TTGTGTTAACTGTTTGGATGACAGA-AGTGGGAACACTGTAGAGAA | ||||
| F2 | 25 | 56.51 | −4.85 | −4.51 | 0.32 | GGGATTTGTTAAAATATGACTTCAC |
| F1c | 25 | 60.56 | −6 | −2.28 | 0.4 | GGTGGTATGTCTGATCCCAATATTT |
| B2 | 20 | 57.27 | −5.84 | −4.1 | 0.45 | AGTGGGAACACTGTAGAGAA |
| B1c | 25 | 60.88 | −4.72 | −4.76 | 0.36 | TTGTGTTAACTGTTTGGATGACAGA |
| LF | 25 | 60.92 | −6.19 | −4.1 | 0.4 | CGGTCAAAGAGTTTTAACCTCTCTT |
| LB | 21 | 60.35 | −4.96 | −5.17 | 0.43 | TGCATTCTGCATTGTGCAAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, E.d.R.; Balzan, L.d.R.; Inamine, E.; Pancotto, L.R.; Gaboardi, G.; Cantarelli, V.V. Performance of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Targeting the RNA Polymerase Gene for the Direct Detection of SARS-CoV2 in Nasopharyngeal Swabs. Int. J. Mol. Sci. 2023, 24, 13056. https://doi.org/10.3390/ijms241713056
Hoffmann EdR, Balzan LdR, Inamine E, Pancotto LR, Gaboardi G, Cantarelli VV. Performance of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Targeting the RNA Polymerase Gene for the Direct Detection of SARS-CoV2 in Nasopharyngeal Swabs. International Journal of Molecular Sciences. 2023; 24(17):13056. https://doi.org/10.3390/ijms241713056
Chicago/Turabian StyleHoffmann, Elias da Rosa, Lisiane da Rocha Balzan, Everton Inamine, Lisiane Rech Pancotto, Guilherme Gaboardi, and Vlademir Vicente Cantarelli. 2023. "Performance of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Targeting the RNA Polymerase Gene for the Direct Detection of SARS-CoV2 in Nasopharyngeal Swabs" International Journal of Molecular Sciences 24, no. 17: 13056. https://doi.org/10.3390/ijms241713056
APA StyleHoffmann, E. d. R., Balzan, L. d. R., Inamine, E., Pancotto, L. R., Gaboardi, G., & Cantarelli, V. V. (2023). Performance of Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Targeting the RNA Polymerase Gene for the Direct Detection of SARS-CoV2 in Nasopharyngeal Swabs. International Journal of Molecular Sciences, 24(17), 13056. https://doi.org/10.3390/ijms241713056





