Cyclosporine A Decreases Dryness-Induced Hyperexcitability of Corneal Cold-Sensitive Nerve Terminals
Abstract
1. Introduction
2. Results
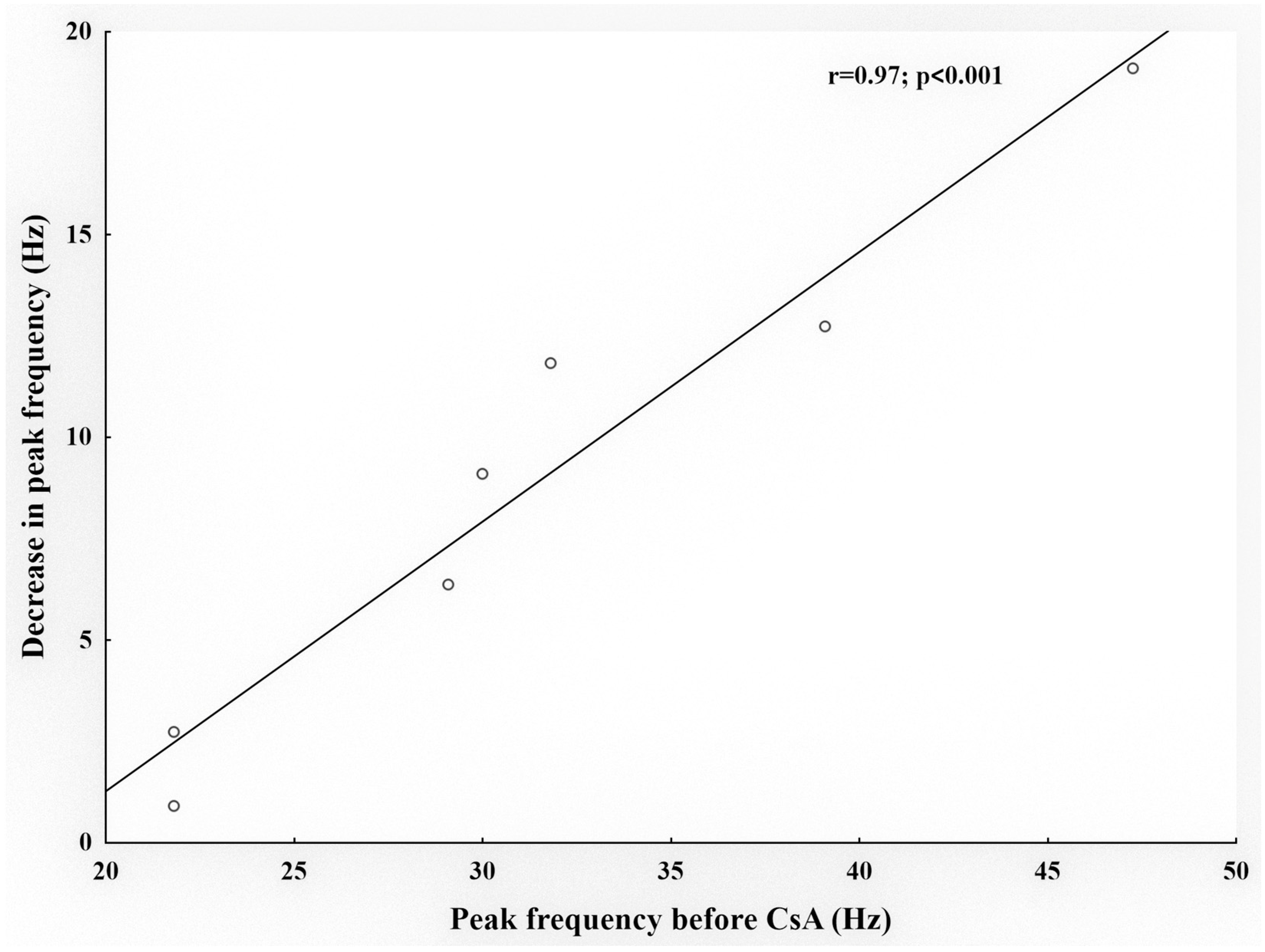
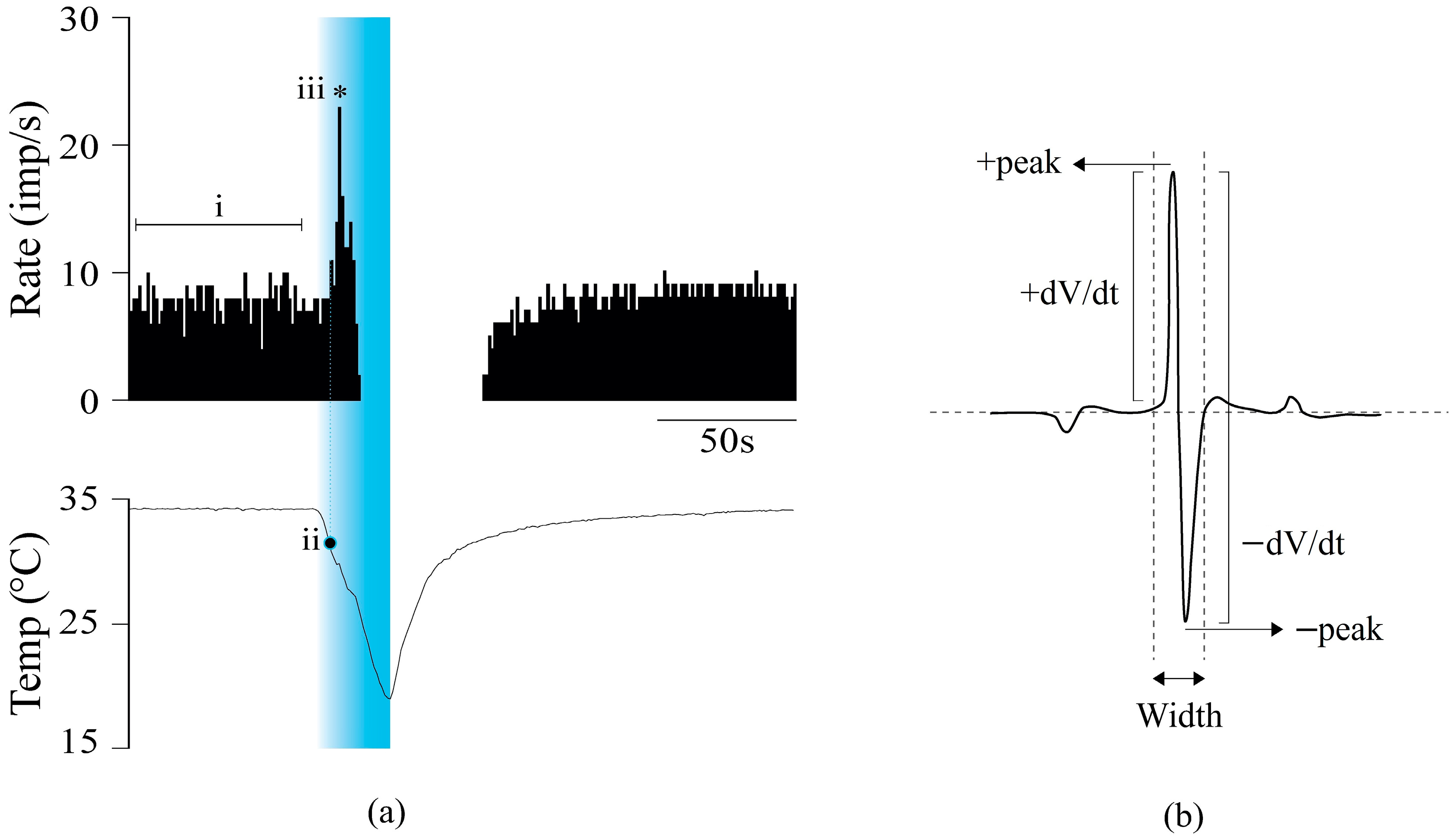
2.1. Effects of CsA on Cold Thermoreceptors’ Nerve Activity
2.2. Effects of CsA on the Shape of NTIs of Cold Thermoreceptors
2.3. Effect of CsA on Blinking and Tearing
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure
4.3. Cyclosporine A
4.4. Recording of the Electrical Activity of Corneal Cold Thermosensitive Nerve Terminals
4.5. Electrophysiological Recording Analysis
4.6. Blinking Frequency and Tearing Rate in Awake Guinea Pigs
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Begley, C.G.; Caffery, B.; Jones, L.A. Symptoms of ocular irritation in patients diagnosed with dry eye. Optom. Vis. Sci. 1999, 76, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Garcia-Hirschfeled, J.; Gallar, J. Neurobiology of ocular pain. Prog. Retin. Eye Res. 1996, 16, 117–156. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef]
- Belmonte, C.; Aracil, A.; Acosta, M.C.; Luna, C.; Gallar, J. Nerves and sensations from the eye surface. Ocul. Surf. 2004, 2, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.H.; King-Smith, P.E.; Nichols, J.J. Evidence for the major contribution of evaporation to tear film thinning between blinks. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6294–6297. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Gallar, J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3888–3892. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Merayo-Lloves, J.; Gallar, J. What causes eye pain? Curr. Ophthalmol. Rep. 2015, 3, 111–121. [Google Scholar] [CrossRef]
- Kovács, I.; Luna, C.; Quirce, S.; Mizerska, K.; Callejo, G.; Riestra, A.; Fernández-Sánchez, L.; Meseguer, V.M.; Cuenca, N.; Merayo-Lloves, J.; et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain 2016, 157, 399–417. [Google Scholar] [CrossRef]
- Ousler, G.W.; Michaelson, C.; Christensen, M.T. An evaluation of tear film breakup time extension and ocular protection index scores among three marketed lubricant eye drops. Cornea 2007, 26, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Profazio, V.; Campos, E.C. One month use of Systane improves ocular surface parameters in subjects with moderate symptoms of ocular dryness. Clin. Ophthalmol. 2008, 2, 629–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- RESTASIS® (Cyclosporine Ophthalmic Emulsion) 0.05% for Topical Ophthalmic Use. Full Prescribing Information. Available online: https://www.rxabbvie.com/pdf/restasis_pi.pdf (accessed on 31 May 2023).
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Gao, J.; Schwalb, T.A.; Addeo, J.V.; Ghosn, C.R.; Stern, M.E. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: The effect of topical cyclosporin A therapy. Cornea 1998, 17, 654–663. [Google Scholar] [CrossRef]
- Kaswan, R.L.; Salisbury, M.A.; Ward, D.A. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: Treatment with cyclosporine eye drops. Arch. Ophthalmol. 1989, 107, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.V.; Abrams, K.L. Topical administration of cyclosporine for treatment of keratoconjunctivitis sicca in dogs. J. Am. Vet. Med. Assoc. 1991, 199, 1043–1046. [Google Scholar]
- Olivero, D.K.; Davidson, M.G.; English, R.V.; Nasisse, M.P.; Jamieson, V.E.; Gerig, T.M. Clinical evaluation of 1% cyclosporine for topical treatment of keratoconjunctivitis sicca in dogs. J. Am. Vet. Med. Assoc. 1991, 199, 1039–1042. [Google Scholar] [PubMed]
- Laibovitz, R.A.; Solch, S.; Andriano, K.; O’Connell, M.; Silverman, M.H. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea 1993, 12, 311–323. [Google Scholar] [CrossRef]
- Stevenson, D.; Tauber, J.; Reis, B.L. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: A dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology 2000, 107, 967–974. [Google Scholar] [CrossRef]
- Jung, G.T.; Kim, M.; Song, J.S.; Kim, T.I.; Chung, T.Y.; Choi, C.Y.; Kim, H.S.; An, W.J.; Jeong, S.J.; Lee, H.S.; et al. Proteomic analysis of tears in dry eye disease: A prospective, double-blind multicenter study. Ocul. Surf. 2023, 29, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-023_Restasis.cfm (accessed on 31 May 2023).
- Wan, K.H.; Chen, L.J.; Young, A.L. Efficacy and Safety of Topical 0.05% Cyclosporine Eye Drops in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-analysis. Ocul. Surf. 2015, 13, 213–225. [Google Scholar] [CrossRef]
- Namavari, A.; Chaudhary, S.; Chang, J.H.; Yco, L.; Sonawane, S.; Khanolkar, V.; Yue, B.Y.; Sarkar, J.; Jain, S. Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves. Investig. Ophthalmol. Vis. Sci. 2012, 53, 732–740. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Basic-Jukic, N.; Basic-Kes, V.; Kes, P.; Furic-Cunko, V.; BacicBaronica, K. Neurological complications in renal transplant recipients. Acta Med. Croatica 2008, 62, 76–81. [Google Scholar] [PubMed]
- Parra, A.; Gonzalez-Gonzalez, O.; Gallar, J.; Belmonte, C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain 2014, 155, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Dienes, L.; Kiss, H.J.; Perényi, K.; Szepessy, Z.; Nagy, Z.Z.; Barsi, Á.; Acosta, M.C.; Gallar, J.; Kovács, I. The Effect of Tear Supplementation on Ocular Surface Sensations during the Interblink Interval in Patients with Dry Eye. PLoS ONE 2015, 10, e0135629. [Google Scholar] [CrossRef]
- Brock, J.; Acosta, M.C.; Al Abed, A.; Pianova, S.; Belmonte, C. Barium ions inhibit the dynamic response of guinea-pig corneal cold receptors to heating but not to cooling. J. Physiol. 2006, 575, 573–581. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, O.; Bech, F.; Gallar, J.; Merayo-Lloves, J.; Belmonte, C. Functional properties of sensory nerve terminals of the mouse cornea. Investig. Ophthalmol. Vis. Sci. 2017, 58, 404–415. [Google Scholar] [CrossRef]
- Hirata, H.; Meng, I.D. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: Implications for dry eye disease. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3969–3976. [Google Scholar] [CrossRef]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Fakih, D.; Migeon, T.; Moreau, N.; Baudouin, C.; Réaux-Le Goazigo, A.; Mélik Parsadaniantz, S. Transient Receptor Potential Channels: Important Players in Ocular Pain and Dry Eye Disease. Pharmaceutics 2022, 14, 1859. [Google Scholar] [CrossRef]
- Kurose, M.; Meng, I.D. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. J. Neurophysiol. 2013, 109, 2517–2522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Schaumburg, C.S.; Dana, R.; Calonge, M.; Niederkorn, J.Y.; Pflugfelder, S.C. Autoimmunity at the ocular surface: Pathogenesis and regulation. Mucosal Immunol. 2010, 3, 425–442. [Google Scholar] [CrossRef]
- Banzet, S.; Koulmann, N.; Sanchez, H.; Serrurier, B.; Peinnequin, A.; Alonso, A.; Bigard, X. Contraction-induced interleukin-6 transcription in rat slow-type muscle is partly dependent on calcineurin activation. J. Cell Physiol. 2007, 210, 596–601. [Google Scholar] [CrossRef]
- Liddicoat, A.M.; Lavelle, E.C. Modulation of innate immunity by cyclosporine A. Biochem. Pharmacol. 2019, 163, 472–480. [Google Scholar] [CrossRef]
- Lara-Ramírez, R.; Segura-Anaya, E.; Martínez-Gomez, A.; Dent, M.A. Expression of interleukin-6 receptor alpha in normal and injured rat sciatic nerve. Neuroscience 2008, 152, 601–608. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Han, L.; Rumbaut, R.E.; Smith, C.W. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am. J. Pathol. 2011, 178, 1106–1116. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsu, L.W.; Huang, L.T.; Huang, T.L. Chronic administration of cyclosporine A changes expression of BDNF and TrkB in rat hippocampus and midbrain. Neurochem. Res. 2010, 35, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.W.; Im, Y.S.; Seong, G.J.; Lee, H.K. Cyclosporine A induces nerve growth factor expression via activation of MAPK p38 and NFAT5. Cornea 2011, 30 (Suppl. S1), S19–S24. [Google Scholar] [CrossRef]
- Erkutlu, I.; Alptekin, M.; Geyik, S.; Geyik, A.M.; Gezgin, I.; Gök, A. Early Cyclosporine A treatment retards axonal degeneration in an experimental peripheral nerve injection injury model. Neural Regen. Res. 2015, 10, 266–270. [Google Scholar] [CrossRef]
- Tuma Junior, P.T.; Ferreira, M.C.; Nakamoto, H.A.; Milcheski, D.A.; Cheroto Filho, A. Influence of immunosuppression on nerve regeneration using allografts; an experimental study on rats. Acta Ortop. Brasil. 2008, 16, 41–44. [Google Scholar]
- Mohammadi, R.; Heydarian, H.; Amini, K. Effect of local administration of cyclosporine A on peripheral nerve regeneration in a rat sciatic nerve transection model. Chin. J. Traumatol. 2014, 17, 12–18. [Google Scholar] [PubMed]
- Amniattalab, A.; Mohammadi, R. Functional, Histopathological and Immunohistichemical Assessments of Cyclosporine A on Sciatic Nerve Regeneration Using Allografts: A Rat Sciatic Nerve Model. Bull. Emerg. Trauma 2017, 5, 152–159. [Google Scholar]
- Choi, J.S.; Hudmon, A.; Waxman, S.G.; Dib-Hajj, S.D. Calmodulin regulates current density and frequency-dependent inhibition of sodium channel Nav1.8 in DRG neurons. J. Neurophysiol. 2006, 96, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.L.; Hosaka, M.; Janz, R.; Shelton, J.M.; Albright, G.M.; Richardson, J.A.; Südhof, T.C.; Victor, R.G. Cyclosporine A-induced hypertension involves synapsin in renal sensory nerve endings. Proc. Natl. Acad. Sci. USA 2000, 97, 9765–9770. [Google Scholar] [CrossRef] [PubMed]
- Onuma, H.; Lu, Y.F.; Tomizawa, K.; Moriwaki, A.; Tokuda, M.; Hatase, O.; Matsui, H. A calcineurin inhibitor, FK506, blocks voltage-gated calcium channel-dependent LTP in the hippocampus. Neurosci. Res. 1998, 30, 313–319. [Google Scholar] [CrossRef]
- Strong, B.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea 2005, 24, 80–85. [Google Scholar] [CrossRef]
- Carr, R.W.; Pianova, S.; Brock, J.A. The effects of polarizing current on nerve terminal impulses recorded from polymodal and cold receptors in the guinea-pig cornea. J. Gen. Physiol. 2002, 120, 395–405. [Google Scholar] [CrossRef]
- Li, H.; An, J.R.; Seo, M.S.; Ha, K.S.; Han, E.T.; Hong, S.H.; Jung, W.K.; Lee, D.S.; Yim, M.J.; Choi, G.; et al. Inhibitory effect of immunosuppressive drug tacrolimus on voltage-gated K+ current in rabbit coronary arterial smooth muscle cells. Eur. J. Pharmacol. 2019, 849, 59–66. [Google Scholar] [CrossRef]
- Arcas, J.M.; González, A.; Gers-Barlag, K.; González-González, O.; Bech, F.; Demirkhanyan, L.; Zakharian, E.; Belmonte, C.; Gomis, A.; Viana, F. The Immunosuppressant Macrolide Tacrolimus Activates Cold-Sensing TRPM8 Channels. J. Neurosci. 2019, 39, 949–969. [Google Scholar] [CrossRef]
- Toshida, H.; Nguyen, D.H.; Beuerman, R.W.; Murakami, A. Neurologic evaluation of acute lacrimomimetic effect of cyclosporine in an experimental rabbit dry eye model. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2736–2741. [Google Scholar] [CrossRef]
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat. Commun. 2015, 6, 7150. [Google Scholar] [CrossRef]
- Acosta, M.C.; Peral, A.; Luna, C.; Pintor, J.; Belmonte, C.; Gallar, J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2333–2336. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.C.; Gallar, J.; Belmonte, C. The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals. Exp. Eye Res. 1999, 68, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.A.; McLachlan, E.M.; Belmonte, C. Tetrodotoxin-resistant impulses in single nerve terminals signalling pain. J. Physiol. 1998, 512, 211–217. [Google Scholar] [CrossRef]
- Brock, J.A.; Pianova, S.; Belmonte, C. Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea. J. Physiol. 2001, 533, 493–501. [Google Scholar] [CrossRef]
- Acosta, M.C.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J. Changes in impulse activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain 2013, 154, 2353–2362. [Google Scholar] [CrossRef]
- Acosta, M.C.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J. Corneal sensory nerve activity in an experimental model of UV keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3403–3412. [Google Scholar] [CrossRef]
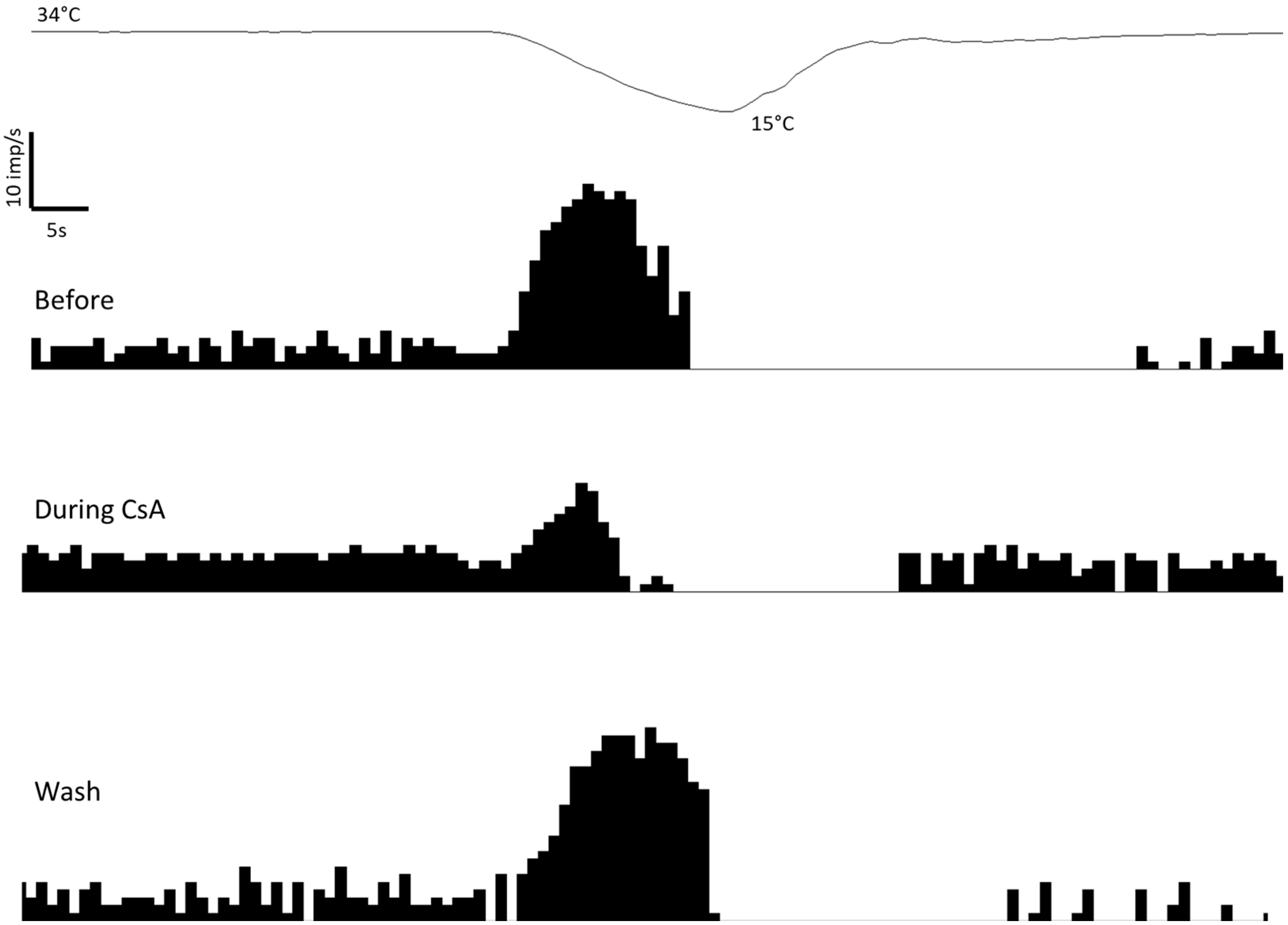
| Control Corneas (n = 7) | Tear-Deficient Corneas (n = 7) | |||
|---|---|---|---|---|
| Before | During CsA | Before | During CsA | |
| Spontaneous activity at 34 °C (imps/s) | 6.4 ± 0.9 | 6.4 ± 0.8 | 10.2 ± 0.3 | 11.4 ± 1.1 |
| Cooling threshold (°C) | 32.3 ± 0.4 | 31.7 ± 0.5 | 32.4 ± 0.2 | 32.4 ± 0.4 |
| Peak response to cold (imp/s) | 28.4 ± 5.4 | 21.6 ± 3.5 | 32.7 ± 4.5 | 23 ± 4.2 * |
| Parameters of NTI Shape | Control Corneas (n = 7) | Tear-Deficient Corneas (n = 3) | ||
|---|---|---|---|---|
| Before | During CsA | Before | During CsA | |
| +peak (µV) | 20.97 ± 2.54 | 25.62 ± 4.28 | 16.72 ± 1.47 | 15.49 ± 0.59 |
| −peak (µV) | 21.57 ± 2.63 | 26.10 ± 3.86 | 17.57 ± 1.86 | 16.56 ± 0.37 |
| Duration (ms) | 2.75 ± 0.14 | 2.85 ± 0.20 | 2.13 ± 0.12 * | 2.27 ± 0.19 |
| +dV/dt max normalized (µV/ms) | 1853 ± 227 | 1766 ± 257 | 2033 ± 251 | 1788 ± 1.7 |
| −dV/dt max normalized (µV/ms) | 2207 ± 202 | 2222 ± 191 | 2472 ± 233 | 2512 ± 190 |
| Control Eyes (n = 24) | Tear-Deficient Eyes (n = 6) | |||
|---|---|---|---|---|
| Before | During CsA | Before | During CsA | |
| Blinking frequency (blinks/min) | 1.7 ± 0.4 | 21.0 ± 1.6 ** | 10 ± 3.7 | 14.7 ± 2.3 |
| Tearing rate (mm) | 12.8 ± 1.2 | 24.0 ± 1.6 ** | 4.0 ± 1.0 | 7.8 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyenes, A.; Tapasztó, Z.; Quirce, S.; Luna, C.; Frutos-Rincón, L.; Gallar, J.; Acosta, M.C.; Kovács, I. Cyclosporine A Decreases Dryness-Induced Hyperexcitability of Corneal Cold-Sensitive Nerve Terminals. Int. J. Mol. Sci. 2023, 24, 13025. https://doi.org/10.3390/ijms241613025
Gyenes A, Tapasztó Z, Quirce S, Luna C, Frutos-Rincón L, Gallar J, Acosta MC, Kovács I. Cyclosporine A Decreases Dryness-Induced Hyperexcitability of Corneal Cold-Sensitive Nerve Terminals. International Journal of Molecular Sciences. 2023; 24(16):13025. https://doi.org/10.3390/ijms241613025
Chicago/Turabian StyleGyenes, Andrea, Zsófia Tapasztó, Susana Quirce, Carolina Luna, Laura Frutos-Rincón, Juana Gallar, M. Carmen Acosta, and Illés Kovács. 2023. "Cyclosporine A Decreases Dryness-Induced Hyperexcitability of Corneal Cold-Sensitive Nerve Terminals" International Journal of Molecular Sciences 24, no. 16: 13025. https://doi.org/10.3390/ijms241613025
APA StyleGyenes, A., Tapasztó, Z., Quirce, S., Luna, C., Frutos-Rincón, L., Gallar, J., Acosta, M. C., & Kovács, I. (2023). Cyclosporine A Decreases Dryness-Induced Hyperexcitability of Corneal Cold-Sensitive Nerve Terminals. International Journal of Molecular Sciences, 24(16), 13025. https://doi.org/10.3390/ijms241613025








