Discovery of α-Linolenic Acid 16(S)-Lipoxygenase: Cucumber (Cucumis sativus L.) Vegetative Lipoxygenase 3
Abstract
:1. Introduction
2. Results
2.1. Hydro(pero)xy Fatty Acid Patterns of Cucumber Seedlings and Fruits—Detection of 16(S)-HPOT
2.2. Specificity of Linoleic Acid Oxidation by LOX from Cucumber Flowers
2.3. Targeted Proteomic Analyses of LOXs from Cucumber Flowers, Leaves, and Fruits
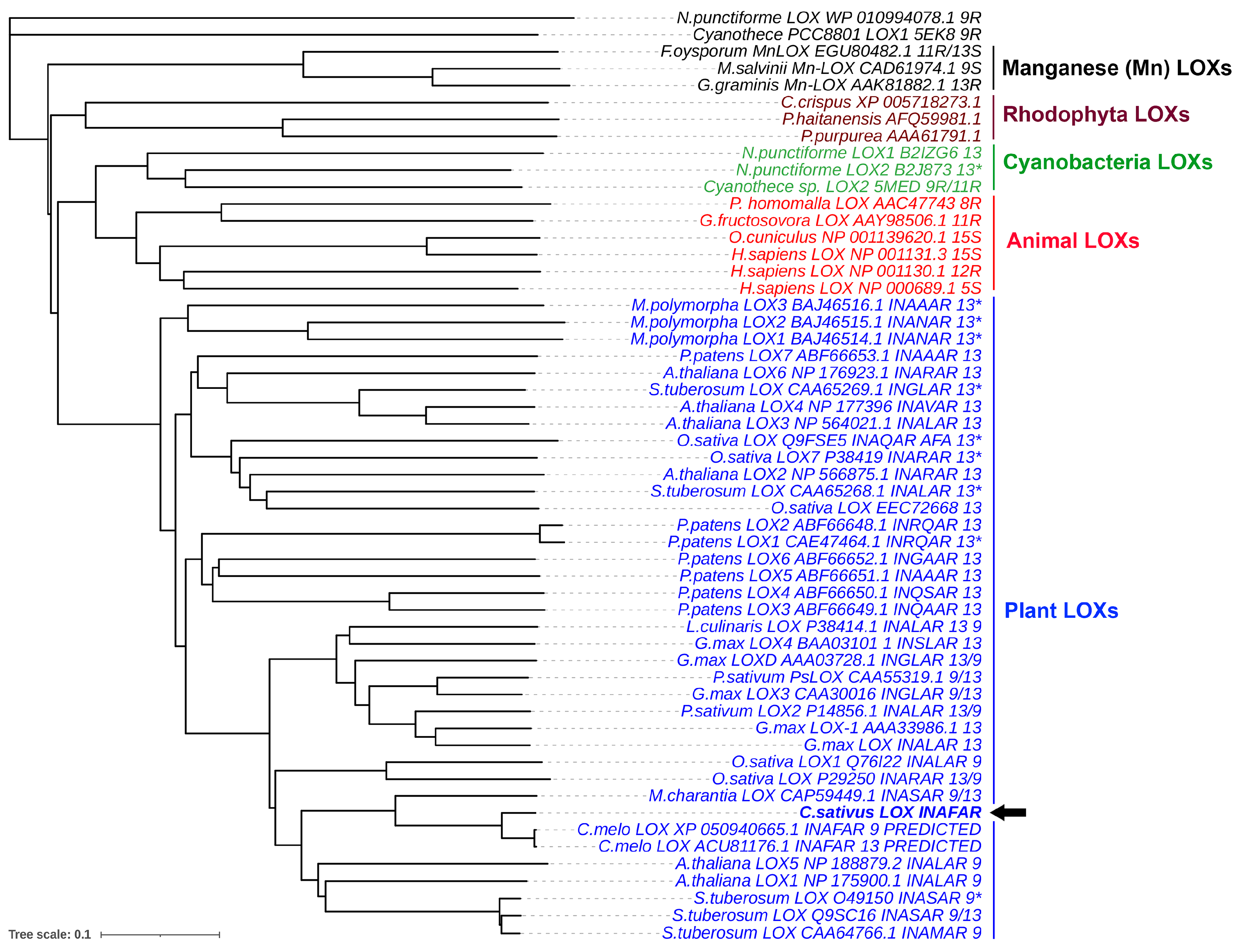
2.4. Molecular Cloning of CsLOX3 and Identification of the Recombinant Protein as 16(S)-LOX
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. In Vitro Incubations of α-Linolenic Acid with Crude Cucumber Enzyme Preparations
4.4. Product Analyses
4.5. Isolation and Identification of Products
4.6. The Targeted Proteomic Analyses of Cucumber Flowers and Fruit Peel Samples
4.7. Bioinformatics and Phylogenetic Analysis
4.8. Molecular Cloning of Cucumber CsLOX3 Gene
4.9. Expression and Purification of Recombinant CsLOX3 Enzyme
4.10. Kinetic Studies of Recombinant CsLOX3
4.11. Micropreparative Isolation and Purification of Selected Products
4.12. GC-MS Analyses
4.13. NMR Spectroscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grechkin, A. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog. Lipid Res. 1998, 37, 317–352. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Ann. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Banthiya, S.; Van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef]
- Umate, P. Genome-wide analysis of lipoxygenase gene family in Arabidopsis and rice. Plant Signal. Behav. 2011, 6, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.W.S.; Levett, G. Autoxidation of methyl linolenate: Analysis of methyl hydroxylinolenate isomers by high performance liquid chromatography. Lipids 1977, 12, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Weichert, H.; Porzel, A.; Wasternack, C.; Kühn, H.; Feussner, I. Enzymatic and non-enzymatic lipid peroxidation in leaf development. Biochim. Biophys. Acta 2001, 1533, 266–276. [Google Scholar] [CrossRef]
- Pollio, A.; Della Greta, M.; Monaco, P.; Pinto, G.; Previtera, L. Lipid composition of the acidophilic alga Dunaliella acidophila (Volvocales, Chlorophyta) I. Non-polar lipids. Biochim. Biophys. Acta 1988, 963, 53–60. [Google Scholar] [CrossRef]
- Aliotta, G.; Monaco, P.; Pinto, G.; Pollio, A.; Previtera, L. Potential allelochemicals from Pistia stratiotes L. J. Chem. Ecol. 1991, 17, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Previtera, L.; Monaco, P. Fatty acid composition in Lemna minor—Characterization of a novel hydroxy C16 acid. Phytochemistry 1983, 22, 1445–1446. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yaoita, Y.; Kikuchi, M. Monohydroxy-substituted polyunsaturated fatty acids from Swertia japonica. Helv. Chim. Acta 2008, 91, 1857–1862. [Google Scholar] [CrossRef]
- Pagani, A.; Scala, F.; Chianese, G.; Grassi, G.; Appendino, G.; Taglialatela-Scafati, O. Cannabioxepane, a novel tetracyclic cannabinoid from hemp, Cannabis sativa L. Tetrahedron 2011, 67, 3369–3373. [Google Scholar] [CrossRef]
- Awouafack, M.D.; Aimaiti, S.; Tane, P.; Morita, H. Clerodendrumol, a new triterpenoid from Clerodendrum yaundense Gürke (Lamiaceae). Helv. Chim. Acta 2016, 99, 161–164. [Google Scholar] [CrossRef]
- Kato, T.; Yamaguchi, Y.; Hirano, T.; Yokoyama, T.; Uyehara, T.; Namai, T.; Yamanaka, S.; Harada, N. Unsaturated hydroxy fatty acids, the self defensive substances in rice plant against rice blast disease. Chem. Lett. 1984, 13, 409–412. [Google Scholar] [CrossRef]
- Gao, B.; Boeglin, W.E.; Brash, A.R. Omega-3 fatty acids are oxygenated at the n-7 carbon by the lipoxygenase domain of a fusion protein in the cyanobacterium Acaryochloris marina. Biochim. Biophys. Acta 2010, 1801, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Newie, J.; Andreou, A.; Neumann, P.; Einsle, O.; Feussner, I.; Ficner, R. Crystal structure of a lipoxygenase from Cyanothece sp. may reveal novel features for substrate acquisition. J. Lipid Res. 2016, 57, 276–287. [Google Scholar] [CrossRef] [PubMed]
- An, J.U.; Lee, I.G.; Ko, Y.J.; Oh, D.K. Microbial synthesis of linoleate 9S-lipoxygenase derived plant C18 oxylipins from C18 polyunsaturated fatty acids. J. Agric. Food Chem. 2019, 67, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Schlag, E.W.; Peatman, W.B. The thermal cyclization of hexafluorobutadiene to hexafluorocyclobutene. J. Am. Chem. Soc. 1964, 86, 1676–1679. [Google Scholar] [CrossRef]
- Shumate, K.M.; Neuman, P.N.; Fonken, G.J. The Thermal Cyclization of 1, 3-Dienes to Cyclobutenes. J. Am. Chem. Soc. 1965, 87, 3996. [Google Scholar] [CrossRef]
- Ogletree, M.L.; Schlesinger, K.; Nettleman, M.; Hubbard, W.C. Measurement of 5-hydroxyeicosatetraenoic acid (5-HETE) in biological fluids by GC-MS. Methods Enzymol. 1982, 86, 607–612. [Google Scholar] [PubMed]
- Matsui, K.; Nishioka, M.; Ikeyoshi, M.; Matsumura, Y.; Mori, T.; Kajiwara, T. Cucumber root lipoxygenase can act on acyl groups in phosphatidylcholine. Biochim. Biophys. Acta 1998, 1390, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Small, M.; Serhan, C.N.; Libreros, S.; Nshimiyimana, R. E-series resolvin metabolome, biosynthesis and critical role of stereochemistry of specialized pro-resolving mediators (SPMs) in inflammation-resolution: Preparing SPMs for long COVID-19, human clinical trials, and targeted precision nutrition. Semin. Immunol. 2022, 59, 101597. [Google Scholar]
- Kato, T.; Nakai, T.; Ishikawa, R.; Karasawa, A.; Namai, T. Preparation of the enantiomers of hydroxy-C18 fatty acids and their anti-rice blast fungus activities. Tetrahedron Asymmetry 2001, 12, 2695–2701. [Google Scholar] [CrossRef]
- Trapp, M.A.; Kai, M.; Mithöfer, A.; Rodrigues-Filho, E. Antibiotic oxylipins from Alternanthera brasiliana and its endophytic bacteria. Phytochemistry 2015, 110, 72–82. [Google Scholar] [CrossRef]
- Dong, M.; Oda, Y.; Hirota, M. (10E,12Z,15Z)-9-Hydroxy-10,12,15-octadecatrienoic acid methyl ester as an anti-inflammatory compound from Ehretia dicksonii. Biosci. Biotechnol. Biochem. 2000, 64, 882–886. [Google Scholar] [CrossRef]
- Youn, B.; Sellhorn, G.E.; Mirchel, R.J.; Gaffney, B.J.; Grimes, H.D.; Kang, C. Crystal Structures of Vegetative Soybean Lipoxygenase VLX-B and VLX-D, and Comparisons with Seed Isoforms LOX-1 and LOX-3. Proteins Struct. Funct. Genet. 2006, 65, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; De Paolis, A.; Gallo, A.; Quarta, A.; Casey, R.; Mita, G. Biochemical and molecular characterization of hazelnut (Corylus avellana) seed lipoxygenases. Eur. J. Biochem. 2003, 270, 4365–4375. [Google Scholar] [CrossRef]
- Liu, S.; Han, B. Differential expression pattern of an acidic 9/13-lipoxygenase in flower opening and senescence and in leaf response to phloem feeders in the tea plant. BMC Plant Biol. 2010, 10, 228. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Tarchevsky, I.A.; Egorova, A.M. Proteomic analysis of cycloheximide influence on pea roots. Rus. J. Plant Phys. 2015, 62, 883–895. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Rzhetsky, A.; Nei, M. A simple method for estimating and testing minimum evolution trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
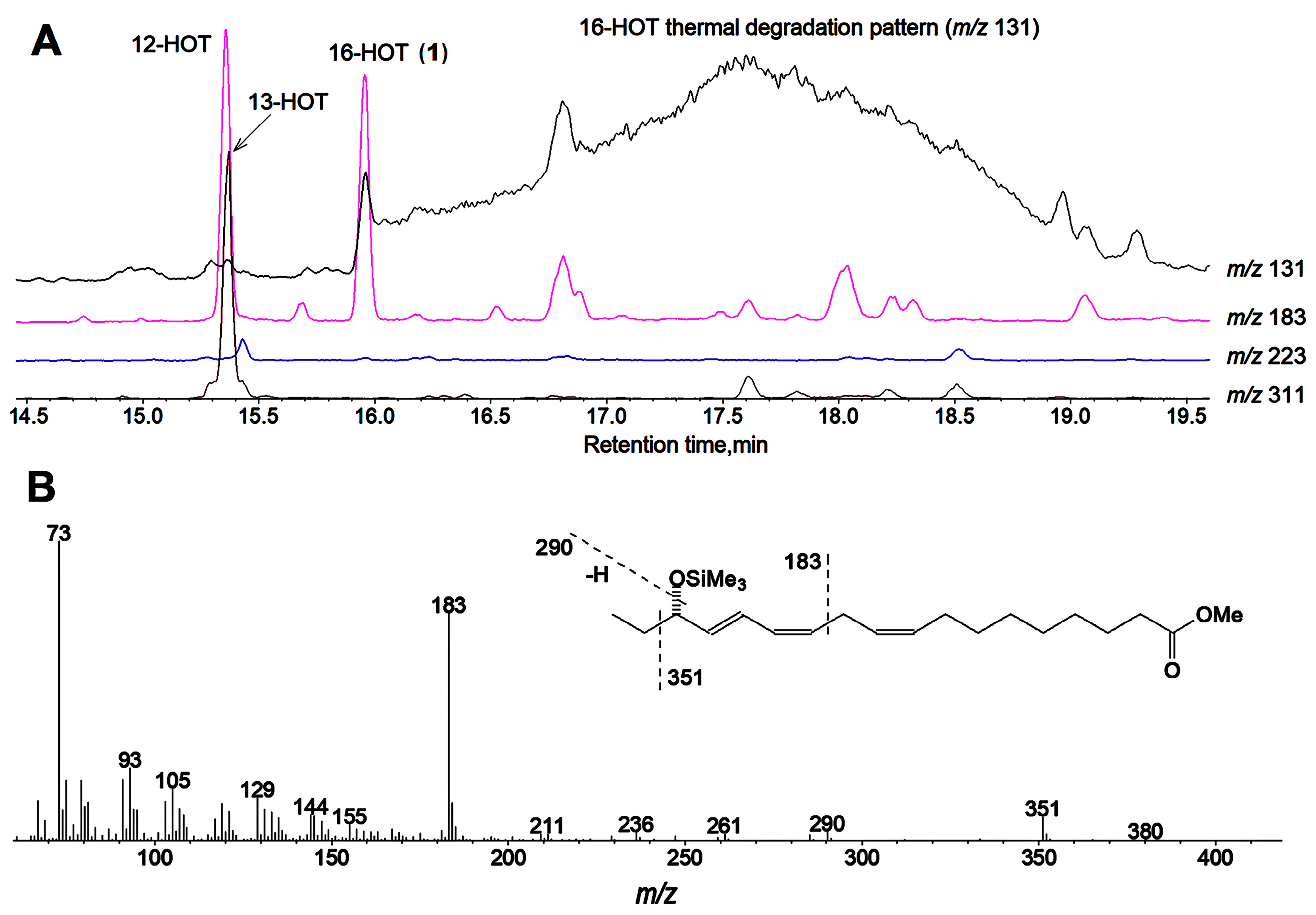
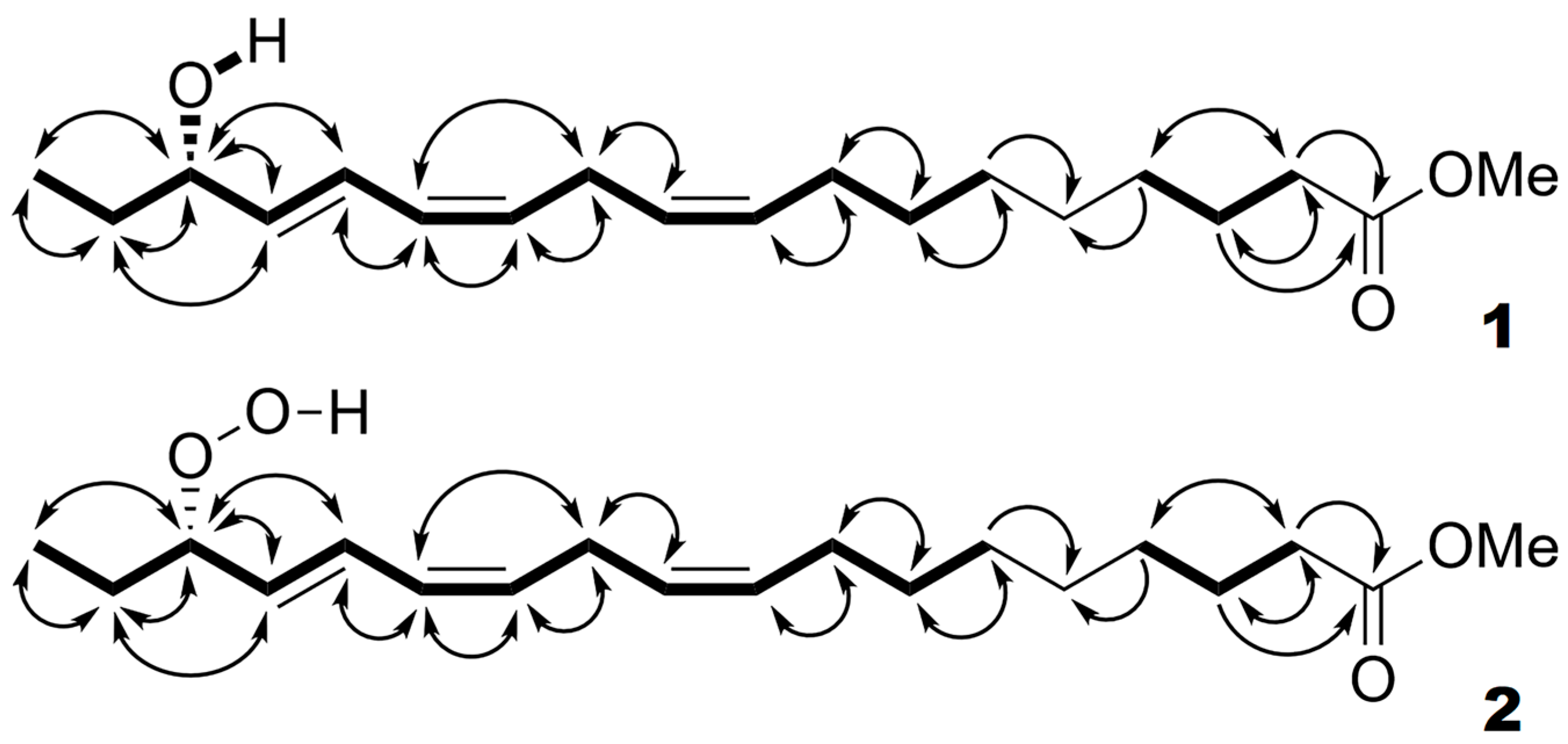


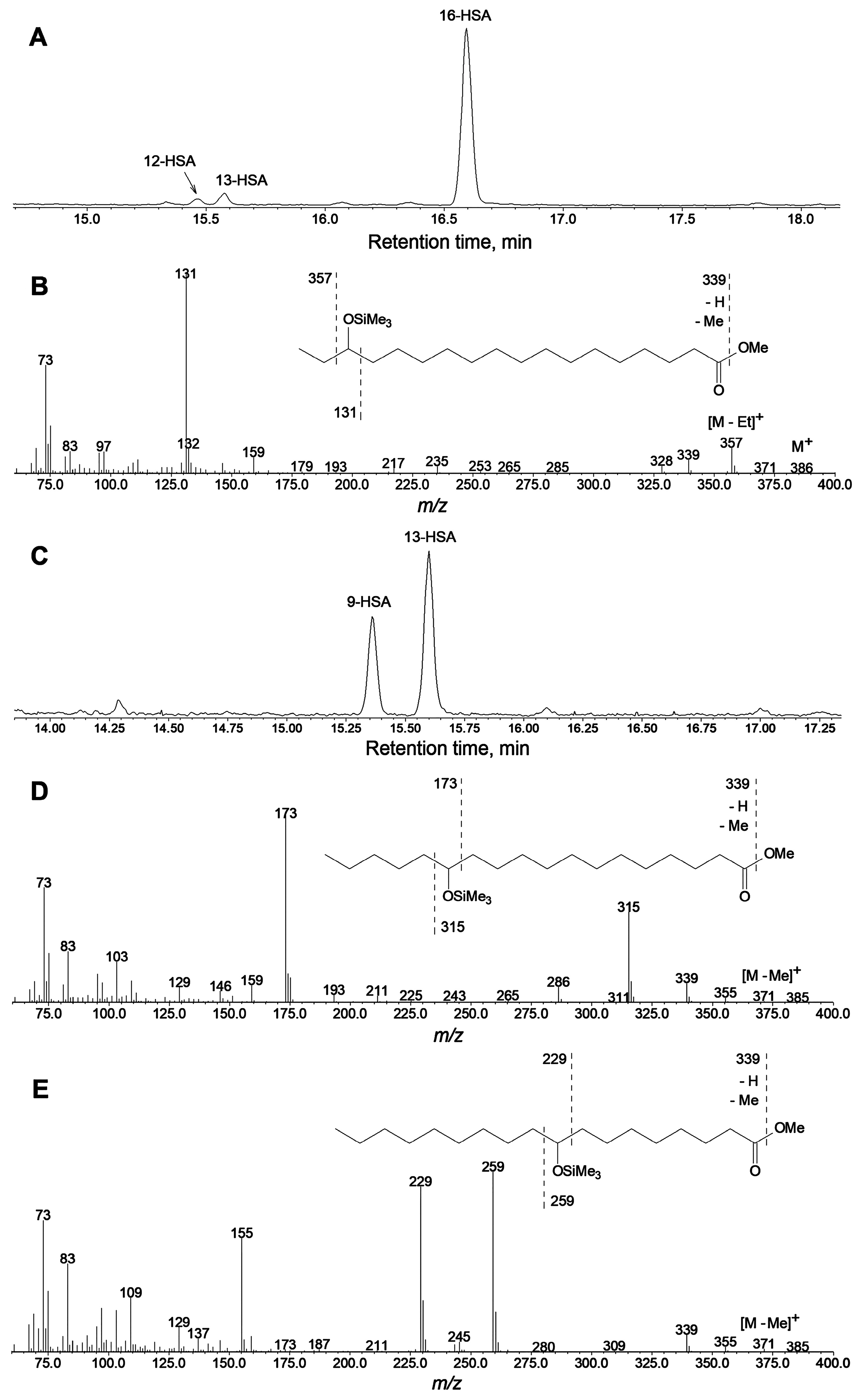
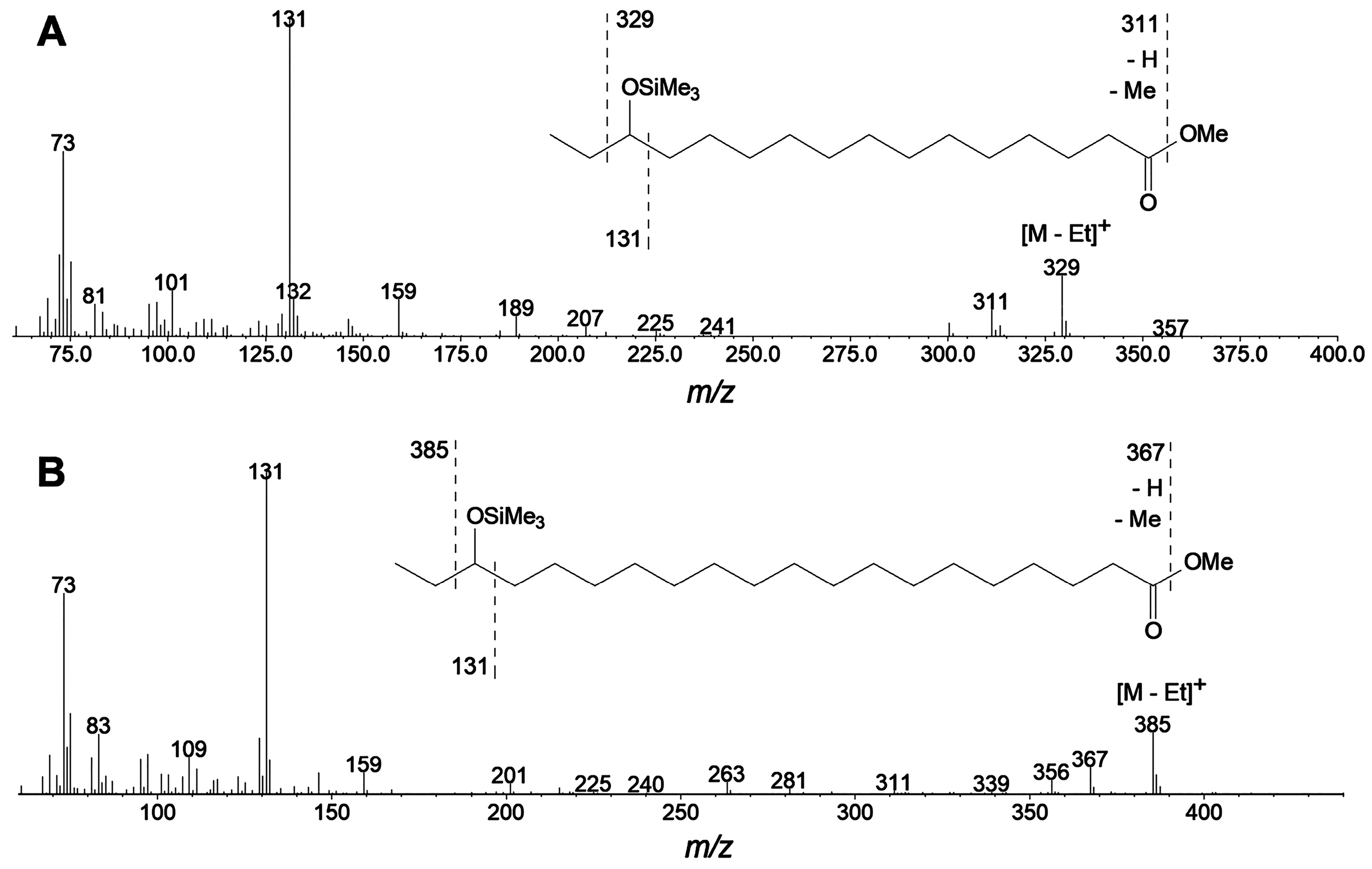


| Position Number | 13C Chemical Shifts (ppm), Functional Group | 1H Chemical Shifts (ppm), Multiplicity, Coupling Constant (Hz) | 2D NMR Correlations | ||
|---|---|---|---|---|---|
| COSY | TOCSY | HMBC | |||
| 1 | 173.74, COOMe | COOMe | |||
| 2 | 34.04, CH2 | 2.10, t, 7.5 (H3) | H3 | H3, H4 | C1, C3, C4 |
| 3 | 25.17, CH2 | 1.54, m | H2, H4 | H2, H4–H8 | C1, C2, C4 |
| 4 | 29.0–29.5, CH2 | 1.16, m | H3 | H2, H3, H7, H8 | C2, C3 |
| 5 | 29.0–29.5, CH2 | 1.15–1.20, m | |||
| 6 | 29.20, CH2 | 1.16, m | H7 | H2–H11 | |
| 7 | 29.68, CH2 | 1.27, m | H6, H8 | H2–H6, H8–H11 | C8, C9 |
| 8 | 27.43, CH2 | 2.01, m | H7, H9 | H3–H7, H9–H11 | C7, C9, C10 |
| 9 | 131.15, CH | 5.42–5.46, AB | H8, H10 | H6–H8, H11–H131 | C7, C8, C11 |
| 10 | 127.58, CH | 5.42–5.46, AB | H8, H9, H11 | H6–H9, H11–H14 | C8, C11, C12 |
| 11a | 26.46, CH | 2.87–2.98, AB | H9, H10, H12, H15 | H6–H10, H12, H13–H16 | C9, C10, C12, C13 |
| 11b | 2.87–2.98, AB | ||||
| 12 | 131.15, CH | 5.44, m | H11, H13 | H11, H13–H15 | C10, C11, C14 |
| 13 | 128.41, CH | 6.02, dddt, 11.1 (H14), 10.9 (H12), 0.8 (H15), 1.6 (H11a,b) | H12, H14 | H11, H12, H14–H16 | C11, C14, C15 |
| 14 | 129.11, CH | 6.65, dddd, 15.3 (H15), 11.1 (H13), 1.0 (H12), 1.0 (H16) | H13, H15 | H11–H13, H15–H18 | C12, C13, C15, C16 |
| 15 | 133.08, CH | 5.54, dd, 15.3 (H14), 7.8 (H16) | H11, H14, H16 | H12–H14, H16–H18 | C13, C16, C17 |
| 16 | 87.43, CH | 4.24, dddd, 7.8 (H15), 7.0 (H17a), 6.8 (H17b), 0.9 (H14) | H15, H17, H18 | H14, H15, H17, H18 | C14, C15, C17, C18 |
| 17a | 25.98, CH2 | 1.43, ddq, 14.1 (H17b), 7.0 (H16), 7.5 (H18) | H16, H18 | H13–H18 | C15, C16, C18 |
| 17b | 1.63, ddq, 14.1 (H17a), 6.8 (H16), 7.5 (H18) | ||||
| 18 | 9.68, CH3 | 0.84, t, 7.5 (H17a,b) | H16, H17 | H16, H17a, 17b | C16, C17 |
| (1) | 50.98; COOMe | 3.36, s | COOMe | ||
| Position Number | 13C Chemical Shifts (ppm), Functional Group | 1H Chemical Shifts (ppm), Multiplicity, Coupling Constant (Hz) | 2D NMR Correlations | ||
|---|---|---|---|---|---|
| COSY | TOCSY | HMBC | |||
| 1 | 173.62, COOMe | COOMe | |||
| 2 | 34.04, CH2 | 2.11, t, 7.5 (H3) | H3 | H3, H4 | C1, C3, C4 |
| 3 | 25.17, CH2 | 1.55, m | H2, H4 | H2, H4–H8 | C1, C2, C4 |
| 4 | 29.0–29.6, CH2 | 1.16, m | H3 | H2, H3, H7, H8 | C2, C3 |
| 5 | 29.0–29.6, CH2 | 1.15–1.20, m | |||
| 6 | 29.36, CH2 | 1.19, m | H7 | H2, H3, H4–11 | |
| 7 | 29.68, CH2 | 1.28, m | H6, H8 | H2–H6, H8–H11 | C8, C9 |
| 8 | 27.42, CH2 | 2.02, dt, 7.0 (H7), 7.0 (H9) | H7, H9 | H3–H7, H9–H11 | C7, C9, C10 |
| 9 | 130.99, CH | 5.43–5.46, AB | H8, H10 | H6–H8, H11–H13 | C8, C11 |
| 10 | 127.76, CH | 5.43–5.46, AB | H8, H9, H11 | H6–H9, H11–H14 | C8, C11 |
| 11 | 26.46, CH2 | 2.97, m | H9, H10, H12, H15 | H6–H10, H12–H16 | C9, C10, C12, C13, C14 |
| 12 | 130.18, CH | 5.45, m | H11, H13 | H11, H13–H15 | C11, C14 |
| 13 | 128.73, CH | 6.04, dddt, 11.0 (H14), 10.9 (H12), 0.8 (H15), 1.6 (H11a,b) | H12, H14 | H11, H12, H14–H16 | C14, C15 |
| 14 | 125.34, CH | 6.60, dddd, 15.2 (H15), 11.0 (H13), 1.2 (H12), 1.2 (H16) | H13, H15 | H11–H13, H15–H18, CHOH | C12, C13, C16 |
| 15 | 137.44, CH | 5.58, dd, 15.2 (H14), 6.3 (H16) | H11, H14, H16 | H12–H14, H16–H18, CHOH | C12, C13, C16, C17 |
| 16 | 73.72, CH | 3.88, m | H15, H17, H18 | H11, H14, H15, H17, H18, CHOH | |
| 17a | 30.65, CH2 | 1.41, m | H16, H18 | H14–H18, CHOH | C15, C16, C18 |
| 17b | 1.46, m | ||||
| 18 | 9.68, CH3 | 0.85 t, 7.5 (H17a,b) | H16, H17 | H15, H16, H17, CHOH | C16, C17 |
| (1) | 50.81; COOMe | 3.35, s | COOMe | ||
| (16) | CHOH | 1.04, d, 4.1 (H16) | H14–H18 | ||
| Substrate | kcat (s−1) | KM (μM) | kcat/KM (μM−1∙s−1) | Specificity (%) |
|---|---|---|---|---|
| 18:2, linoleic acid | 46 | 21 | 2.2 | 30.6 |
| 18:3, α-linolenic acid | 43 | 6 | 7.2 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorina, S.S.; Egorova, A.M.; Lantsova, N.V.; Toporkova, Y.Y.; Grechkin, A.N. Discovery of α-Linolenic Acid 16(S)-Lipoxygenase: Cucumber (Cucumis sativus L.) Vegetative Lipoxygenase 3. Int. J. Mol. Sci. 2023, 24, 12977. https://doi.org/10.3390/ijms241612977
Gorina SS, Egorova AM, Lantsova NV, Toporkova YY, Grechkin AN. Discovery of α-Linolenic Acid 16(S)-Lipoxygenase: Cucumber (Cucumis sativus L.) Vegetative Lipoxygenase 3. International Journal of Molecular Sciences. 2023; 24(16):12977. https://doi.org/10.3390/ijms241612977
Chicago/Turabian StyleGorina, Svetlana S., Alevtina M. Egorova, Natalia V. Lantsova, Yana Y. Toporkova, and Alexander N. Grechkin. 2023. "Discovery of α-Linolenic Acid 16(S)-Lipoxygenase: Cucumber (Cucumis sativus L.) Vegetative Lipoxygenase 3" International Journal of Molecular Sciences 24, no. 16: 12977. https://doi.org/10.3390/ijms241612977
APA StyleGorina, S. S., Egorova, A. M., Lantsova, N. V., Toporkova, Y. Y., & Grechkin, A. N. (2023). Discovery of α-Linolenic Acid 16(S)-Lipoxygenase: Cucumber (Cucumis sativus L.) Vegetative Lipoxygenase 3. International Journal of Molecular Sciences, 24(16), 12977. https://doi.org/10.3390/ijms241612977




