Deficiency of Caspase-1 Attenuates HIV-1-Associated Atherogenesis in Mice
Abstract
1. Introduction
2. Results
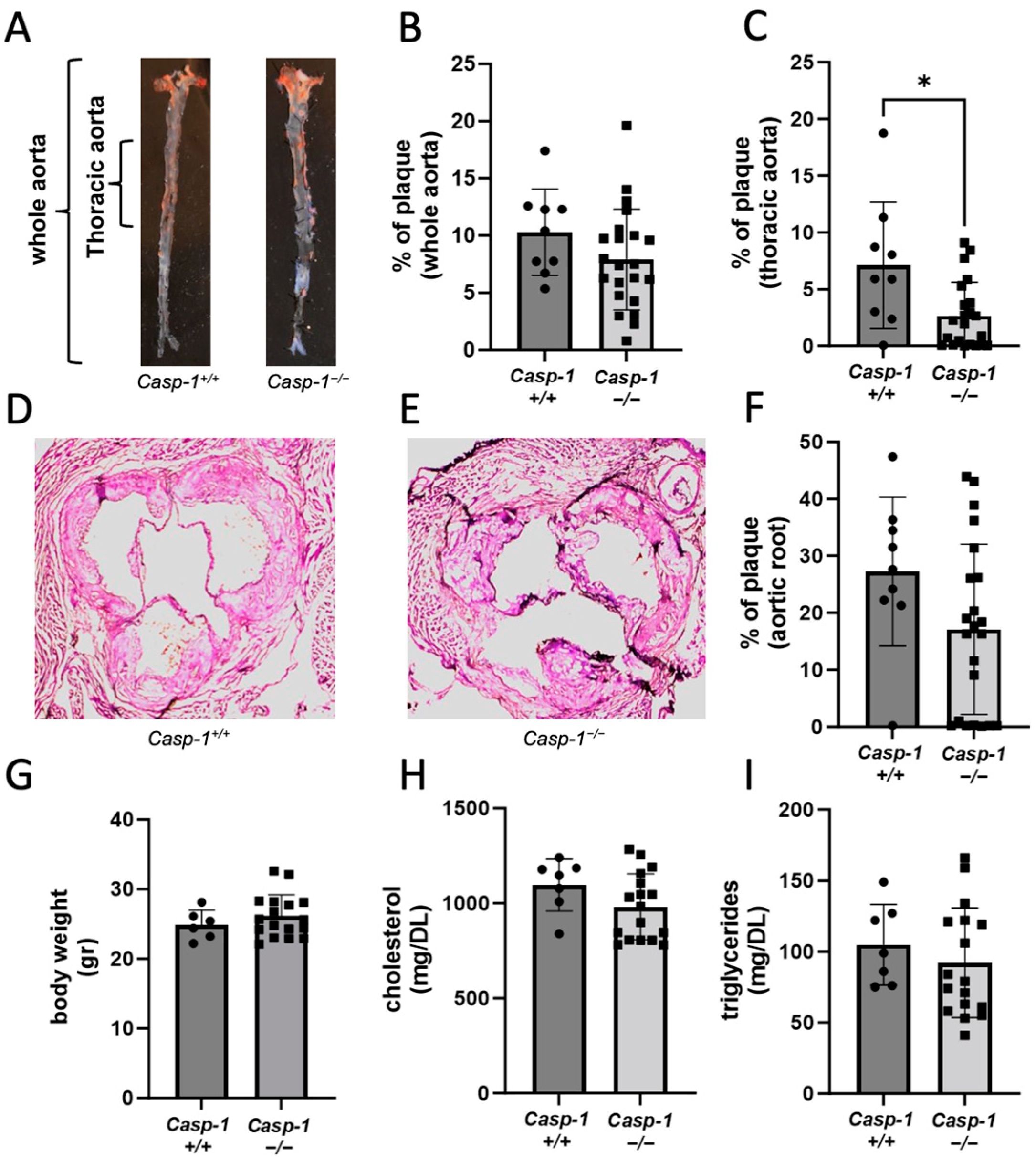
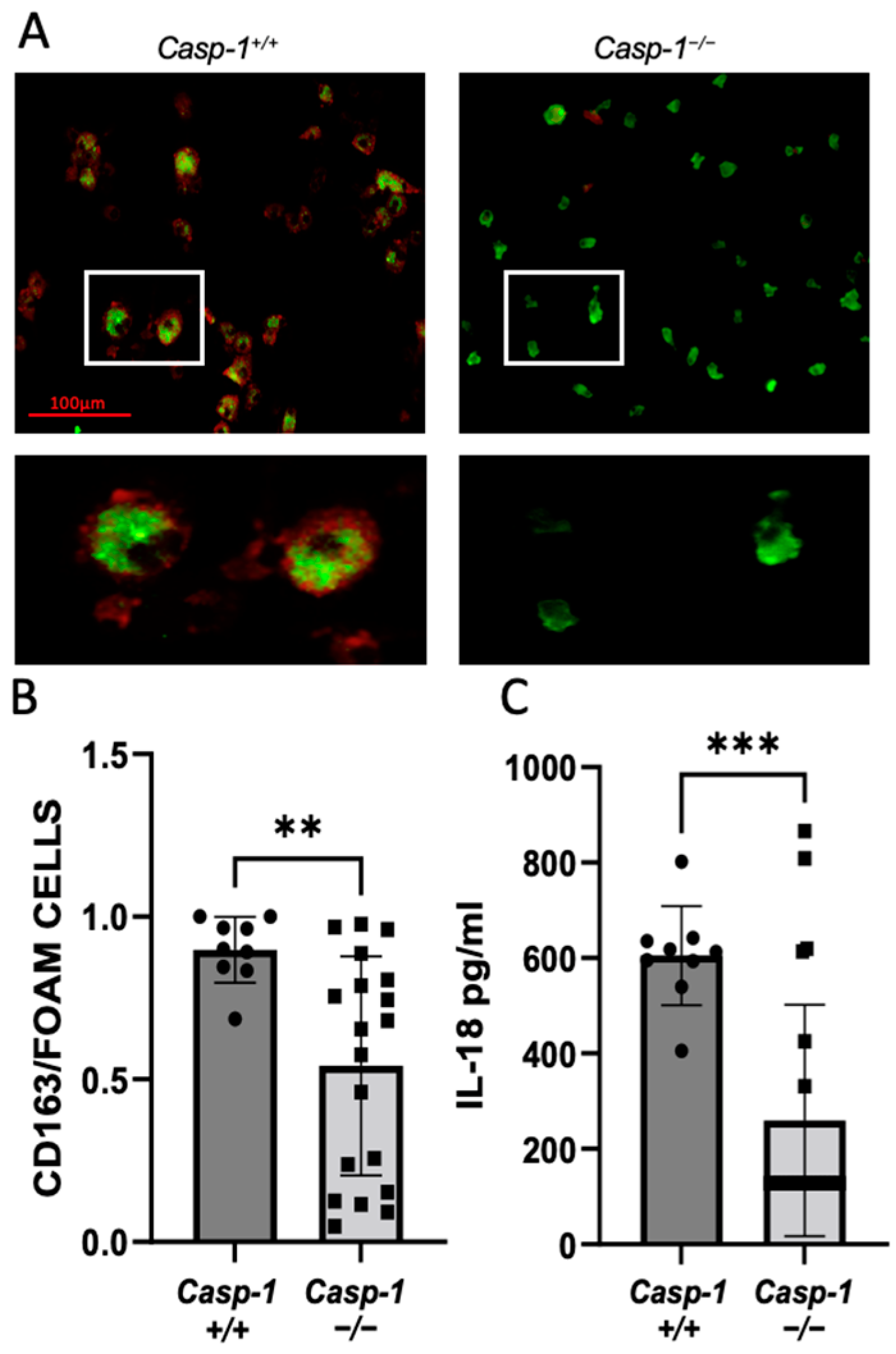
2.1. Global Caspase-1 Knockout Decreases Plaque Size Only in the Thoracic Region of the Aorta in Tg26+/−/ApoE−/−/Casp-1−/− Mice and Reduces Foam Cell Formation
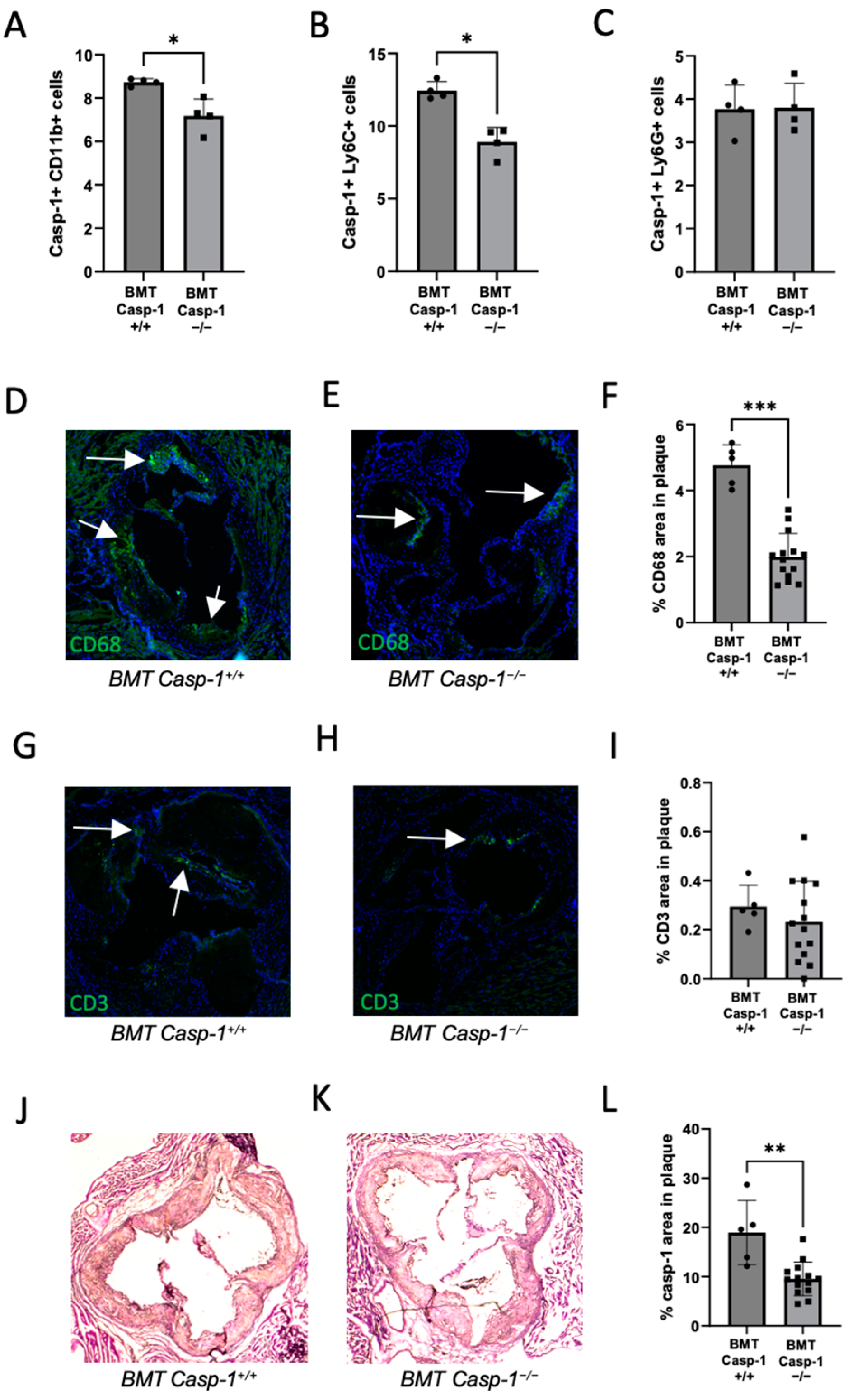
2.2. Deficiency of Caspase-1 in Immune Cells Attenuates Atherogenesis in HIV Transgenic Mice
2.3. Reduced Caspase-1 Activation Is Associated with Reduced Macrophage Content in the Plaque of Chimeric ApoE−/− Mice Reconstituted with Tg26+/−/ApoE−/−/Casp-1−/− Bone Marrow
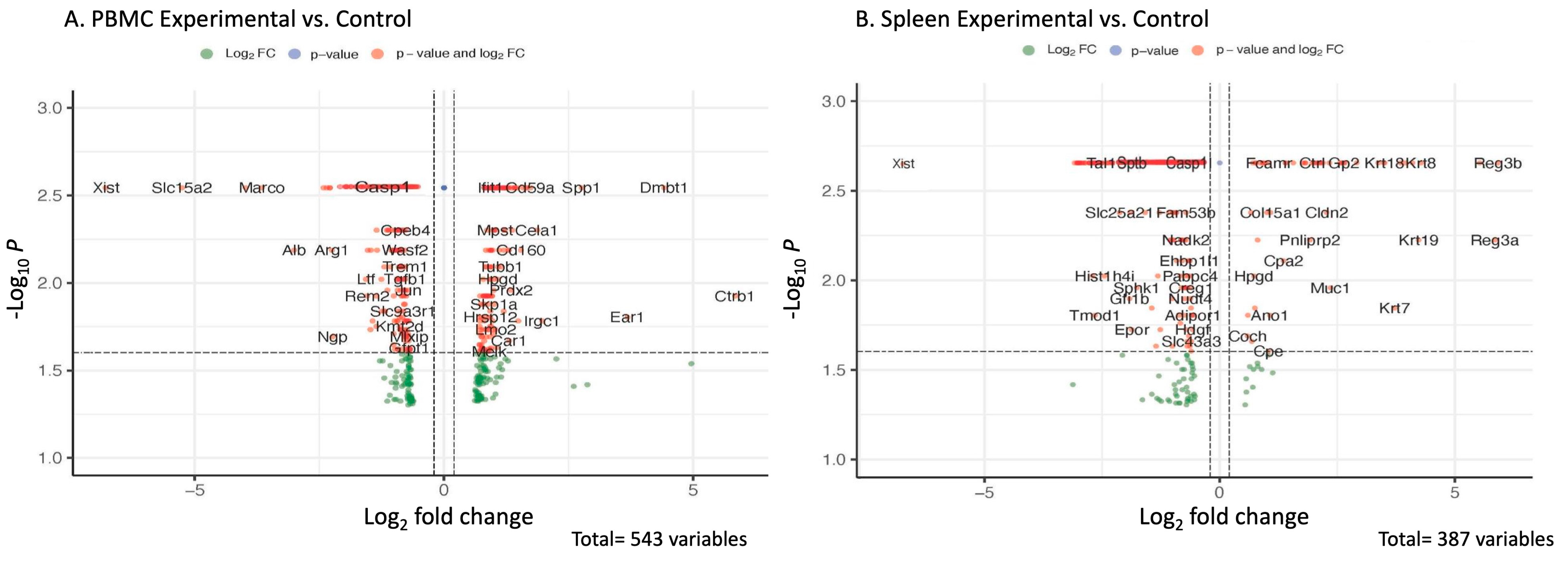
2.4. Transcriptomic and Pathway Changes in PBMCs and Spleens of Chimeric ApoE−/− Mice Reconstituted with Caspase-1-Deficient (Tg26+/−/ApoE−/−/Casp-1−/−) vs. Caspase-1-Sufficient (Tg26+/−/ApoE−/−/Casp-1+/+) Bone Marrow
3. Discussion
4. Materials and Methods
4.1. Mouse Treatment, Bone Marrow Transplantation, and Atherosclerotic Plaque Analyses
4.2. Serum Lipid Analysis
4.3. Atherosclerotic Plaque Analysis in Aorta
4.4. Histological Analysis of Plaque Progression in Aortic Sinus
4.5. Immunohistochemistry
4.6. FAM-FLICA Caspase-1 Staining and Flow Cytometry Analysis
4.7. Foam Cell Formation Assay
4.8. qRT-PCR
4.9. ELISA and Meso Scale Discovery (MSD) Assay
4.10. RNA Extraction, Library Preparation, Sequencing, and Analysis
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrestha, S.; Irvin, M.R.; Grunfeld, C.; Arnett, D.K. Hiv, inflammation, and calcium in atherosclerosis. Arter. Thromb. Vasc. Biol. 2014, 34, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Plutzky, J. The biology of atherosclerosis: General paradigms and distinct pathogenic mechanisms among hiv-infected patients. J. Infect. Dis. 2012, 205 (Suppl. S3), S368–S374. [Google Scholar] [CrossRef] [PubMed]
- Vachiat, A.; McCutcheon, K.; Tsabedze, N.; Zachariah, D.; Manga, P. Hiv and ischemic heart disease. J. Am. Coll. Cardiol. 2017, 69, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Ryom, L.; Weber, R.; Morlat, P.; Pradier, C.; Reiss, P.; Kowalska, J.D.; de Wit, S.; Law, M.; el Sadr, W.; et al. Trends in underlying causes of death in people with hiv from 1999 to 2011 (d:A:D): A multicohort collaboration. Lancet 2014, 384, 241–248. [Google Scholar] [CrossRef]
- Lewden, C.; May, T.; Rosenthal, E.; Burty, C.; Bonnet, F.; Costagliola, D.; Jougla, E.; Semaille, C.; Morlat, P.; Salmon, D.; et al. Changes in causes of death among adults infected by hiv between 2000 and 2005: The “mortalite 2000 and 2005” surveys (anrs en19 and mortavic). J. Acquir. Immune Defic. Syndr. 2008, 48, 590–598. [Google Scholar] [CrossRef]
- Kearns, A.; Burdo, T.H.; Qin, X. Editorial commentary: Clinical management of cardiovascular disease in hiv-infected patients. Trends Cardiovasc. Med. 2017, 27, 564–566. [Google Scholar] [CrossRef]
- Kearns, A.; Gordon, J.; Burdo, T.H.; Qin, X. Hiv-1-associated atherosclerosis: Unraveling the missing link. J. Am. Coll. Cardiol. 2017, 69, 3084–3098. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Hunt, P.W.; Schnell, A.; Kalapus, S.C.; Hoh, R.; Ganz, P.; Martin, J.N.; Deeks, S.G. Role of viral replication, antiretroviral therapy, and immunodeficiency in hiv-associated atherosclerosis. AIDS 2009, 23, 1059–1067. [Google Scholar] [CrossRef]
- Chivero, E.T.; Guo, M.L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. Hiv-1 tat primes and activates microglial nlrp3 inflammasome-mediated neuroinflammation. J. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef]
- Walsh, J.G.; Reinke, S.N.; Mamik, M.K.; McKenzie, B.A.; Maingat, F.; Branton, W.G.; Broadhurst, D.I.; Power, C. Rapid inflammasome activation in microglia contributes to brain disease in hiv/aids. Retrovirology 2014, 11, 35. [Google Scholar] [CrossRef]
- Hernandez, J.C.; Latz, E.; Urcuqui-Inchima, S. Hiv-1 induces the first signal to activate the nlrp3 inflammasome in monocyte-derived macrophages. Intervirology 2014, 57, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gao, J.; Taxman, D.J.; Ting, J.P.; Su, L. Hiv-1 infection induces interleukin-1beta production via tlr8 protein-dependent and nlrp3 inflammasome mechanisms in human monocytes. J. Biol. Chem. 2014, 289, 21716–21726. [Google Scholar] [CrossRef] [PubMed]
- Lisco, A.; Wong, C.S.; Lage, S.L.; Levy, I.; Brophy, J.; Lennox, J.; Manion, M.; Anderson, M.V.; Mejia, Y.; Grivas, C.; et al. Identification of rare hiv-1-infected patients with extreme cd4+ t cell decline despite art-mediated viral suppression. JCI Insight 2019, 4, e127113. [Google Scholar] [CrossRef]
- Pereyra, F.; Lo, J.; Triant, V.A.; Wei, J.; Buzon, M.J.; Fitch, K.V.; Hwang, J.; Campbell, J.H.; Burdo, T.H.; Williams, K.C.; et al. Increased coronary atherosclerosis and immune activation in hiv-1 elite controllers. AIDS 2012, 26, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.V.; Srinivasa, S.; Abbara, S.; Burdo, T.H.; Williams, K.C.; Eneh, P.; Lo, J.; Grinspoon, S.K. Noncalcified coronary atherosclerotic plaque and immune activation in hiv-infected women. J. Infect. Dis. 2013, 208, 1737–1746. [Google Scholar] [CrossRef]
- Tawakol, A.; Ishai, A.; Li, D.; Takx, R.A.; Hur, S.; Kaiser, Y.; Pampaloni, M.; Rupert, A.; Hsu, D.; Sereti, I.; et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol. 2017, 2, 163–171. [Google Scholar] [CrossRef]
- Zanni, M.V.; Toribio, M.; Robbins, G.K.; Burdo, T.H.; Lu, M.T.; Ishai, A.E.; Feldpausch, M.N.; Martin, A.; Melbourne, K.; Triant, V.A.; et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol. 2016, 1, 474–480. [Google Scholar] [CrossRef]
- Currier, J.S.; Hsue, P. The role of inflammation in hiv-associated atherosclerosis—One size may not fit all. J. Infect. Dis. 2019, 221, 495–497. [Google Scholar] [CrossRef]
- Kearns, A.; Velasquez, S.; Liu, F.; Dai, S.; Chen, Y.; Lehmicke, G.; Gordon, J.; Rappaport, J.; Qin, X. Elevated indoleamine-2,3-dioxygenase (ido) enzyme activity in a novel mouse model of hiv-associated atherosclerosis. AIDS 2019, 33, 1557–1564. [Google Scholar] [CrossRef]
- De, S.K.; Wohlenberg, C.R.; Marinos, N.J.; Doodnauth, D.; Bryant, J.L.; Notkins, A.L. Human chorionic gonadotropin hormone prevents wasting syndrome and death in hiv-1 transgenic mice. J. Clin. Investig. 1997, 99, 1484–1491. [Google Scholar] [CrossRef]
- Dickie, P.; Felser, J.; Eckhaus, M.; Bryant, J.; Silver, J.; Marinos, N.; Notkins, A.L. Hiv-associated nephropathy in transgenic mice expressing hiv-1 genes. Virology 1991, 185, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.Y.; Gordon, J.; Wang, J.; Song, J.; Zhang, X.Q.; Tilley, D.G.; Gao, E.; Koch, W.J.; Rabinowitz, J.; Klotman, P.E.; et al. Cardiac dysfunction in hiv-1 transgenic mouse: Role of stress and bag3. Clin. Transl. Sci. 2015, 8, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.C.; Liu, F.; Dai, S.; Robinson, J.A.; Kiernan, E.; Tesfaye Cheru, L.; Peng, X.; Gordon, J.; Morgello, S.; Abuova, A.; et al. Caspase-1 activation is related with hiv-associated atherosclerosis in an hiv transgenic mouse model and hiv patient cohort. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.C.; Robinson, J.A.; Shekarabi, M.; Liu, F.; Qin, X.; Burdo, T.H. Caspase-1-associated immune activation in an accelerated siv-infected rhesus macaque model. J. Neurovirol. 2018, 24, 420–431. [Google Scholar] [CrossRef]
- Niu, Z.; Tang, J.; Zhang, W.; Chen, Y.; Huang, Y.; Chen, B.; Li, J.; Shen, P. Caspase-1 promotes monocyte-macrophage differentiation by repressing pparγ. FEBS J. 2017, 284, 568–585. [Google Scholar] [CrossRef]
- Kondreddy, V.; Magisetty, J.; Keshava, S.; Rao, L.V.M.; Pendurthi, U.R. Gab2 (grb2-associated binder2) plays a crucial role in inflammatory signaling and endothelial dysfunction. Arter. Thromb. Vasc. Biol. 2021, 41, 1987–2005. [Google Scholar] [CrossRef]
- Proctor, B.M.; Ren, J.; Chen, Z.; Schneider, J.G.; Coleman, T.; Lupu, T.S.; Semenkovich, C.F.; Muslin, A.J. Grb2 is required for atherosclerotic lesion formation. Arter. Thromb. Vasc. Biol. 2007, 27, 1361–1367. [Google Scholar] [CrossRef]
- Rabolli, V.; Wallemme, L.; Lo Re, S.; Uwambayinema, F.; Palmai-Pallag, M.; Thomassen, L.; Tyteca, D.; Octave, J.N.; Marbaix, E.; Lison, D.; et al. Critical role of aquaporins in interleukin 1β (il-1β)-induced inflammation. J. Biol. Chem. 2014, 289, 13937–13947. [Google Scholar] [CrossRef]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. Nlrp3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell. Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef]
- Rheinheimer, J.; de Souza, B.M.; Cardoso, N.S.; Bauer, A.C.; Crispim, D. Current role of the nlrp3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism 2017, 74, 1–9. [Google Scholar] [CrossRef]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation-the negative regulation of nf-kappab and the nlrp3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Usui, F.; Shirasuna, K.; Kimura, H.; Tatsumi, K.; Kawashima, A.; Karasawa, T.; Hida, S.; Sagara, J.; Taniguchi, S.; Takahashi, M. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in western diet-fed apolipoprotein e-deficient mice. Biochem. Biophys. Res. Commun. 2012, 425, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.; Hasu, M.; Thabet, M.; Whitman, S.C. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein e-null mice. Can. J. Cardiol. 2012, 28, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Menu, P.; Pellegrin, M.; Aubert, J.F.; Bouzourene, K.; Tardivel, A.; Mazzolai, L.; Tschopp, J. Atherosclerosis in apoe-deficient mice progresses independently of the nlrp3 inflammasome. Cell Death Dis. 2011, 2, e137. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, T.; Jeurissen, M.L.; van Gorp, P.J.; Gijbels, M.J.; Walenbergh, S.M.; Houben, T.; van Gorp, R.; Pottgens, C.C.; Stienstra, R.; Netea, M.G.; et al. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in ldlr−/− mice. FEBS J. 2015, 282, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Elhage, R.; Jawien, J.; Rudling, M.; Ljunggren, H.G.; Takeda, K.; Akira, S.; Bayard, F.; Hansson, G.K. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein e-knockout mice. Cardiovasc. Res. 2003, 59, 234–240. [Google Scholar] [CrossRef]
- Yearley, J.H.; Xia, D.; Pearson, C.B.; Carville, A.; Shannon, R.P.; Mansfield, K.G. Interleukin-18 predicts atherosclerosis progression in siv-infected and uninfected rhesus monkeys (macaca mulatta) on a high-fat/high-cholesterol diet. Lab. Investig. 2009, 89, 657–667. [Google Scholar] [CrossRef][Green Version]
- Mallat, Z.; Corbaz, A.; Scoazec, A.; Besnard, S.; Lesèche, G.; Chvatchko, Y.; Tedgui, A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 2001, 104, 1598–1603. [Google Scholar] [CrossRef]
- Gerdes, N.; Sukhova, G.K.; Libby, P.; Reynolds, R.S.; Young, J.L.; Schönbeck, U. Expression of interleukin (il)-18 and functional il-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for atherogenesis. J. Exp. Med. 2002, 195, 245–257. [Google Scholar] [CrossRef]
- Iwata, H.; Manabe, I.; Nagai, R. Lineage of bone marrow-derived cells in atherosclerosis. Circ. Res. 2013, 112, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ferris, S.P.; Tweten, R.K.; Wu, G.; Radaeva, S.; Gao, B.; Bronson, R.T.; Halperin, J.A.; Qin, X. Rapid conditional targeted ablation of cells expressing human cd59 in transgenic mice by intermedilysin. Nat. Med. 2008, 14, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Dai, S.; Liu, F.; Ohtake, Y.; Zhou, Z.; Wang, H.; Zhang, Y.; Kearns, A.; Peng, X.; Zhu, F.; et al. Cre-inducible human cd59 mediates rapid cell ablation after intermedilysin administration. J. Clin. Investig. 2016, 126, 2321–2333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, F.; Dai, S.; Feng, D.; Qin, Z.; Peng, X.; Sakamuri, S.; Ren, M.; Huang, L.; Cheng, M.; Mohammad, K.E.; et al. Distinct fate, dynamics and niches of renal macrophages of bone marrow or embryonic origins. Nat. Commun. 2020, 11, 2280. [Google Scholar] [CrossRef]
- Chuang, J.P.; Kao, C.Y.; Lee, J.C.; Ling, P.; Maa, M.C.; Leu, T.H. Eps8 regulates an nlrp3 inflammasome-independent caspase-1 activation pathway in monosodium urate crystal-treated raw264.7 macrophages. Biochem. Biophys. Res. Commun. 2020, 530, 487–493. [Google Scholar] [CrossRef]
- Hu, Y.; Song, F.; Jiang, H.; Nuñez, G.; Smith, D.E. Slc15a2 and slc15a4 mediate the transport of bacterially derived di/tripeptides to enhance the nucleotide-binding oligomerization domain-dependent immune response in mouse bone marrow-derived macrophages. J. Immunol. 2018, 201, 652–662. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyazaki, A. Emerging roles of calpain proteolytic systems in macrophage cholesterol handling. Cell. Mol. Life Sci. 2017, 74, 3011–3021. [Google Scholar] [CrossRef]
- Ji, J.; Su, L.; Liu, Z. Critical role of calpain in inflammation. Biomed. Rep. 2016, 5, 647–652. [Google Scholar] [CrossRef]
- Molla, M.D.; Akalu, Y.; Geto, Z.; Dagnew, B.; Ayelign, B.; Shibabaw, T. Role of caspase-1 in the pathogenesis of inflammatory-associated chronic noncommunicable diseases. J. Inflamm. Res. 2020, 13, 749–764. [Google Scholar] [CrossRef]
- da Silva, I.V.; Soveral, G. Aquaporins in immune cells and inflammation: New targets for drug development. Int. J. Mol. Sci. 2021, 22, 1845. [Google Scholar] [CrossRef]
- Wu, G.; Chen, T.; Shahsafaei, A.; Hu, W.; Bronson, R.T.; Shi, G.P.; Halperin, J.A.; Aktas, H.; Qin, X. Complement regulator cd59 protects against angiotensin ii-induced abdominal aortic aneurysms in mice. Circulation 2010, 121, 1338–1346. [Google Scholar] [CrossRef]
- Wu, G.; Hu, W.; Shahsafaei, A.; Song, W.; Dobarro, M.; Sukhova, G.K.; Bronson, R.R.; Shi, G.P.; Rother, R.P.; Halperin, J.A.; et al. Complement regulator cd59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ. Res. 2009, 104, 550–558. [Google Scholar] [CrossRef]
- Liu, F.; Wu, L.; Wu, G.; Wang, C.; Zhang, L.; Tomlinson, S.; Qin, X. Targeted mouse complement inhibitor cr2-crry protects against the development of atherosclerosis in mice. Atherosclerosis 2014, 234, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Liu, F.; Ren, M.; Qin, Z.; Rout, N.; Yang, X.-F.; Wang, H.; Tomlinson, S.; Qin, X. Complement inhibition targeted to injury specific neoepitopes attenuates atherogenesis in mice. Front. Cardiovasc. Med. 2021, 8, 731315. [Google Scholar] [CrossRef] [PubMed]
- Seidel, F.; Kleemann, R.; van Duyvenvoorde, W.; van Trigt, N.; Keijzer, N.; van der Kooij, S.; van Kooten, C.; Verschuren, L.; Menke, A.; Kiliaan, A.J.; et al. Therapeutic intervention with anti-complement component 5 antibody does not reduce nash but does attenuate atherosclerosis and mif concentrations in ldlr−/− Leiden mice. Int. J. Mol. Sci. 2022, 23, 10736. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; Sha, X.; Xi, H.; Li, Y.F.; Shao, Y.; Mai, J.; Virtue, A.; Lopez-Pastrana, J.; Meng, S.; et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arter. Thromb. Vasc. Biol. 2015, 35, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Winkels, H.; Ehinger, E.; Vassallo, M.; Buscher, K.; Dinh, H.Q.; Kobiyama, K.; Hamers, A.A.J.; Cochain, C.; Vafadarnejad, E.; Saliba, A.E.; et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell rna-sequencing and mass cytometry. Circ. Res. 2018, 122, 1675–1688. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, F.; Blair, R.; Wang, C.; Yang, H.; Mudd, J.; Currey, J.M.; Iwanaga, N.; He, J.; Mi, R.; et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics 2021, 11, 8076–8091. [Google Scholar] [CrossRef]


| Gene | Chromosome Location | Fold Change | p-Value |
|---|---|---|---|
| Casp-1 | Chr-9 | −1.36 | 0.002 |
| Eps8I1 | Chr-7 | −4.93 | 1.04 × 10−18 |
| Slc15a2 | Chr-16 | −5.42 | 3.37 × 10−40 |
| Capn11 | Chr-17 | −6.15 | 7.72 × 10−6 |
| Gene | Chromosome Location | Fold Change (PBMC) | Fold Change (Spleen) | p Value |
|---|---|---|---|---|
| GRB2 | Chr-11 | 1.87 | 0.004 | |
| Aqp1 | Chr-6 | 2.05 | 0.036 | |
| Ppp3cc | Chr-8 | −0.89 | 0.024 | |
| CD14 | Chr-18 | −1.50 | 0.004 | |
| Myc | Chr-11 | −1.55 | 0.013 | |
| Ear1 | Chr-14 | 3.94 | 6.31 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.A.; Caocci, M.; Ren, M.; Chen, Z.; Liu, F.; Khatun, M.S.; Kolls, J.K.; Qin, X.; Burdo, T.H. Deficiency of Caspase-1 Attenuates HIV-1-Associated Atherogenesis in Mice. Int. J. Mol. Sci. 2023, 24, 12871. https://doi.org/10.3390/ijms241612871
Alam MA, Caocci M, Ren M, Chen Z, Liu F, Khatun MS, Kolls JK, Qin X, Burdo TH. Deficiency of Caspase-1 Attenuates HIV-1-Associated Atherogenesis in Mice. International Journal of Molecular Sciences. 2023; 24(16):12871. https://doi.org/10.3390/ijms241612871
Chicago/Turabian StyleAlam, Mohammad Afaque, Maurizio Caocci, Mi Ren, Zheng Chen, Fengming Liu, Mst Shamima Khatun, Jay K. Kolls, Xuebin Qin, and Tricia H. Burdo. 2023. "Deficiency of Caspase-1 Attenuates HIV-1-Associated Atherogenesis in Mice" International Journal of Molecular Sciences 24, no. 16: 12871. https://doi.org/10.3390/ijms241612871
APA StyleAlam, M. A., Caocci, M., Ren, M., Chen, Z., Liu, F., Khatun, M. S., Kolls, J. K., Qin, X., & Burdo, T. H. (2023). Deficiency of Caspase-1 Attenuates HIV-1-Associated Atherogenesis in Mice. International Journal of Molecular Sciences, 24(16), 12871. https://doi.org/10.3390/ijms241612871






