Comprehensive Analysis of Photoreceptor Outer Segments: Flow Cytometry Characterization and Stress-Driven Impact on Retinal Pigment Epithelium Phagocytosis
Abstract
1. Introduction
2. Results
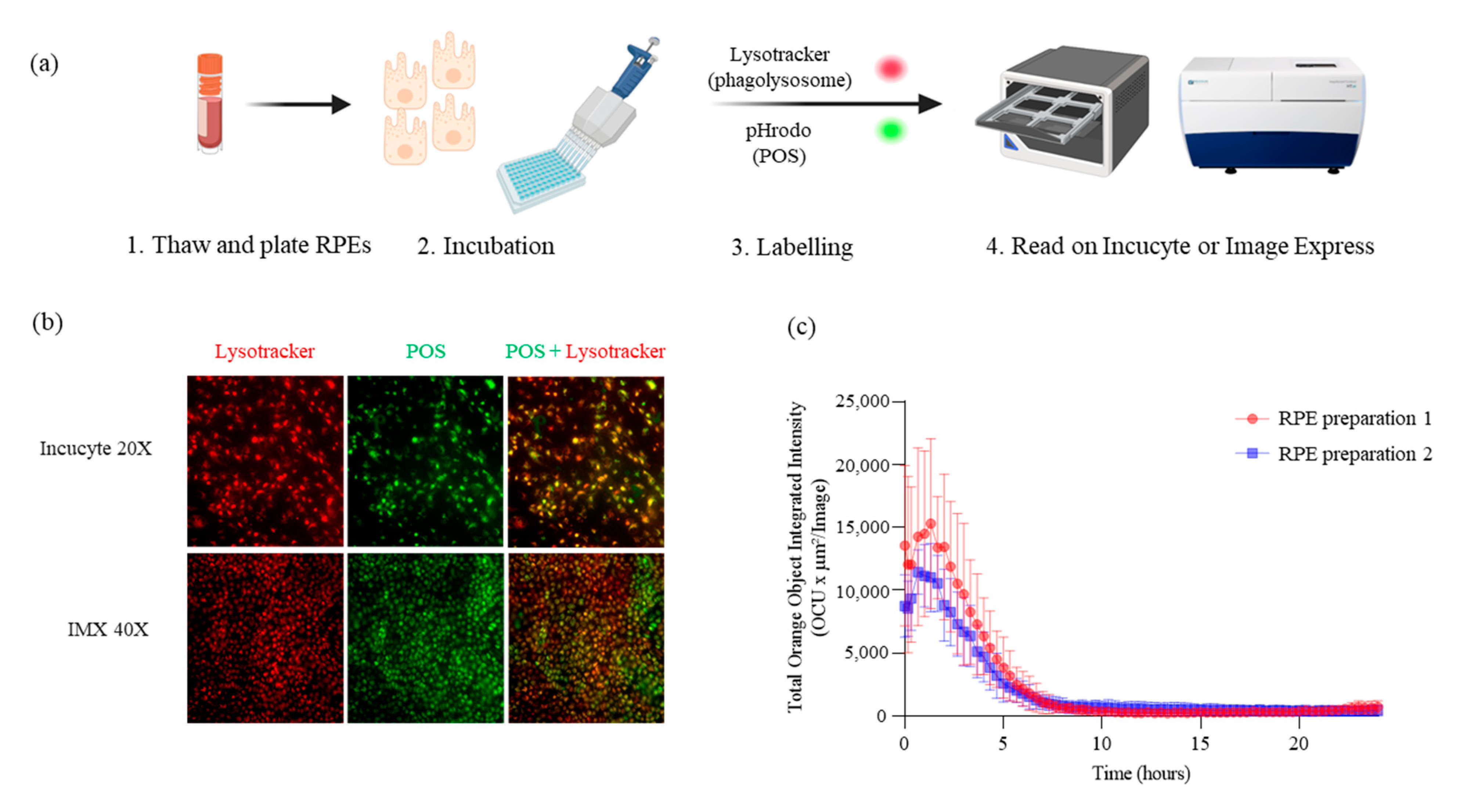
2.1. Phagocytosis Assay
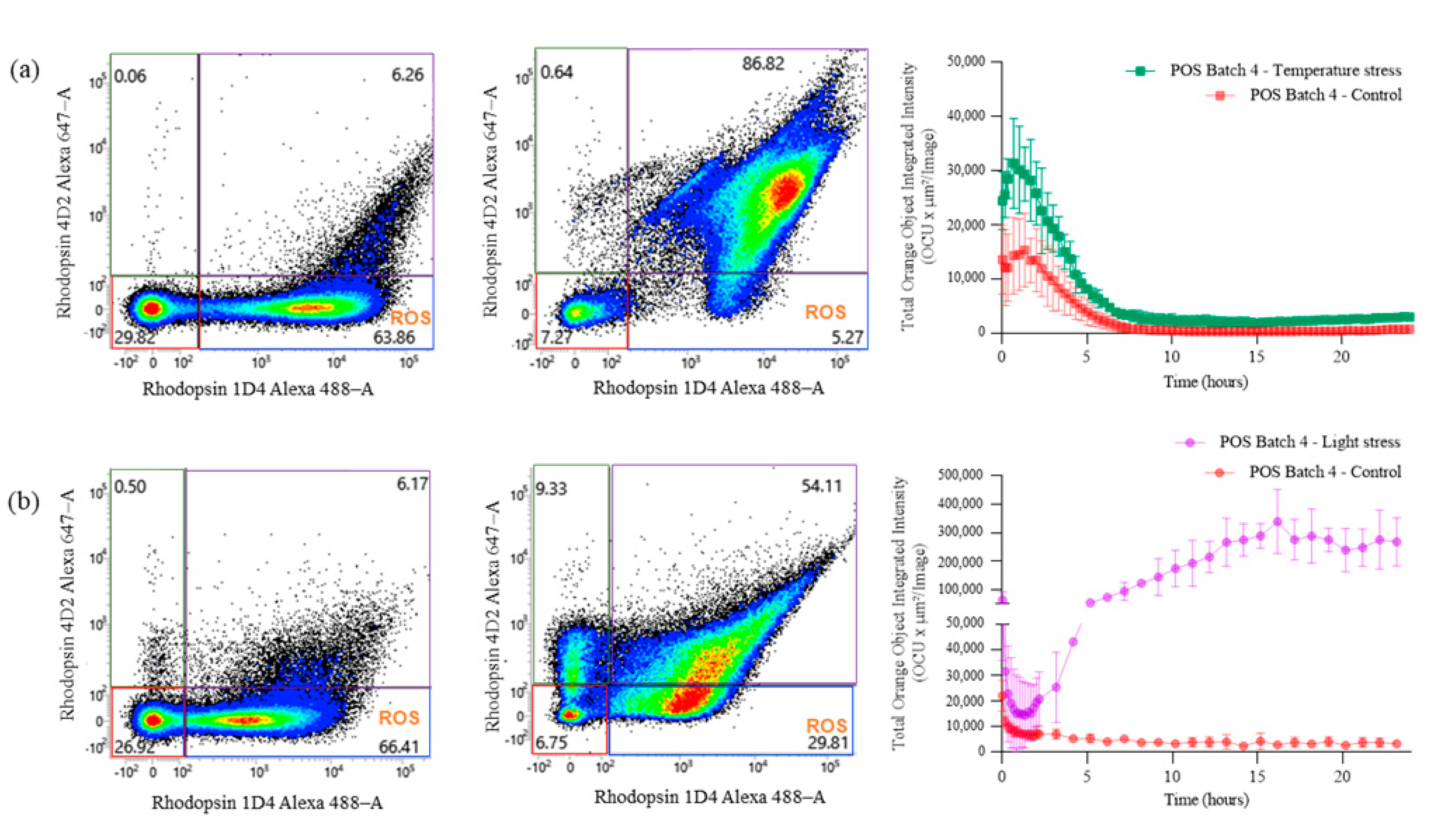
2.2. Characterization of POS Purity Using a Flow-Cytometry-Based Method Targeting Specific ROSs and COS Markers
2.3. Study of POS Stability under Several Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Cell Media, Solvents, and Reagents
4.2. RPE Cells Preparation
4.3. Phagocytosis Assay
4.3.1. POS Batches
4.3.2. POS Conjugation with pHrodo™ Dye
4.3.3. Phagocytosis Assay
4.4. POS Sample Preparation
4.4.1. Heat-Stress-Treated Samples
4.4.2. Light-Stress-Treated Samples
4.4.3. In-House POS Extraction and Purification
4.5. Characterization of POS Population Using Flow Cytometry
4.5.1. POS Antibody Staining
4.5.2. Flow Cytometry
4.5.3. Analysis of Intracellular and Extracellular Soluble Proteins via HPLC/UV
4.5.4. Characterization of POSs Using Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vyawahare, H.; Shinde, P. Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus. Cureus. 2022, 14, e29583. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Barthelmes, D.; Gillies, M.C. Neovascular age-related macular degeneration: A review of findings from the real-world Fight Retinal Blindness! registry. Clin. Exp. Ophthalmol. 2021, 49, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Inana, G.; Murat, C.; An, W.; Yao, X.; Harris, I.R.; Cao, J.R.P.E. phagocytic function declines in age-related macular degeneration and is rescued by human umbilical tissue derived cells. J. Transl. Med. 2018, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Marcheselli, V.L.; de Rivero Vaccari, J.C.; Gordon, W.C.; Jackson, F.E.; Bazan, N.G. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13158–13163. [Google Scholar] [CrossRef]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]
- Uyama, H.; Mandai, M.; Takahashi, M. Stem-cell-based therapies for retinal degenerative diseases: Current challenges in the establishment of new treatment strategies. Dev. Growth Differ. 2021, 63, 59–71. [Google Scholar] [CrossRef]
- Maeda, T.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Trends of Stem Cell Therapies in Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 1785. [Google Scholar] [CrossRef]
- Idelson, M.; Alper, R.; Obolensky, A.; Ben-Shushan, E.; Hemo, I.; Yachimovich-Cohen, N.; Khaner, H.; Smith, Y.; Wiser, O.; Gropp, M.; et al. Directed Differentiation of Human Embryonic Stem Cells into Functional Retinal Pigment Epithelium Cells. Cell Stem Cell 2009, 5, 396–408. [Google Scholar] [CrossRef]
- Akrami, H.; Soheili, Z.S.; Khalooghi, K.; Ahmadieh, H.; Rezaie-Kanavi, M.; Samiei, S.; Davari, M.; Ghaderi, S.; Sanie-Jahromi, F. Retinal pigment epithelium culture; a potential source of retinal stem cells. J. Ophthalmic Vis. Res. 2009, 4, 134–141. [Google Scholar]
- Uygun, B.E.; Sharma, N.; Yarmush, M. Retinal Pigment Epithelium Differentiation of Stem Cells: Current Status and Challenges. Crit. Rev. Biomed. Eng. 2009, 37, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Rastoin, O.; Pagès, G.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, S.; Mirshahi, R.; Shoae-Hassani, A.; Naseripour, M. Human-induced pluripotent stem cells-derived retinal pigmented epithelium, a new horizon for cells-based therapies for age-related macular degeneration. Stem Cell Res. Ther. 2022, 13, 217. [Google Scholar] [CrossRef]
- Duarri, A.; Rodríguez-Bocanegra, E.; Martínez-Navarrete, G.; Biarnés, M.; García, M.; Ferraro, L.L.; Kuebler, B.; Aran, B.; Izquierdo, E.; Aguilera-Xiol, E.; et al. Transplantation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in a Swine Model of Geographic Atrophy. Int. J. Mol. Sci. 2021, 22, 10497. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.; Zimmermann, S.J.; Milenkovic, V.M.; Rosendahl, S.M.G.; Grassmann, F.; Milenkovic, A.; Hehr, U.; Federlin, M.; Wetzel, C.H.; Helbig, H.; et al. In-Depth Characterisation of Retinal Pigment Epithelium (RPE) Cells Derived from Human Induced Pluripotent Stem Cells (hiPSC). NeuroMolecular Med. 2014, 16, 551–564. [Google Scholar] [CrossRef]
- Kokkinaki, M.; Sahibzada, N.; Golestaneh, N. Human Induced Pluripotent Stem-Derived Retinal Pigment Epithelium (RPE) Cells Exhibit Ion Transport, Membrane Potential, Polarized Vascular Endothelial Growth Factor Secretion, and Gene Expression Pattern Similar to Native RPE. Stem Cells 2011, 29, 825–835. [Google Scholar] [CrossRef]
- Liao, J.L.; Yu, J.; Huang, K.; Hu, J.; Diemer, T.; Ma, Z.; Dvash, T.; Yang, X.-J.; Travis, G.H.; Williams, D.S.; et al. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum. Mol. Genet. 2010, 19, 4229–4238. [Google Scholar] [CrossRef]
- Mazzoni, F.; Safa, H.; Finnemann, S.C. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Exp. Eye Res. 2014, 126, 51–60. [Google Scholar]
- Voisin, A.; Gaillard, A.; Balbous, A.; Leveziel, N. Proteins Associated with Phagocytosis Alteration in Retinal Pigment Epithelial Cells Derived from Age-Related Macular Degeneration Patients. Antioxidants 2022, 11, 713. [Google Scholar] [CrossRef]
- Sethna, S.; Finnemann, S.C. Analysis of Photoreceptor Rod Outer Segment Phagocytosis by RPE Cells In Situ. In Weber BHF; Langmann, T., Ed.; Retinal Degeneration [Internet]; (Methods in Molecular Biology); Humana Press: Totowa, NJ, USA, 2012; Volume 935, pp. 245–254. Available online: http://link.springer.com/10.1007/978-1-62703-080-9_17 (accessed on 1 May 2023).
- Lasansky, A.; de Robertis, E. Electron Microscopy of Retinal Photoreceptors. J. Biophys. Biochem. Cytol. 1960, 7, 493–497. [Google Scholar] [CrossRef]
- Corley, S.C.; Albert, A.D. Assessment of bovine rod outer segment disk membrane heterogeneity utilizing flow cytometry. Exp. Eye Res. 2011, 92, 20–27. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.T.M.; Väkeväinen-Anttila, M.; Käyhty, H.; Nahm, M.; Bakker, N.; Verhoef, J.; Snippe, H.; Verheul, A.F.M. Comparison of a Classical Phagocytosis Assay and a Flow Cytometry Assay for Assessment of the Phagocytic Capacity of Sera from Adults Vaccinated with a Pneumococcal Conjugate Vaccine. Clin. Diagn. Lab. Immunol. 2001, 8, 245–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.S.; Kefalov, V.J. The Cone-specific visual cycle. Prog. Retin. Eye Res. 2011, 30, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Molday, L.L.; Cheng, C.L.; Molday, R.S. Cell-Specific Markers for the Identification of Retinal Cells and Subcellular Organelles by Immunofluorescence Microscopy. In Weber BHF; Langmann, T., Ed.; Retinal Degeneration [Internet]; (Methods in Molecular Biology); Springer: New York, NY, USA, 2019; Volume 1834, pp. 293–310. Available online: http://link.springer.com/10.1007/978-1-4939-8669-9_19 (accessed on 11 May 2023).
- Parinot, C.; Rieu, Q.; Chatagnon, J.; Finnemann, S.C.; Nandrot, E.F. Large-Scale Purification of Porcine or Bovine Photoreceptor Outer Segments for Phagocytosis Assays on Retinal Pigment Epithelial Cells. J. Vis. Exp. 2014, 94, e52100. [Google Scholar]
- Panfoli, I.; Musante, L.; Bachi, A.; Ravera, S.; Calzia, D.; Cattaneo, A.; Bruschi, M.; Bianchini, P.; Diaspro, A.; Morelli, A.; et al. Proteomic Analysis of the Retinal Rod Outer Segment Disks. J. Proteome Res. 2008, 7, 2654–2669. [Google Scholar] [CrossRef]
- Sun, M.; Finnemann, S.C.; Febbraio, M.; Shan, L.; Annangudi, S.P.; Podrez, E.A.; Hoppe, G.; Darrow, R.; Organisciak, D.T.; Salomon, R.G.; et al. Light-induced Oxidation of Photoreceptor Outer Segment Phospholipids Generates Ligands for CD36-mediated Phagocytosis by Retinal Pigment Epithelium. J. Biol. Chem. 2006, 281, 4222–4230. [Google Scholar] [CrossRef]
- Papermaster, D.S. Preparation of retinal rod outer segments. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1982; pp. 48–52. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0076687982810100 (accessed on 2 May 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Wu, Q.; Guo, X.V.; Chan, L.; Mao, T.; Stella, C.; Guilbaud, A.; Camperi, J. Comprehensive Analysis of Photoreceptor Outer Segments: Flow Cytometry Characterization and Stress-Driven Impact on Retinal Pigment Epithelium Phagocytosis. Int. J. Mol. Sci. 2023, 24, 12889. https://doi.org/10.3390/ijms241612889
Liang H, Wu Q, Guo XV, Chan L, Mao T, Stella C, Guilbaud A, Camperi J. Comprehensive Analysis of Photoreceptor Outer Segments: Flow Cytometry Characterization and Stress-Driven Impact on Retinal Pigment Epithelium Phagocytosis. International Journal of Molecular Sciences. 2023; 24(16):12889. https://doi.org/10.3390/ijms241612889
Chicago/Turabian StyleLiang, Haoqian, Qiang Wu, Xinzheng Victor Guo, Linda Chan, Tin Mao, Cinzia Stella, Axel Guilbaud, and Julien Camperi. 2023. "Comprehensive Analysis of Photoreceptor Outer Segments: Flow Cytometry Characterization and Stress-Driven Impact on Retinal Pigment Epithelium Phagocytosis" International Journal of Molecular Sciences 24, no. 16: 12889. https://doi.org/10.3390/ijms241612889
APA StyleLiang, H., Wu, Q., Guo, X. V., Chan, L., Mao, T., Stella, C., Guilbaud, A., & Camperi, J. (2023). Comprehensive Analysis of Photoreceptor Outer Segments: Flow Cytometry Characterization and Stress-Driven Impact on Retinal Pigment Epithelium Phagocytosis. International Journal of Molecular Sciences, 24(16), 12889. https://doi.org/10.3390/ijms241612889






