Quality Control Optimization for Minimizing Security Risks Associated with Mesenchymal Stromal Cell-Based Product Development
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Advanced Therapy Medicinal Product
4.3. Quality Control
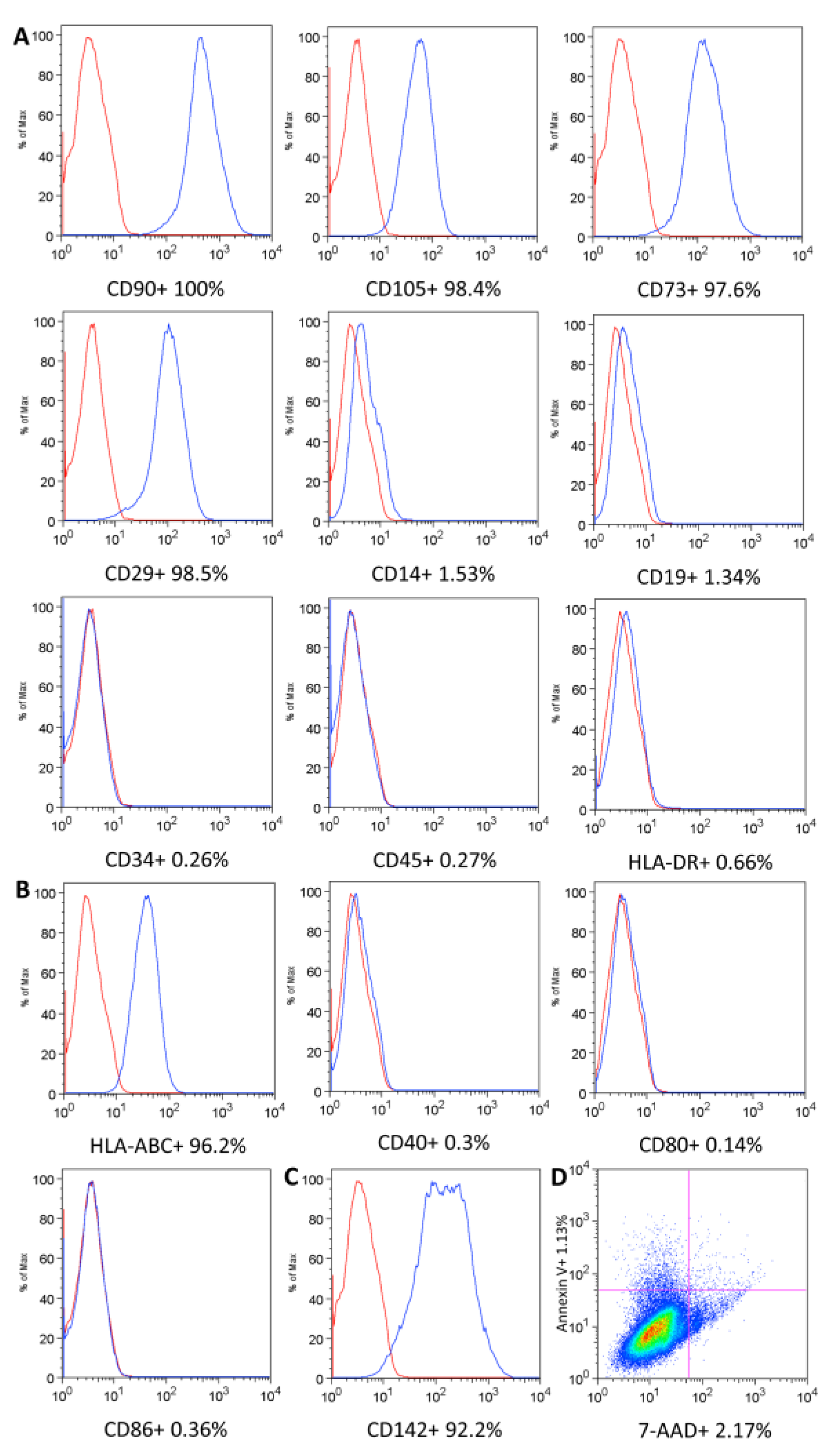
4.3.1. UC-MSC Surface Marker Expression, Cell Viability, and Immunogenicity
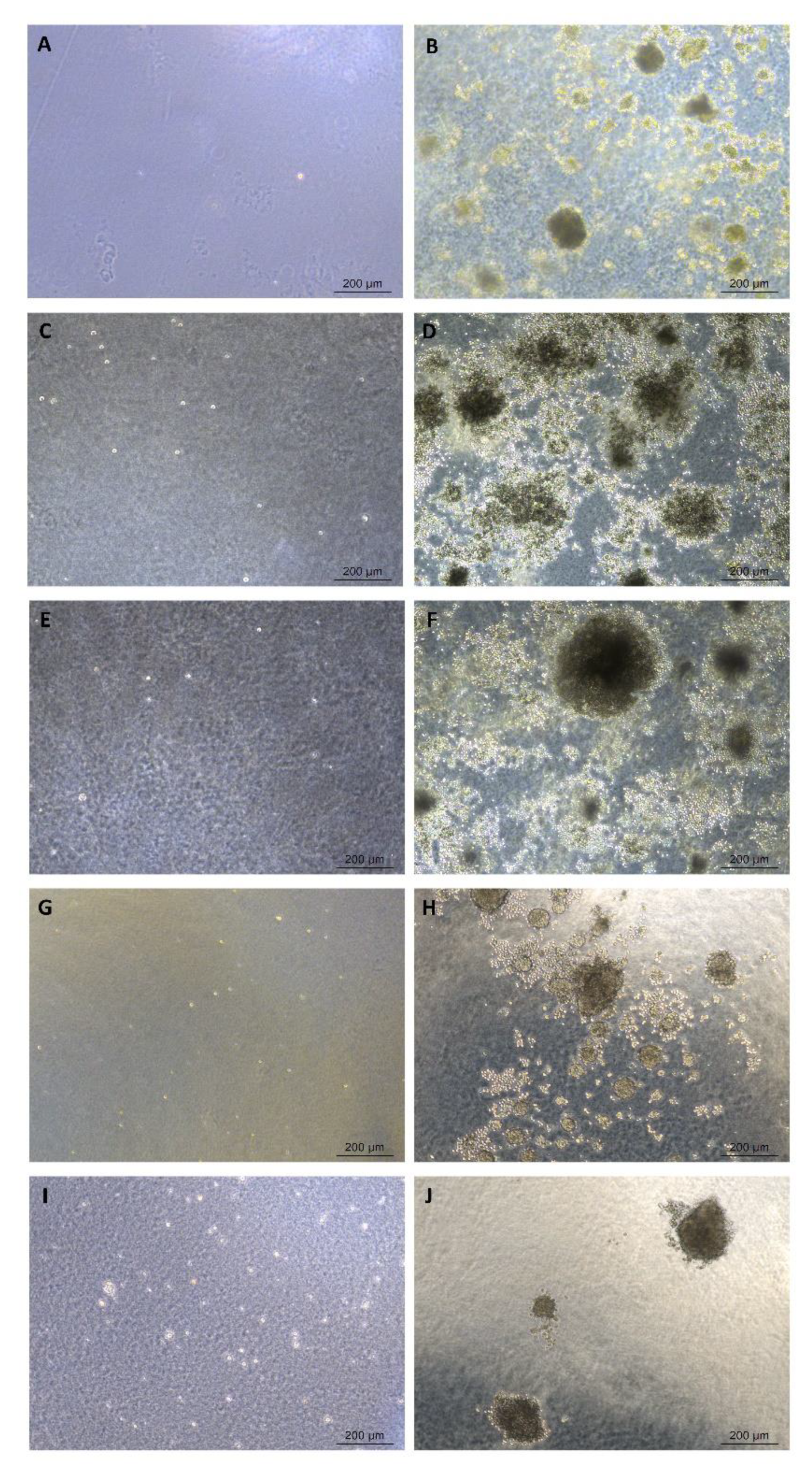
4.3.2. Cell Differentiation
4.3.3. Microbiological Tests
4.3.4. Purity Test
4.3.5. Genomic Stability
4.3.6. Cytokinesis-Block Micronucleus Assay
4.3.7. Soft Agar Colony Formation Assay
4.3.8. Potency Test—Inhibition of T-Lymphocyte Proliferation Assay
4.3.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Parekkadan, B.; Milwid, J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Guadix, J.A.; Zugaza, J.L.; Gálvez-Martín, P. Characteristics, applications and prospects of mesenchymal stem cells in cell therapy. Med. Clin. 2017, 148, 408–414. [Google Scholar] [CrossRef]
- ANVISA Brazilian Health Regulatory Agency. Resolution RDC No 508, 27 May 2021. Provides for Good Practices in Human Cells for Therapeutic Use and Clinical Research and Makes Other Provisions. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2020/rdc0508_27_05_2021.pdf (accessed on 1 February 2023).
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016, 2016, 6901286. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jeong, S.Y.; Ha, J.; Kim, M.; Jin, H.J.; Kwon, S.J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2014, 446, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.K.; Ogando, C.R.; See, C.W.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Cuende, N.; Rasko, J.E.J.; Koh, M.B.C.; Dominici, M.; Ikonomou, L. Cell, tissue and gene products with marketing authorization in 2018 worldwide. Cytotherapy 2018, 20, 1401–1413. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Rousseau, C.F.; Mačiulaitis, R.; Śladowski, D.; Narayanan, G. Cell and gene therapies: European view on challenges in translation and how to address them. Front. Med. 2018, 5, 158. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Concise review: Challenges in clinical development of mesenchymal stromal/stem cells. Stem Cells Transl. Med. 2019, 11, 1135–1148. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Guadix, J.A.; López-Beas, J.; Clares, B.; Soriano-Ruiz, J.L.; Zugaza, J.L.; Gálvez-Martín, P. Principal criteria for evaluating the quality, safety and efficacy of hmsc-based products in clinical practice: Current approaches and challenges. Pharmaceutics 2019, 11, 552. [Google Scholar] [CrossRef]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021, 12, 152. [Google Scholar] [CrossRef]
- Santilli, F.; Fabrizi, J.; Pulcini, F.; Santacroce, C.; Sorice, M.; Delle-Monache, S.; Mattei, V. Gangliosides and their role in multilineage differentiation of mesenchymal stem cells. Biomedicines 2022, 10, 3112. [Google Scholar] [CrossRef] [PubMed]
- Mattar, P.; Bieback, K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front. Immunol. 2015, 6, 560. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wangs, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef]
- Song, S.W.; Kim, K.E.; Choi, J.W.; Lee, C.; Lee, J.; Seo, H.H.; Lim, K.H.; Lim, S.; Lee, S.; Kim, S.W.; et al. Proteomic analysis and identifcation of paracrine factors in mesenchymal stem cell-conditioned media under hypoxia. Cell Physiol. Biochem. 2016, 40, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Romaldini, A.; Mastrogiacomo, M.; Cancedda, R.; Descalzi, F. Platelet lysate activates human subcutaneous adipose tissue cells by promoting cell proliferation and their paracrine activity toward epidermal keratinocytes. Front. Bioeng. Biotechnol. 2018, 6, 203. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef]
- Shaer, A.; Azarpira, N.; Aghdaie, M.H.; Esfandiari, E. Isolation and characterization of human mesenchymal stromal cells derived from placental decidua basalis; umbilical cord wharton’s jelly and amniotic membrane. Pak. J. Med. Sci. 2014, 30, 1022–1026. [Google Scholar] [PubMed]
- Voisin, C.; Cauchois, G.; Reppel, L.; Laroye, C.; Louarn, L.; Schenowitz, C.; Sonon, P.; Poras, I.; Wang, V.; Carosella, E.D.; et al. Are the immune properties of mesenchymal stem cells from wharton’s jelly maintained during chondrogenic differentiation? J. Clin. Med. 2020, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Mei, S.H.J.; Wolfe, D.; Champagne, J.; Fergusson, D.; Stewart, D.J.; Sullivan, K.J.; Doxtator, E.; Lalu, M.; English, S.W.; et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 19, 100249. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Z.H.; Wei, L.F.; Yang, H.N.; Yang, S.N.; Zhu, Z.Y.; Wamg, P.; Zhao, C.P.; Bi, J.Z. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res. Ther. 2013, 4, 76. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Cong, X.; Liu, G.; Zhou, J.; Bai, B.; Li, Y.; Bai, W.; Li, M.; Ji, H.; et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem Cells Dev. 2013, 22, 3192–3202. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Manuchehrabadi, N.; Weir, M.D.; Zhu, Z.; Xu, H.H. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials 2013, 34, 9917–9925. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J. The mesenchymal stromal cells dilema-does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 2013, 15, 2–8. [Google Scholar] [CrossRef]
- Phinney, D.G. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J. Cell. Biochem. 2012, 113, 2806–2812. [Google Scholar] [CrossRef]
- Can, A.; Celikkan, F.T.; Cinar, O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy 2017, 19, 1351–1382. [Google Scholar] [CrossRef]
- Secco, M.; Zucconi, E.; Vieira, N.M.; Fogaça, L.L.Q.; Cerqueira, A.; Carvalho, M.D.F. Mesenchymal stem cells from umbilical cord: Do not discard the cord. Neuromuscul. Disord. 2008, 18, 17–18. [Google Scholar] [CrossRef]
- Cooper, K.; Viswanathan, C. Establishment of a mesenchymal stem cell bank. Stem Cells Int. 2011, 2011, 905621. [Google Scholar] [CrossRef]
- Mori, Y.; Ohshimo, J.; Shimazu, T.; He, H.; Takahashi, A.; Yamamoto, Y.; Tsunoda, H.; Tojo, A.; Nagamura-Inoue, T. Improved explant method to isolate umbilical cord-derived mesenchymal stem cells and their immunosuppressive properties. Tissue Eng. Part C Methods 2015, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Salehinejad, P.; Alitheen, N.B.; Ali, A.M.; Omar, A.R.; Mohit, M.; Janzamin, E.; Samani, F.S.; Troshizi, Z.; Nematollahi-Mahani, S.N. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. Vitr. Cell Dev. Biol. Anim. 2012, 48, 75–83. [Google Scholar] [CrossRef]
- Han, Y.F.; Tao, R.; Sun, T.J.; Chai, J.K.; Xu, G.; Liu, J. Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology 2013, 65, 819–827. [Google Scholar] [CrossRef]
- Margossian, T.; Reppel, L.; Makdissy, N.; Stoltz, J.F.; Bensoussan, D.; Huselstein, C. Mesenchymal stem cells derived from Wharton’s jelly: Comparative phenotype analysis between tissue and in vitro expansion. Biomed. Mater. Eng. 2012, 22, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Majore, I.; Moretti, P.; Stahl, F.; Hass, R.; Kasper, C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2011, 7, 17–31. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.B.; Song, Y.P.; Han, Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012, 2012, 652034. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le-Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kim, J.H.; Jo, C.H.; Kim, H.R.; Wang, Y. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018, 2018, 8429042. [Google Scholar] [CrossRef]
- Fazzina, R.; Iudicone, P.; Fioravanti, D.; Bonanno, G.; Totta, P.; Zizzari, I.G.; Pierelli, L. Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues. Stem Cell Res. Ther. 2016, 7, 122. [Google Scholar] [CrossRef]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Pro-coagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83, S164–S169. [Google Scholar] [CrossRef]
- Moll, G.; Ankrum, J.A.; Olson, S.D.; Nolta, J.A. Improved MSC minimal criteria to maximize patient safety: A call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem Cells Transl. Med. 2022, 11, 2–13. [Google Scholar] [CrossRef]
- Can, A.; Karahuseyinoglu, S. Concise review: Human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Datta, I.; Mishra, S.; Mohanty, L.; Pulikkot, S.; Joshi, P.G. Neuronal plasticity of human Wharton’s jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells. Cytotherapy 2011, 13, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.S.; Bhonde, R.R. Islet neogenesis from the constitutively nestin expressing human umbilical cord matrix derived mesenchymal stem cells. Islets 2010, 2, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Li, D.R.; Cai, J.H. Methods of isolation, expansion, differentiating induction and preservation of human umbilical cord mesenchymal stem cells. Chin. Med. J. 2012, 125, 4504–4510. [Google Scholar]
- Han, Y.; Chai, J.; Sun, T.; Li, D.; Tao, R. Differentiation of human umbilical cord mesenchymal stem cells into dermal fibroblasts in vitro. Biochem. Biophys. Res. Commun. 2011, 413, 561–565. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, T.; Xu, F.; Li, Y.; Wu, M.; Zhu, D.; Cong, X.; Liu, Y. How important is differentiation in the therapeutic effect of mesenchymal stromal cells in liver disease? Cytotherapy 2014, 16, 309–318. [Google Scholar] [CrossRef]
- Yang, S.; Ma, K.; Feng, C.; Wu, Y.; Wang, Y.; Huang, S.; Fu, X. Capacity of human umbilical cord-derived mesenchymal stem cells to differentiate into sweat gland-like cells: A preclinical study. Front. Med. 2013, 7, 345–353. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Chen, L.; Ye, L.; Cui, J.; Sun, Q.; Li, K.; Li, Z.; Liu, L. Human umbilical cord mesenchymal stem cells: A new therapeutic option for tooth regeneration. Stem Cells Int. 2015, 2015, 549432. [Google Scholar] [CrossRef]
- Reppel, L.; Schiavi, J.; Charif, N.; Leger, L.; Yu, H.; Pinzano, A.; Henrionnet, C.; Stoltz, J.F.; Bensoussan, D.; Huselstein, C. Chondrogenic induction of mesenchymal stromal/stem cells from Wharton’s jelly embedded in alginate hydrogel and without added growth factor: An alternative stem cell source for cartilage tissue engineering. Stem Cell Res. Ther. 2015, 6, 260. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Fan, Z.; Liu, D.; Wang, F.; Zhou, Y. IGFBP2 enhances adipogenic differentiation potentials of mesenchymal stem cells from Wharton’s jelly of the umbilical cord via JNK and Akt signaling pathways. PLoS ONE 2017, 12, e0184182. [Google Scholar] [CrossRef]
- Messerli, M.; Wagner, A.; Sager, R.; Mueller, M.; Baumann, M.; Surbek, D.V.; Schoeberlein, A. Stem cells from umbilical cord Wharton’s jelly from preterm birth have neuroglial differentiation potential. Reprod. Sci. 2013, 20, 1455–1464. [Google Scholar] [CrossRef]
- Kim, J.; Piao, Y.; Pak, Y.K.; Chung, D.; Han, Y.M.; Hong, J.S.; Jun, E.J.; Shim, J.Y.; Choi, J.; Kim, C.J. Umbilical cord mesenchymal stromal cells affected by gestational diabetes mellitus display premature aging and mitochondrial dysfunction. Stem Cells Dev. 2015, 24, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. MSC Committee of the International Society for Cellular Therapy (ISCT): Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Bovenkamp, M.; Hoefnagel, M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy 2017, 19, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.C.; Tucker, H.A.; Bunnell, B.A.; Andreeff, M.; Schober, W.; Gaynor, A.S.; Stricker, K.L.; Lin, S.; Lacey, M.R.; O’Connor, K.C. Cell-surface expression of neuron-glial antigen 2 (NG2) and melanoma cell adhesion molecule (CD146) in heterogeneous cultures of marrow-derived mesenchymal stem cells. Tissue Eng. Part A 2013, 19, 2253–2266. [Google Scholar] [CrossRef]
- Wang, L.T.; Liu, K.J.; Sytwu, H.K.; Yen, M.L.; Yen, B.L. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl. Med. 2021, 10, 1288–1303. [Google Scholar] [CrossRef]
- Song, Y.; Lim, J.Y.; Lim, T.; Im, K.I.; Kim, N.; Nam, Y.S.; Jeon, Y.W.; Shin, J.C.; Ko, H.S.; Park, I.Y.; et al. Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms. World J. Stem Cells 2020, 12, 1032–1049. [Google Scholar] [CrossRef]
- Shawki, S.; Gaafar, T.; Erfan, H.; El-Khateeb, E.; El-Sheikhah, A.; El-Hawary, R. Immunomodulatory effects of umbilical cord-derived mesenchymal stem cells. Microbiol. Immunol. 2015, 59, 348–356. [Google Scholar] [CrossRef]
- Wagner, W.; Frobel, J.; Goetzke, R. Epigenetic quality check–How good are your mesenchymal stromal cells? Epigenomics 2016, 8, 889–894. [Google Scholar] [CrossRef]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef]
- Catalina, P.; Montes, R.; Ligero, G.; Sanchez, L.; Cueva, T.; Bueno, C.; Leone, P.E.; Menendez, P. Human ESCs predisposition to karyotypic instability: Is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol. Cancer 2008, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Sensebé, L.; Bourin, P. Mesenchymal stem cells for therapeutic purposes. Transplantation 2009, 87, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, T.; Solarewicz, M.M.; Vaz, I.M.; Daga, D.R.; Rebelatto, C.L.K.; Senegaglia, A.C.; Ribeiro, E.; Cavalli, I.J.; Brofman, P.R.S. Emergence of clonal chromosomal alterations during the mesenchymal stromal cell cultivation. Mol. Cytogenet. 2015, 8, 94. [Google Scholar] [CrossRef]
- Karahuseyinoglu, S.; Cinar, O.; Kilic, E.; Kara, F.; Akay, G.G.; Demiralp, D.O.; Tukun, A.; Uckan, D.; Can, A. Biology of stem cells in human umbilical cord stroma: In situ and in vitro surveys. Stem Cells 2007, 25, 319–331. [Google Scholar] [CrossRef]
- Ruan, Z.B.; Zhu, L.; Yin, Y.G.; Chen, G.C. Karyotype stability of human umbilical cord-derived mesenchymal stem cells during in vitro culture. Exp. Ther. Med. 2014, 8, 1508–1512. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Venkatesan, V.; Prakhya, B.M.; Bhonde, R. Human mesenchymal stem cells as a novel platform for simultaneous evaluation of cytotoxicity and genotoxicity of pharmaceuticals. Mutagenesis 2015, 30, 391–399. [Google Scholar] [CrossRef]
- Cornelio, D.A.; Tavares, J.C.M.; Pimentel, T.V.C.A.; Cavalcanti-Jr, G.B.; Medeiros, S.R.B. Cytokinesis-block micronucleus assay adapted for analyzing genomic instability of human mesenchymal stem cells. Stem Cells Dev. 2014, 23, 8. [Google Scholar] [CrossRef]
- Bonassi, S.; El-Zein, R.; Bolognesi, C.; Fenech, M. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: Evidence from human studies. Mutagenesis 2011, 26, 93–100. [Google Scholar] [CrossRef]
- Sharma, S.; Bhonde, R. Influence of nuclear blebs and micronuclei status on the growth kinetics of human mesenchymal stem cells. J. Cell. Physiol. 2015, 230, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhonde, R. Genetic and epigenetic stability of stem cells: Epigenetic modifiers modulate the fate of mesenchymal stem cells. Genomics 2020, 112, 3615–3623. [Google Scholar] [CrossRef]
- Horibata, S.; Vo, T.V.; Subramanian, V.; Thompson, P.R.; Coonrod, S.A. Utilization of the soft agar colony formation assay to identify inhibitors of tumorigenicity in breast cancer cells. J. Vis. Exp. 2015, 99, E52727. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.J.; Kim, W.; Kim, O.S.; Lee, S.; Han, S.Y.; Jeong, E.J.; Park, H.S.; Kim, H.W.; Moon, K.S. Tumorigenicity Evaluation of Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Toxicol. Res. 2016, 32, 251–258. [Google Scholar] [CrossRef]
- Subramanian, A.; Shu-Uin, G.; Ngo, K.S.; Gauthaman, K.; Biswas, A.; Choolani, M.; Bongso, A.; Chui-Yee, F. Human umbilical cord Wharton’s jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1886–1895. [Google Scholar] [CrossRef]
- Blázquez-Prunera, A.; Díez, J.M.; Gajardo, R.; Grancha, S. Human mesenchymal stem cells maintain their phenotype, multipotentiality, and genetic stability when cultured using a defined xeno-free human plasma fraction. Stem Cell Res. Ther. 2017, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- McGowan-Jordan, J.; Simons, A.; Schmid, M. ISCN 2016: An International System for Human Cytogenomic Nomenclature; Karger Publishers: Basel, Switzerland, 2016; p. 139. [Google Scholar]
- Rebelatto, C.L.K.; Senegaglia, A.C.; Franck, C.L.; Daga, D.R.; Shigunov, P.; Stimamiglio, M.A.; Marsaro, D.B.; Schaidt, B.; Micosky, A.; Azambuja, A.P.; et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: A randomized clinical trial. Stem Cell Res. Ther. 2022, 13, 122. [Google Scholar] [CrossRef]
- Rebelatto, C.L.K.; Aguiar, A.M.; Moretão, M.P.; Senegaglia, A.C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Utumi, P.H.; Fracaro, L.; Senegaglia, A.C.; Fragoso, F.Y.I.; Miyasaki, D.M.; Rebelatto, C.L.K.; Brofman, P.R.S.; Villanova-Junior, J.A. Canine dental pulp and umbilical cord-derived mesenchymal stem cells as alternative sources for cell therapy in dogs. Res. Vet. Sci. 2021, 140, 117–124. [Google Scholar] [CrossRef]
- Borgonovo, T.; Vaz, I.M.; Senegaglia, A.C.; Rebelatto, C.L.K.; Brofman, P.R.S. Genetic evaluation of mesenchymal stem cells by G-banded karyotyping in a cell technology center. Rev. Bras. Hematol. Hemoter. 2014, 36, 202–207. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Andriolo, G.; Provasi, E.; Brambilla, A.; Cicero, V.L.; Sonsin, S.; Barile, L.; Turchetto, L.; Radrizzani, M. GMP-Grade Methods for Cardiac Progenitor Cells: Cell Bank Production and Quality Control. Methods Mol. Biol. 2021, 2286, 131–166. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peinado, P.; Pascual-García, S.; Roche, E.; Sempere-Ortells, J.M. Differences of Clonogenic Mesenchymal Stem Cells on Immunomodulation of Lymphocyte Subsets. J. Immunol. Res. 2018, 2018, 7232717. [Google Scholar] [CrossRef] [PubMed]




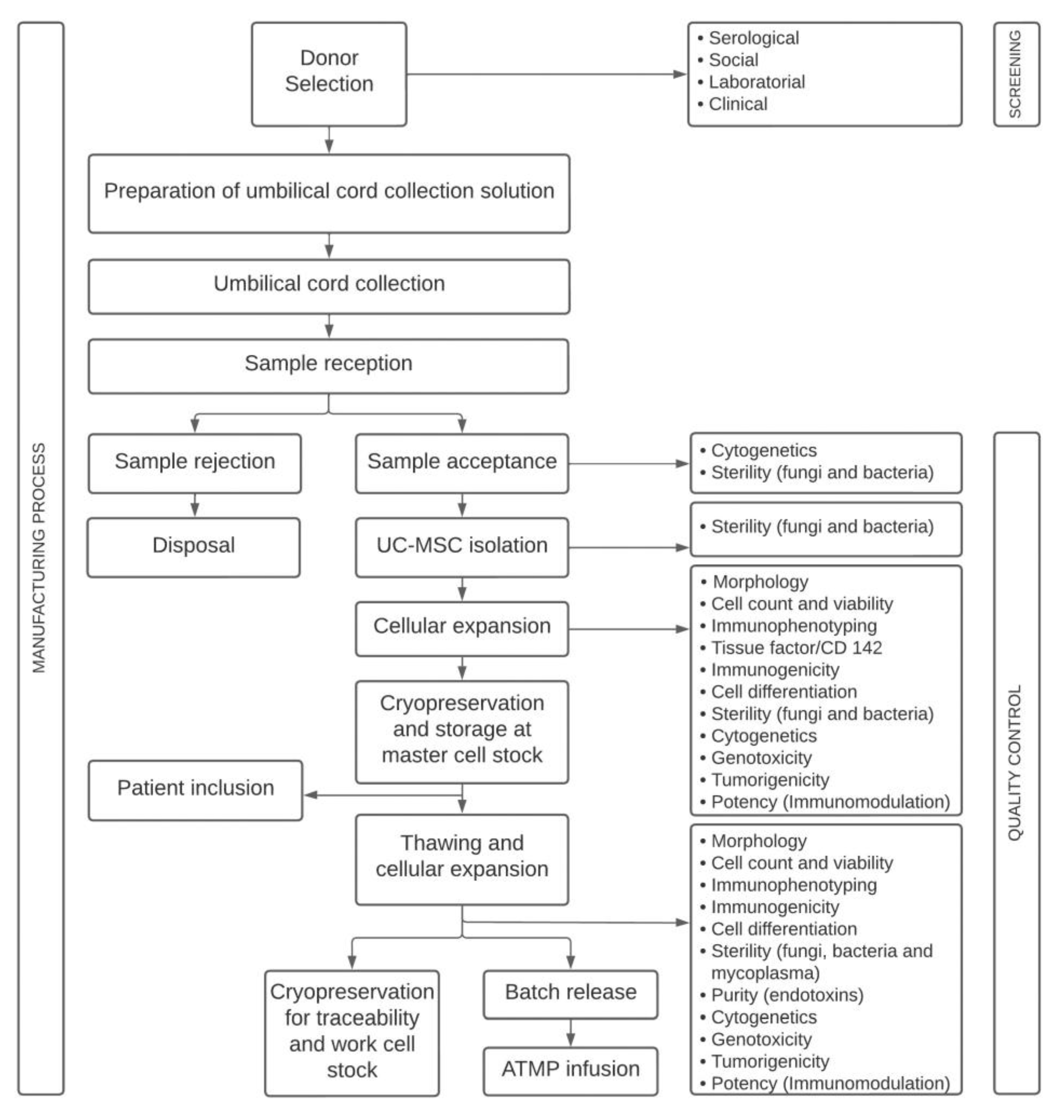
| Process | Quality Controls | Methods | Acceptance Criteria | Reference |
|---|---|---|---|---|
| UC donor selection | Screening | N/A | Eligible donor |
|
| Serology for HBV, HCV, HIV, HTLV, Treponema pallidum and Trypanossoma cruzi | Serology and nucleic acid detection | Negative |
| |
| UC isolation | Sterility | Automated growth-based | Absence of bacterial or fungal growth |
|
| UC-MSC expansion | Adherence to plastic | Microscope observation | Adherent |
|
| Viability | Flow cytometry | ≥70% |
| |
| Phenotypic markers | Flow cytometry | ≥95% for CD90, CD105, CD73, CD29 and ≤2% for CD14, CD19, CD34, CD45, HLA-DR |
| |
| Immunogenicity | Flow cytometry | ≥90% for HLA-ABC ≤2% for CD40, CD80, CD86, HLA-DR | N/A | |
| Differential potential assay | Cell differentiation assay | Differentiate into osteoblasts, adipocytes and chondroblasts |
| |
| Sterility | Automated growth-based | Absence of bacterial or fungal growth |
| |
| Genomic stability | G-Banding Technique | Absence clonal chromosomal alterations |
| |
| Potency | Inhibition of T-lymphocyte proliferation assay | Concentration 1:10 (PBMC:MSC) ≥ 50% | N/A | |
| Genotoxicity | Cytokinesis-block micronucleus assay | No significantly DNA damage events | N/A | |
| Tumorigenicity | Soft agar colony formation assay | Non-tumorigenic | N/A | |
| UC-MSC final product | Adherence to plastic | Microscope observation | Adherent cells |
|
| Viability | Flow cytometry | ≥70% |
| |
| Phenotypic markers | Flow cytometry | ≥95% for CD90, CD105, CD73, CD29 and ≤2% for CD14, CD19, CD34, CD45, HLA-DR |
| |
| Procoagulant tissue factor (TF) | Flow cytometry | N/A | N/A | |
| Sterility | Automated growth-based | Absence of bacterial or fungal growth |
| |
| Mycoplasma | PCR-based and bioluminescent assays | Not detected |
| |
| Purity | Chromogenic kinetic | <0.5 EU/mL |
| |
| Genomic stability | G-Banding Technique | Absence clonal chromosomal alterations |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebelatto, C.L.K.; Boldrini-Leite, L.M.; Daga, D.R.; Marsaro, D.B.; Vaz, I.M.; Jamur, V.R.; de Aguiar, A.M.; Vieira, T.B.; Furman, B.P.; Aguiar, C.O.; et al. Quality Control Optimization for Minimizing Security Risks Associated with Mesenchymal Stromal Cell-Based Product Development. Int. J. Mol. Sci. 2023, 24, 12955. https://doi.org/10.3390/ijms241612955
Rebelatto CLK, Boldrini-Leite LM, Daga DR, Marsaro DB, Vaz IM, Jamur VR, de Aguiar AM, Vieira TB, Furman BP, Aguiar CO, et al. Quality Control Optimization for Minimizing Security Risks Associated with Mesenchymal Stromal Cell-Based Product Development. International Journal of Molecular Sciences. 2023; 24(16):12955. https://doi.org/10.3390/ijms241612955
Chicago/Turabian StyleRebelatto, Carmen Lúcia Kuniyoshi, Lidiane Maria Boldrini-Leite, Debora Regina Daga, Daniela Boscaro Marsaro, Isadora May Vaz, Valderez Ravaglio Jamur, Alessandra Melo de Aguiar, Thalita Bastida Vieira, Bianca Polak Furman, Cecília Oliveira Aguiar, and et al. 2023. "Quality Control Optimization for Minimizing Security Risks Associated with Mesenchymal Stromal Cell-Based Product Development" International Journal of Molecular Sciences 24, no. 16: 12955. https://doi.org/10.3390/ijms241612955
APA StyleRebelatto, C. L. K., Boldrini-Leite, L. M., Daga, D. R., Marsaro, D. B., Vaz, I. M., Jamur, V. R., de Aguiar, A. M., Vieira, T. B., Furman, B. P., Aguiar, C. O., & Brofman, P. R. S. (2023). Quality Control Optimization for Minimizing Security Risks Associated with Mesenchymal Stromal Cell-Based Product Development. International Journal of Molecular Sciences, 24(16), 12955. https://doi.org/10.3390/ijms241612955






