Suaeda salsa NRT1.1 Is Involved in the Regulation of Tolerance to Salt Stress in Transgenic Arabidopsis
Abstract
1. Introduction
2. Results
2.1. Structural Analysis of the NRT Proteins from Suaeda salsa and Arabidopsis
2.2. Differential Expression of SsNRT1.1 Genes in Response to Salt Stress in Suaeda salsa
2.3. Subcellular Localization of the SsNRT1.1-GFP Fusion Protein
2.4. Overexpression of the SsNRT1.1 Genes in Transgenic Arabidopsis under Salt Stress
2.5. The Expression of SsNRT1.1C in S. salsa under Different Levels of Salt Stress and Construction of a Yeast Membrane Library
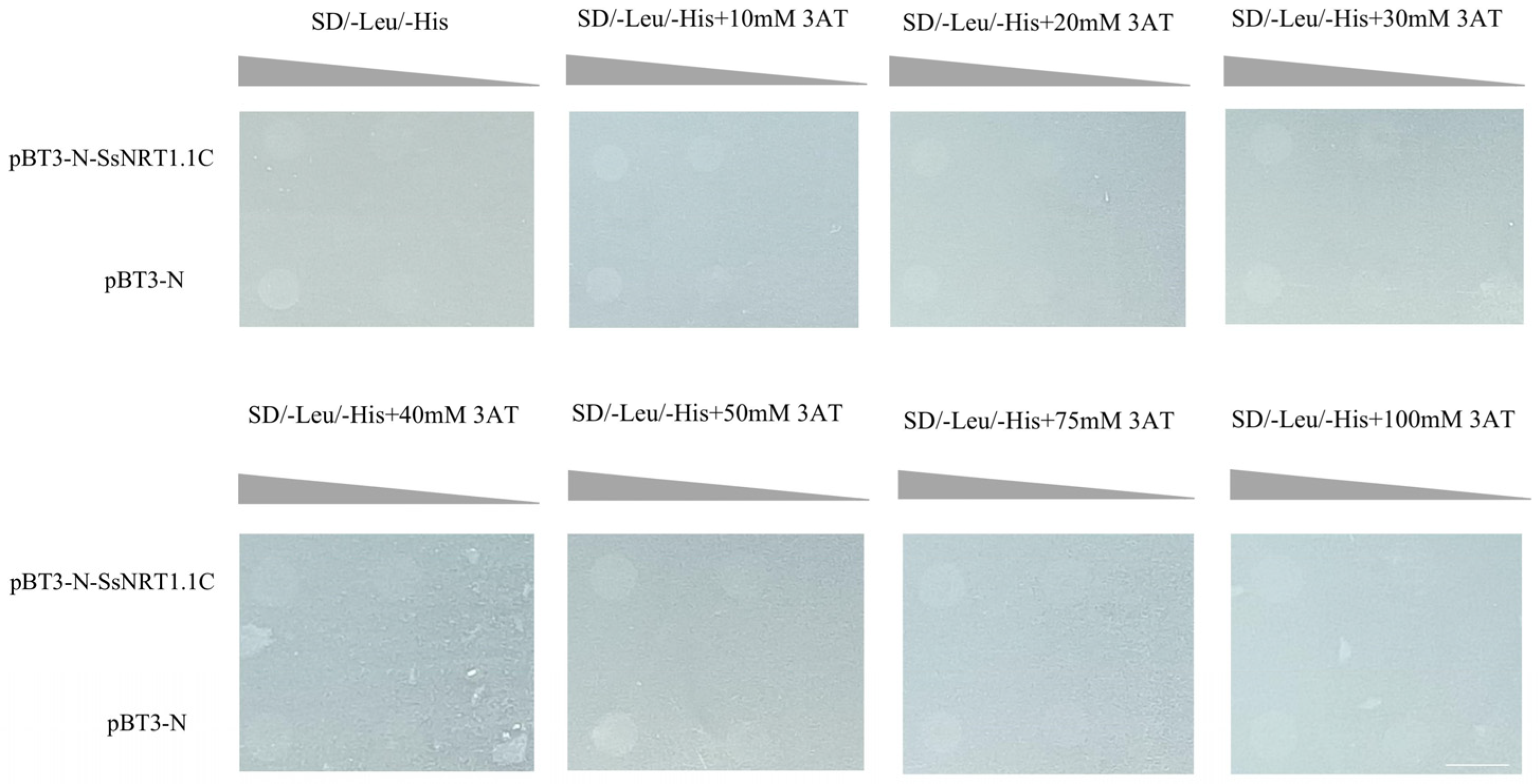
2.6. Constructed SsNRT1.1C Bait Vector
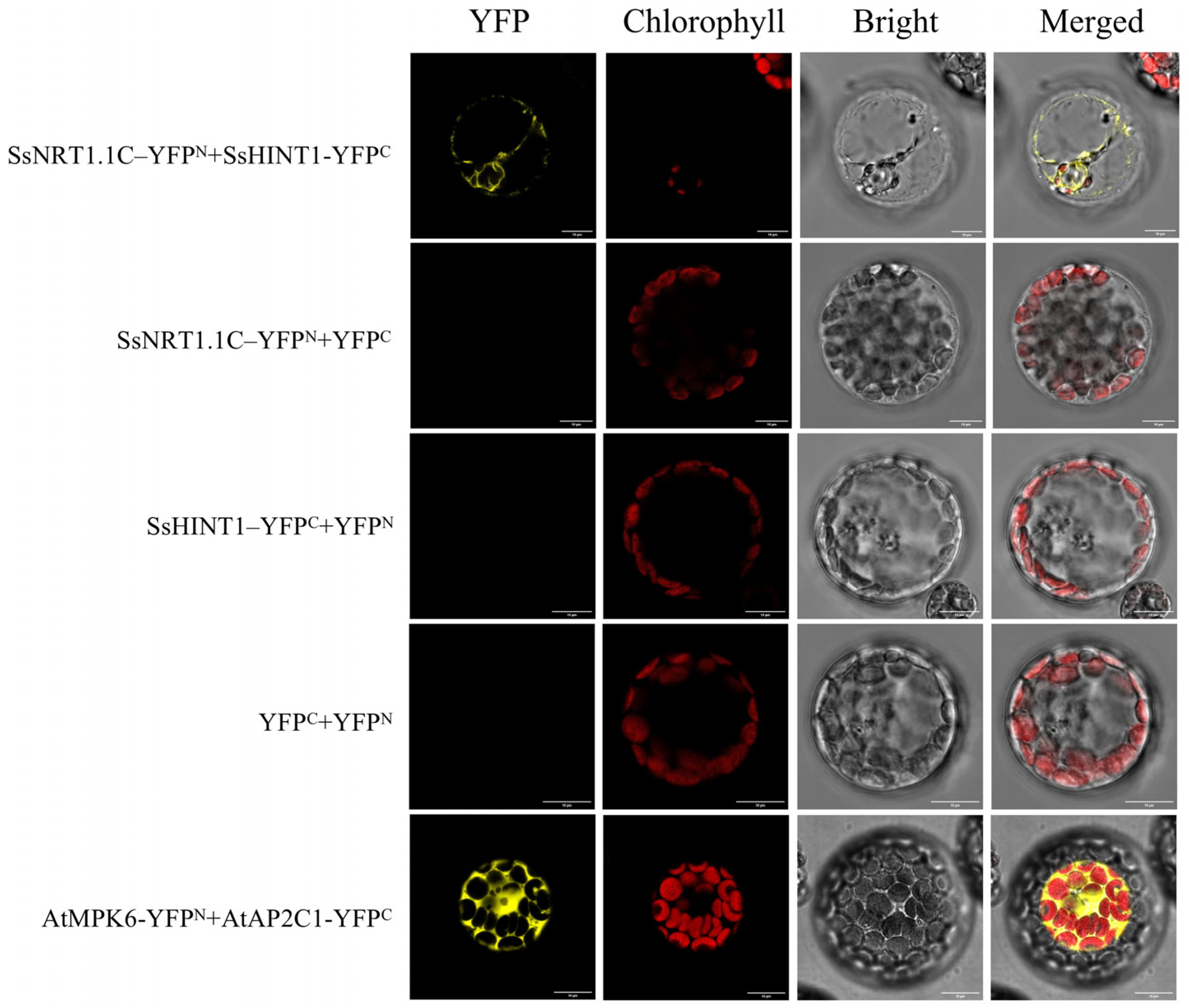
2.7. Yeast Two-Hybrid Screening and BiFC Validation
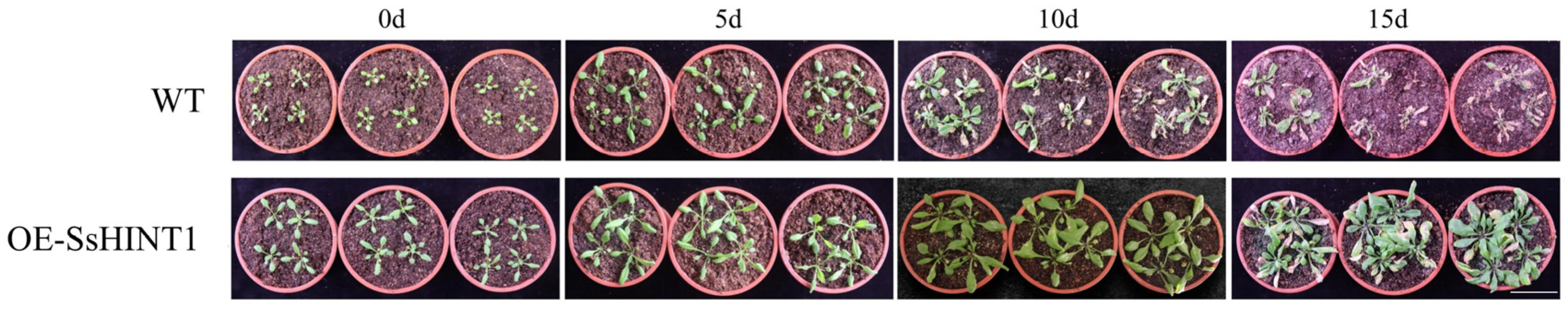
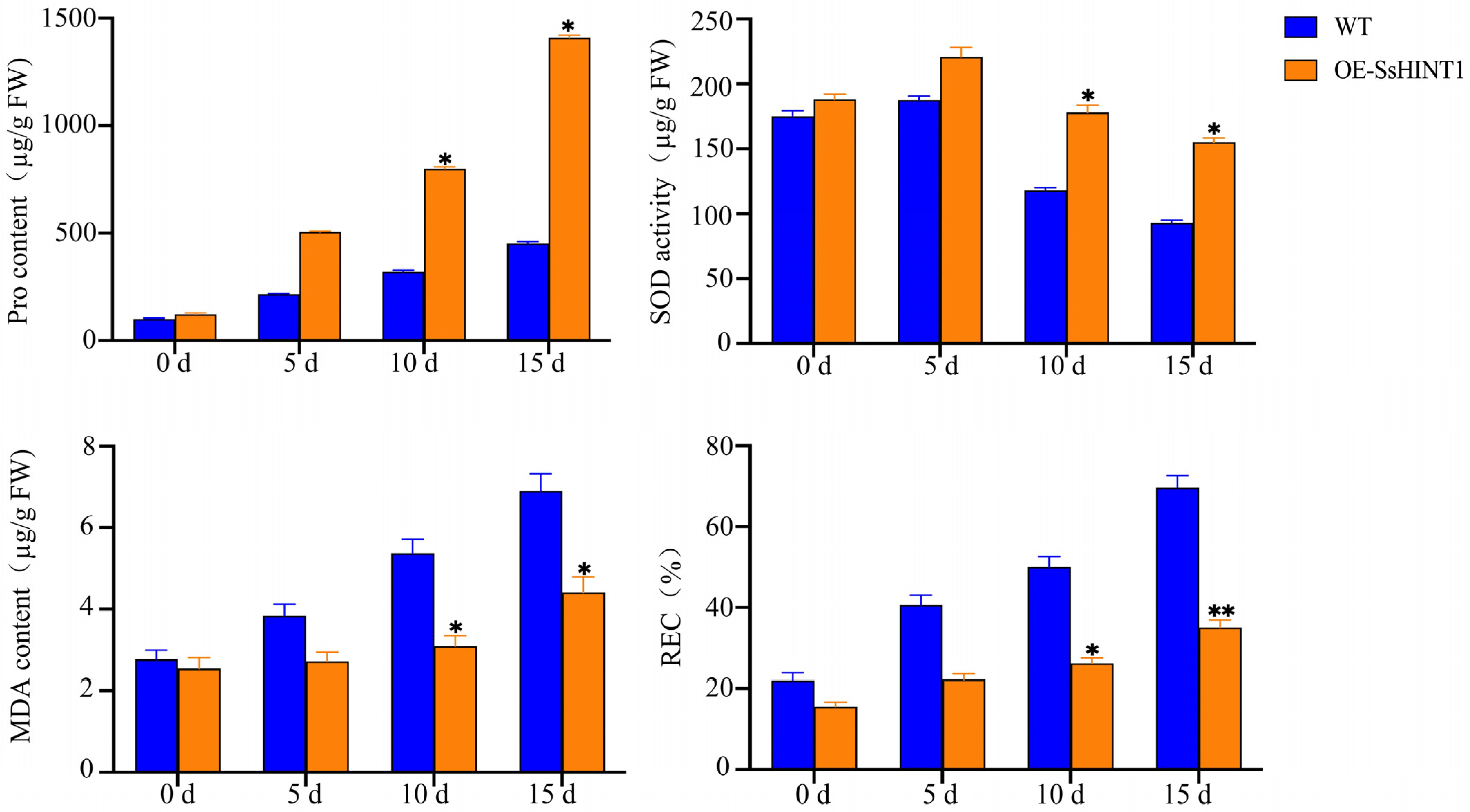
2.8. Overexpression of SsHINT1 in Transgenic Arabidopsis under Salt Stress
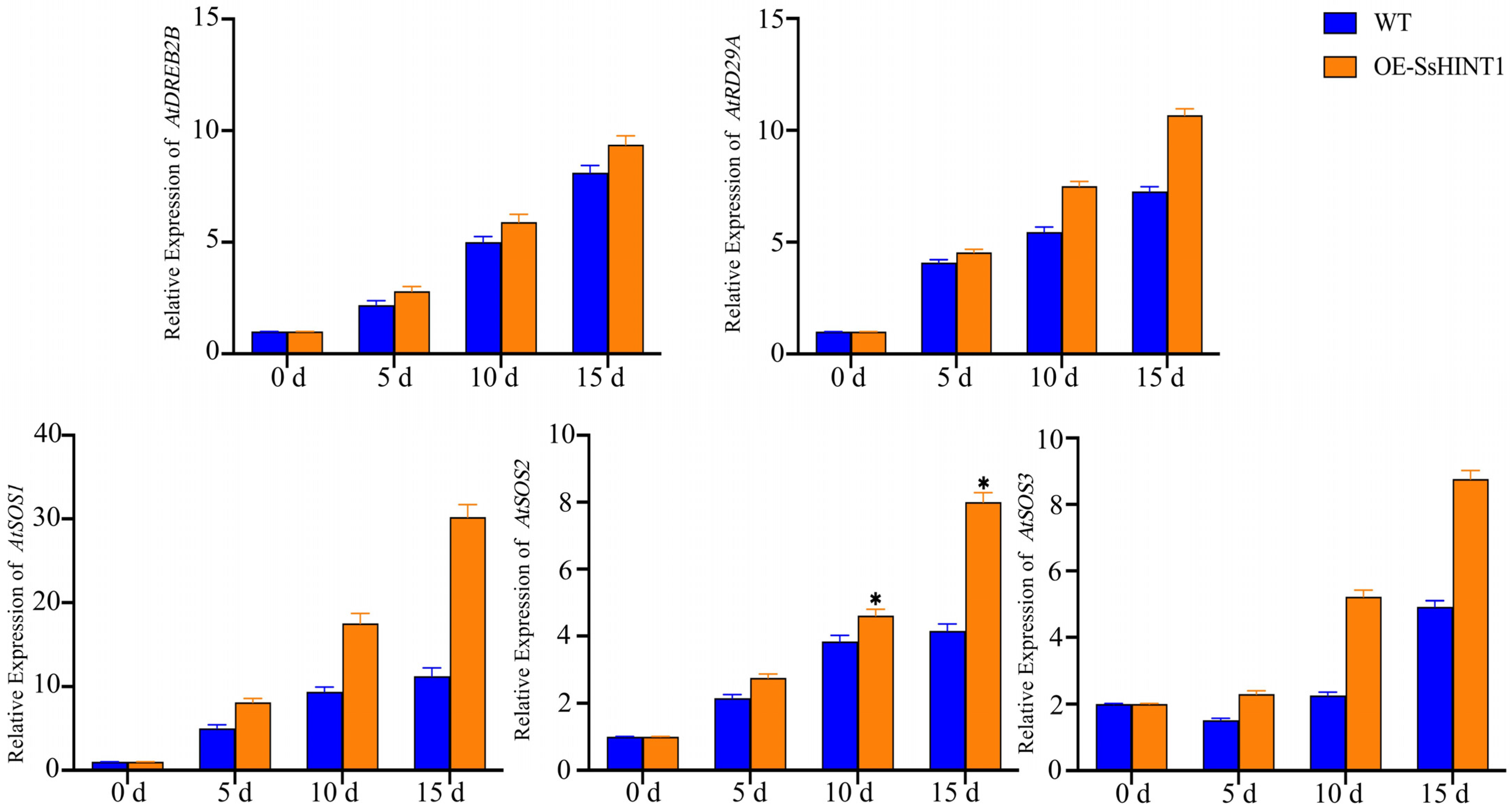
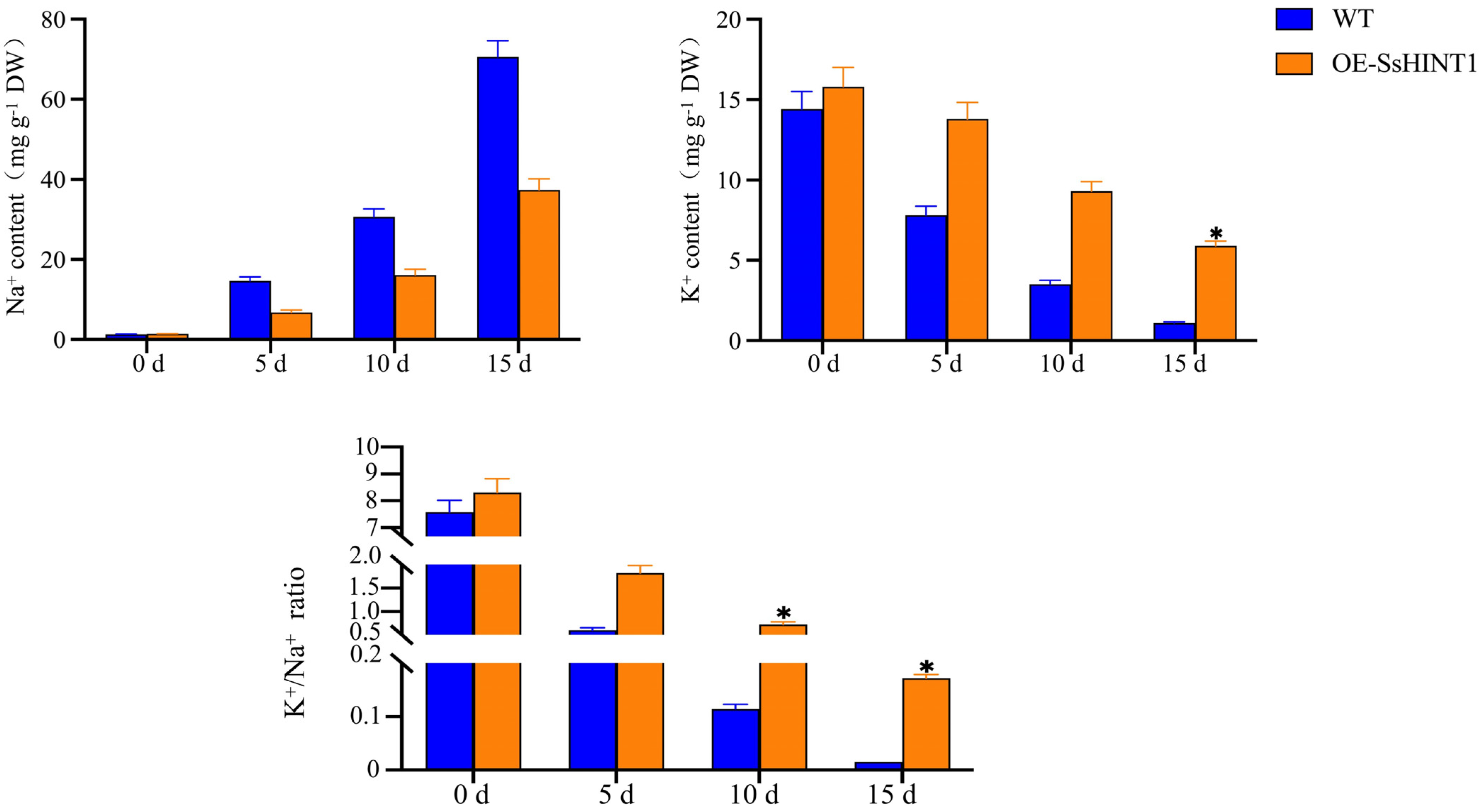
2.9. Expression and Ion Concentration Detection of Salt Resistance Genes Related to Salt Stress in Transgenic SsHINT1 Arabidopsis
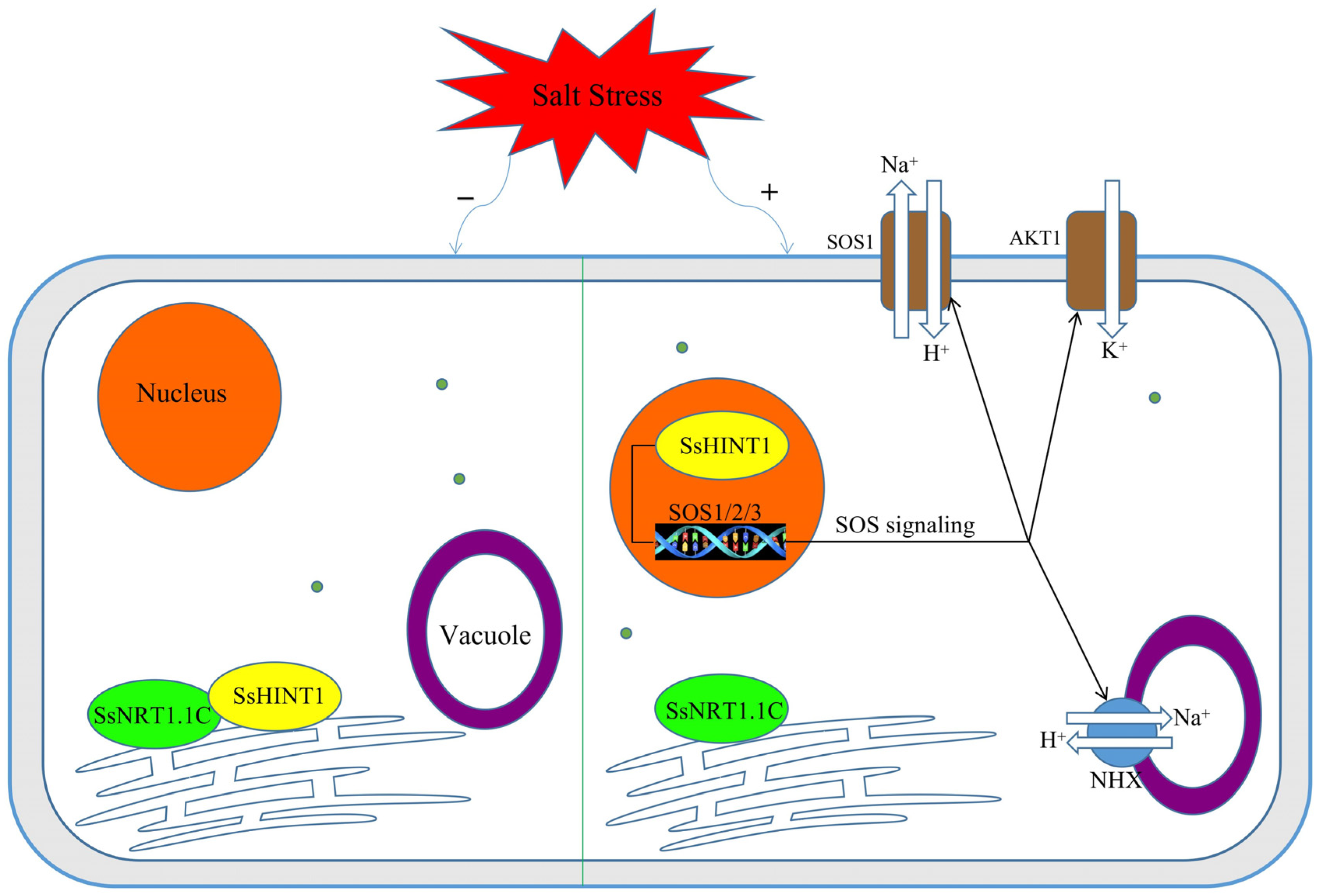
3. Discussion
4. Materials and Methods
4.1. Primers Used in the Experiment
4.2. Salt Stress Treatment of Suaeda salsa Seedlings in Soil
4.3. Search for SsNRT1.1 and Screening in Suaeda salsa
4.4. qRT-PCR Experiment
4.5. Subcellular Localization
4.6. Disinfection and Cultivation of Arabidopsis
4.7. Construction of a Salt Stress Yeast Library for Suaeda salsa in Saline Soil
4.7.1. Purification of mRNA
4.7.2. Amplification and Purification of the cDNA
4.7.3. Homogenization of cDNA and the Remove of Small Fragments
4.7.4. Homologous Recombination of cDNA and pPR3-N
4.7.5. Plasmid Transformation of Escherichia coli
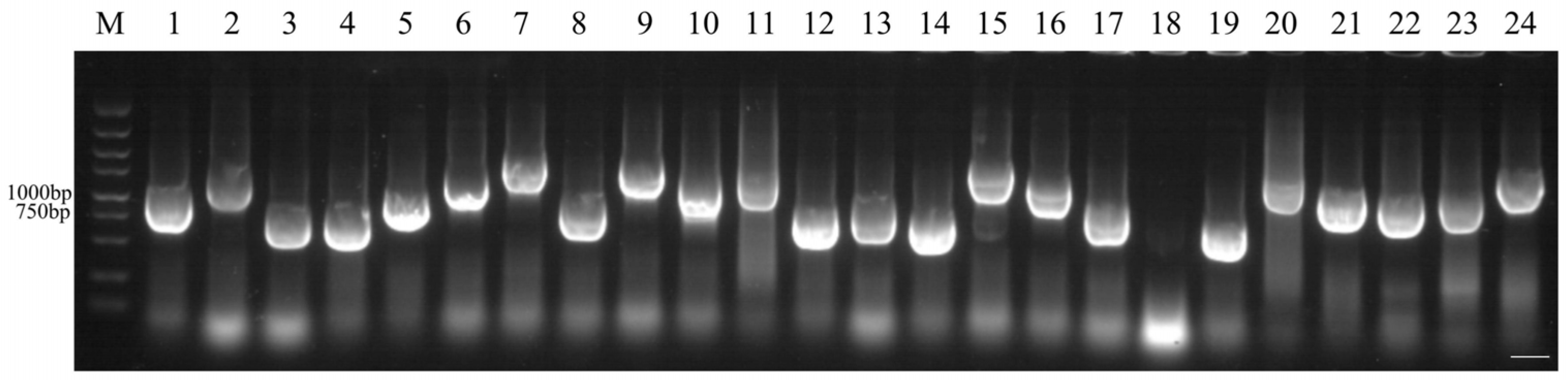
4.7.6. Library Identification
4.7.7. Library Plasmid Extraction
4.8. Yeast Transformation and Detection of Self-Activation
4.8.1. Transfer of pBT3-N-SsNRT1.1C and pBT3-N into NMY51
4.8.2. Detection of Self-Activation
4.8.3. Yeast Library Screening
4.8.4. Library DNA Transformation
4.8.5. Positive Clone Identification and Sequencing Comparison
4.8.6. Rotational Validation of the Positive Yeast Clones
4.9. Double Molecule Fluorescence Complementary Experiment (BiFC) to Detect the Protein Interactions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Yu, C.; Hunag, L.; Wu, M.; Liu, B.; Liu, Y.; Song, G.; Liu, D.; Gan, Y. Overexpression of MADS-box transcription factor OsMADS25 enhances salt stress tolerance in Rice and Arabidopsis. Plant Growth Regul. 2020, 90, 163–171. [Google Scholar] [CrossRef]
- Nabati, J.; Nabati, J.; Kafi, M.; Nezami, A.; Moghaddam, P.R.; Ali, M.; Mehrjerdi, M.Z. Effect of salinity on biomass production and activities of some key enzymatic antioxidants in Kochia (Kochia scoparia). Pak. J. Bot. 2011, 43, 539–548. [Google Scholar]
- Ludwig, L.; Wilmes, P.; Schrader, S. Measuring soil sustainability via soil resilience. Sci. Total Environ. 2018, 626, 1484–1493. [Google Scholar] [CrossRef]
- Yang, S.; Hou, L.L.; Guo, F.; Zhang, J.L.; Geng, Y.; Meng, J.J.; Li, X.G.; Wan, S.B. Effects of exogenous Ca2+ on growth and development, physiology and yield of peanut under salt stress. Chin. J. Appl. Ecol. 2017, 28, 894–900. [Google Scholar]
- Shao, G.; Chen, M.; Wang, W.; Zhang, G. The Effect of Salinity Pretreatment on Cd Accumulation and Cd-Induced Stress in BADH-Transgenic and Nontransgenic Rice Seedlings. J. Plant Growth Regul. 2008, 27, 205–210. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; De Pascale, S. Contrasting Effects of GA3 Treatments on Tomato Plants Exposed to Increasing Salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Ying, M.; Wang, X.; Zhang, S.F.; Shi, D.; Sheng, L.X. Effects of salt and alkali stresses on growth and ions accumulation in shoot of medicinal plant Kochia sieversiana. Biologia. Plantarum. 2016, 60, 774–782. [Google Scholar]
- Lin, Y.H. Effects of potassium behaviour in soils on crop absorption. Afr. J. Biotechnol. 2010, 9, 4638–4643. [Google Scholar]
- Chen, D.; Afriyie, A.J.; Li, R.; Dominic, K.; Li, L.; Li, R.; Zhao, W. Molecular cloning of potassium transporter gene, MaHAK5 of mulberry (Morus alba L.) and gene expression and biochemistry analysis under potassium stress. J. Pomol. Hortic. Sci. 2018, 94, 130–136. [Google Scholar] [CrossRef]
- Ankit, A.; Kamali, S.; Singh, A. Genomic & structural diversity and functional role of potassium (K+) transport proteins in plants. Int. J. Biol. Macromol. 2022, 208, 844–857. [Google Scholar]
- Takahashi, R.; Liu, S.; Takano, T. Cloning and functional comparison of a high-affinity K+ transporter gene PhaHKT1 of salt-tolerant and salt-sensitive reed plants. J. Exp. Bot. 2007, 58, 4387–4395. [Google Scholar] [CrossRef]
- Thompson, A.N.; Kim, I.; Panosian, T.D.; Iverson, T.M.; Allen, T.W.; Nimigean, C.M. Mechanism of potassium-channel selectivity revealed by Na+ and Li+ binding sites within the KcsA pore. Nat. Struct. Mol. Biol. 2009, 16, 1317–1326. [Google Scholar] [CrossRef]
- Verslues, P.E.; Batelli, G.; Grillo, S.; Agius, F.; Kim, Y.-S.; Zhu, J.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.-K. Interaction of SOS2 with Nucleoside Diphosphate Kinase 2 and Catalases Reveals a Point of Connection between Salt Stress and H2O2 Signaling in Arabidopsis thaliana. Mol. Cell. Biol. 2007, 27, 7771–7780. [Google Scholar] [CrossRef]
- Duan, L.; Dietrich, D.; Ng, C.H.; Chan, P.M.Y.; Bhalerao, R.; Bennett, M.J.; Dinneny, J.R. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 2013, 25, 324–341. [Google Scholar] [CrossRef]
- Chen, D.-D.; Chen, M.; Xue, F.-Y.; Xu, Z.-S.; Li, L.-C.; Ma, Y.-Z.; Min, D.-H. Association Analysis of K+ Uptake Features and Salt Stress Responsive Signal Pathway SOS in Arabidopsis. Sci. Agric. Sin. 2012, 45, 4967–4977. [Google Scholar]
- Li, J.; Shen, L.; Han, X.; He, G.; Fan, W.; Li, Y.; Yang, S.; Zhang, Z.; Yang, Y.; Jin, W.; et al. Phosphatidic acid-regulated SOS2 controls sodium and potassium homeostasis in Arabidopsis under salt stress. EMBO J. 2023, 42, e112401. [Google Scholar] [CrossRef]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty Years from Transport to Signaling Networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef]
- Gimeno, V.; Syvertsen, J.; Nieves, M.; Simón, I.; Martínez, V.; García-Sánchez, F. Additional nitrogen fertilization affects salt tolerance of lemon trees on different rootstocks. Sci. Hortic. 2009, 121, 298–305. [Google Scholar] [CrossRef]
- Bai, J.; Jia, J.; Huang, C.; Wang, Q.; Wang, W.; Zhang, G.; Cui, B.; Liu, X. Selective uptake of nitrogen by Suaeda salsa under drought and salt stresses and nitrogen fertilization using 15N. Ecol. Eng. 2017, 102, 542–545. [Google Scholar] [CrossRef]
- Hu, Y.; Bellaloui, N.; Tigabu, M.; Wang, J.; Diao, J.; Wang, K.; Yang, R.; Sun, G. Gaseous NO2 effects on stomatal behavior, photosynthesis and respiration of hybrid poplar leaves. Acta Physiol. Plant. 2015, 37, 39. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the Nitrate Transporter Gene OsNRT1.1A/OsNPF6.3 Confers High Yield and Early Maturation in Rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef]
- Huang, W.; Li, W.; Niu, Z.; Xie, Z.; Liu, X. Interactive Effect of Salinity and Drought on the Germination of Dimorphic Seeds of Suaeda salsa. In Sabkha Ecosystems; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Mori, S.; Yoshiba, M.; Tadano, T. Mechanisms of growth stimulation induced by a high concentration of NaCl in Suaeda salsa (L.) Pall. Int. Plant Nutr. Colloq. 2005, 46, S249. [Google Scholar]
- Zhang, X.; Yao, Y.; Li, X.; Zhang, L.; Fan, S. Transcriptomic analysis identifies novel genes and pathways for salt stress responses in Suaeda salsa leaves. Sci. Rep. 2020, 10, 4236. [Google Scholar] [CrossRef]
- Guo, S.-M.; Tan, Y.; Chu, H.-J.; Sun, M.-X.; Xing, J.-C. Transcriptome sequencing revealed molecular mechanisms underlying tolerance of Suaeda salsa to saline stress. PLoS ONE 2019, 14, e0219979. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Li, A.; Chu, C. NRT1.1 in plants: Functions beyond nitrate transport. J. Exp. Bot. 2019, 71, 4373–4379. [Google Scholar] [CrossRef]
- Wang, P.; Yamaji, N.; Inoue, K.; Mochida, K.; Ma, J.F. Plastic transport systems of rice for mineral elements in response to diverse soil environmental changes. New Phytol. 2020, 226, 156–169. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, J.; Wang, Y.; Wang, F.; Mao, W.; He, Q.; Xu, J.; Wu, Z.; Mao, C. PROTEIN PHOSPHATASE95 Regulates Phosphate Homeostasis by Affecting Phosphate Transporter Trafficking in Rice. Plant Cell 2020, 32, 740–757. [Google Scholar] [CrossRef]
- Zhu, J.; Fang, X.Z.; Dai, Y.J.; Zhu, Y.X.; Chen, H.S.; Lin, X.Y.; Jin, C.W. Nitrate transporter 1.1 alleviates lead toxicity in Arabidopsis by preventing rhizosphere acidification. J. Exp. Bot. 2019, 70, 6363–6374. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Guan, M.Y.; Lu, K.X.; Du, S.T.; Fan, S.K.; Ye, Y.Q.; Lin, X.Y.; Jin, C.W. Inhibition of Nitrate Transporter 1.1-Controlled Nitrate Uptake Reduces Cadmium Uptake in Arabidopsis. Plant Physiol. 2014, 166, 934–944. [Google Scholar] [CrossRef]
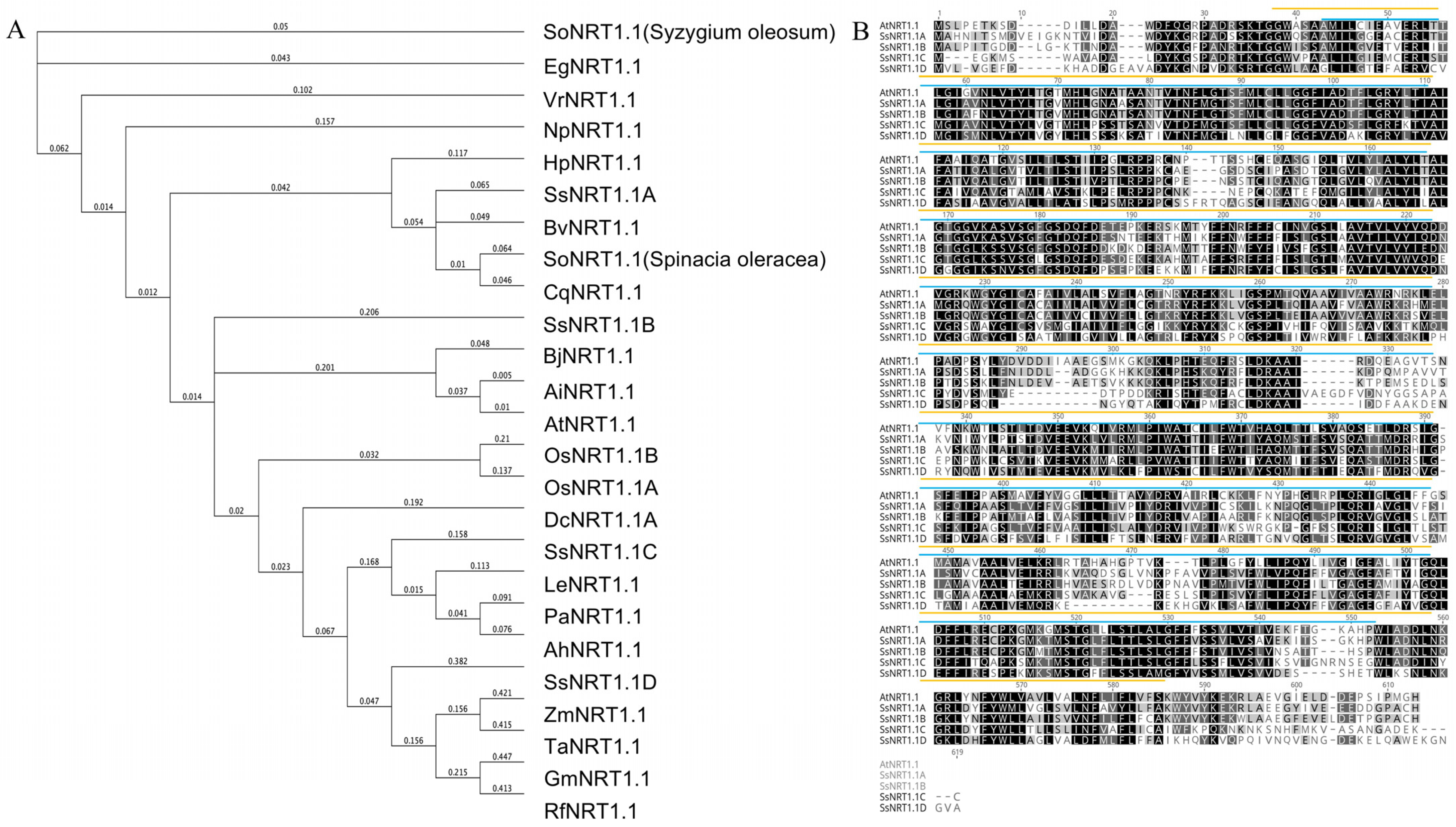
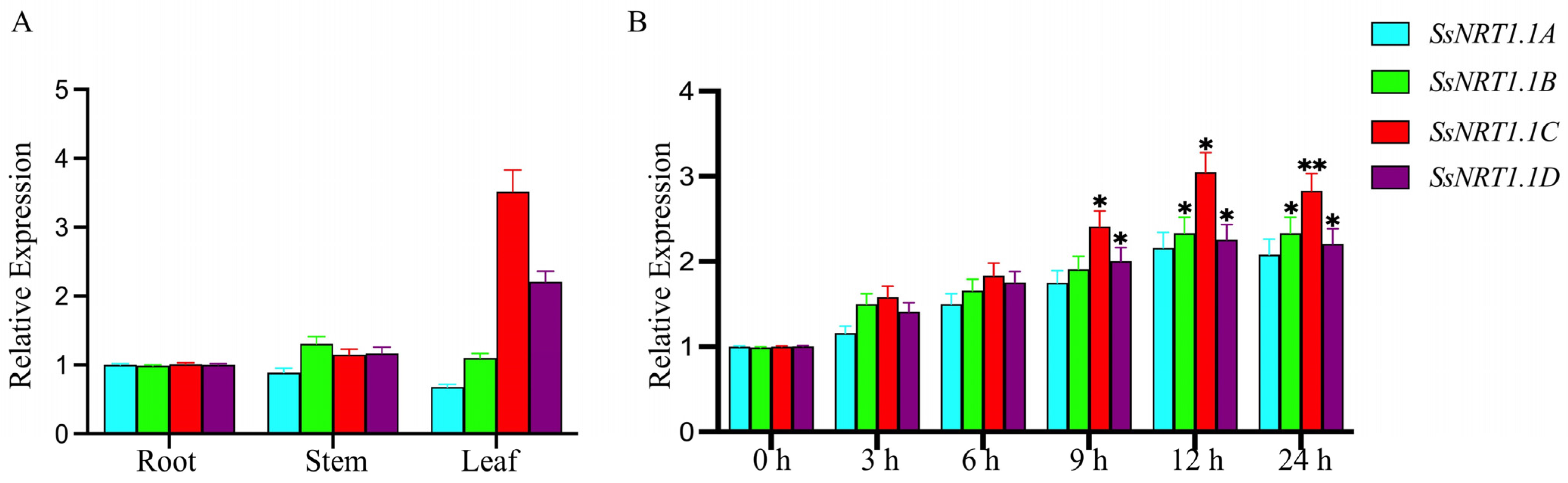

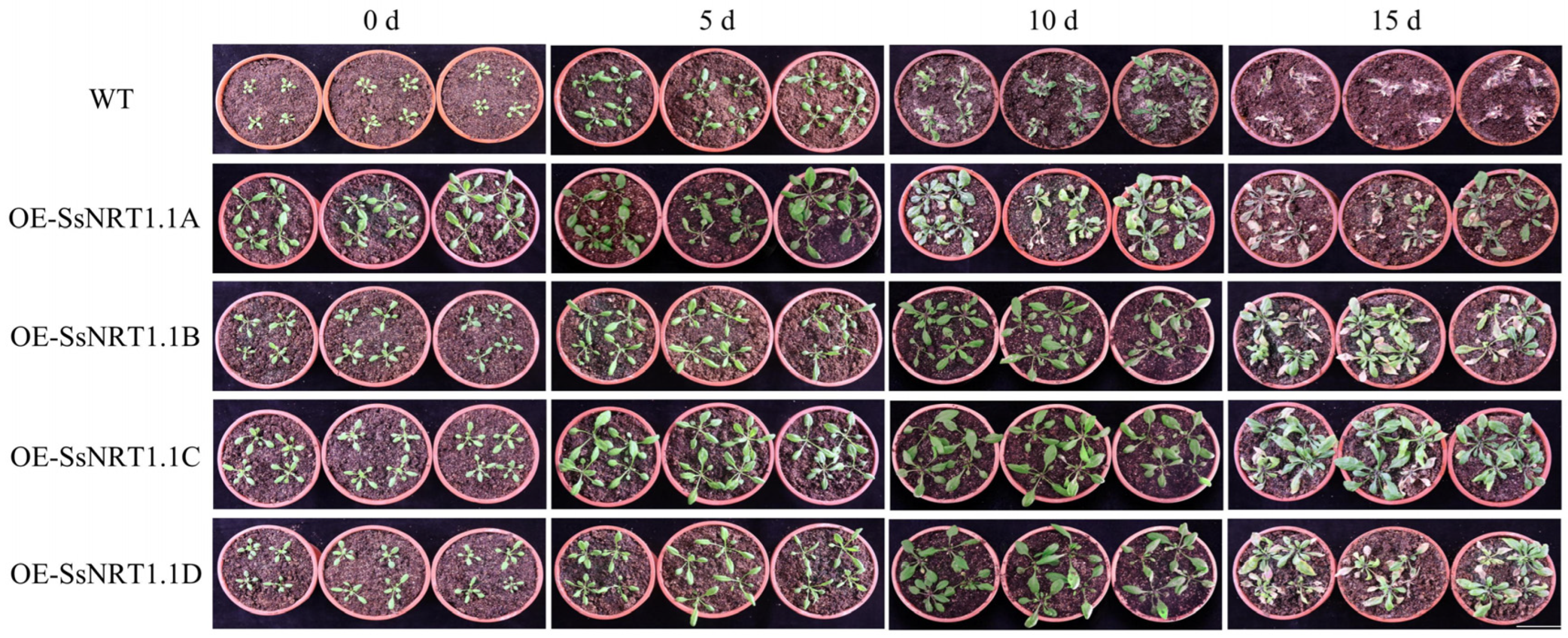
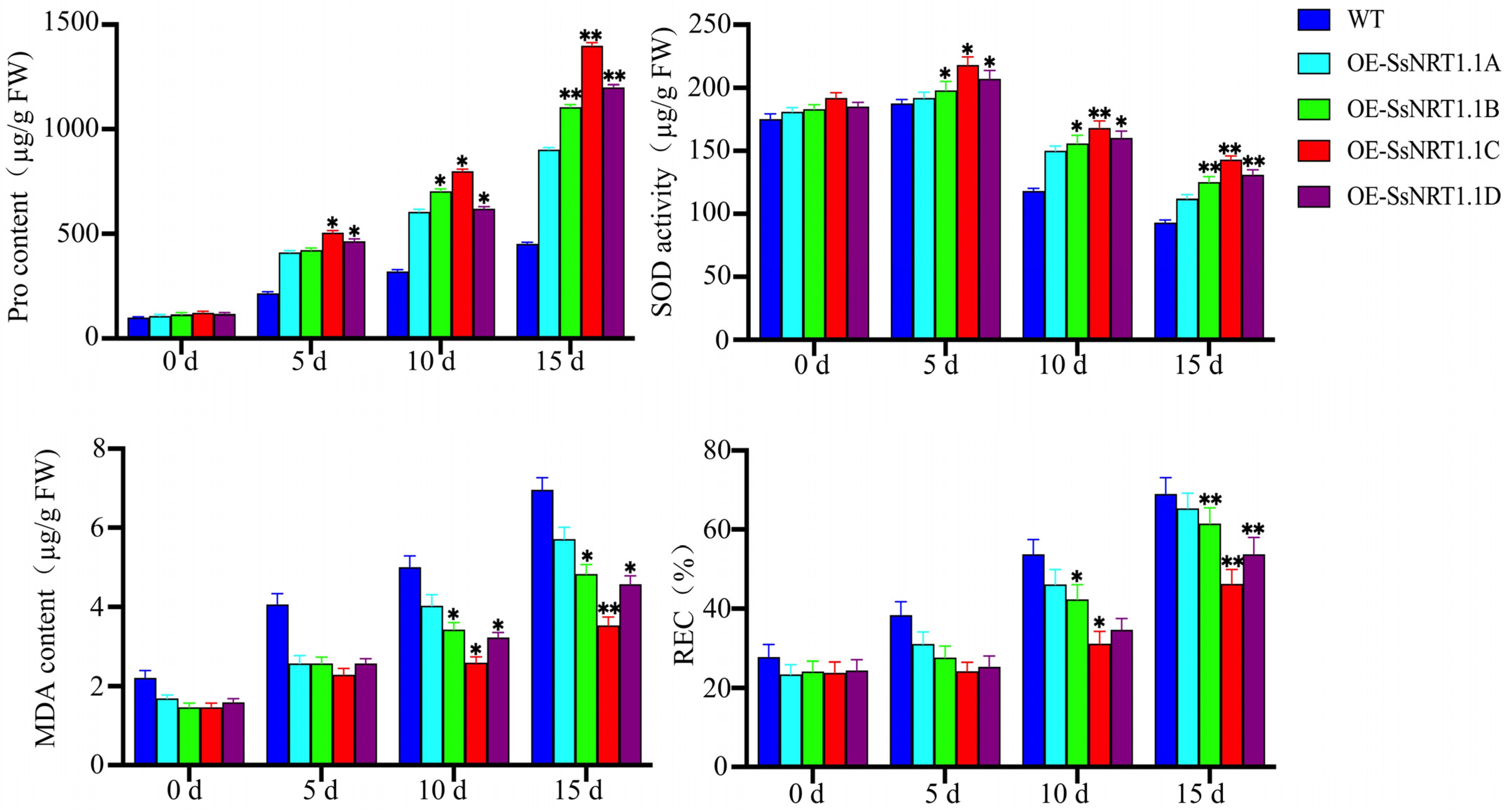




| Gene Name | CDS Length/bp | Amino Acid Number | Molecular Weight/kDa | pI | Instability Coefficient | Hydrophilicity |
|---|---|---|---|---|---|---|
| SsNRT1.1A | 1791 | 596 | 65.777 | 8.71 | 36.93 | 0.391 |
| SsNRT1.1B | 1782 | 593 | 65.186 | 7.54 | 33.37 | 0.398 |
| SsNRT1.1C | 1755 | 584 | 64.006 | 8.87 | 26.57 | 0.363 |
| SsNRT1.1D | 1746 | 581 | 64.185 | 8.51 | 25.00 | 0.291 |
| Primer Name | Sequence |
|---|---|
| OE-SsNRT1.1A-F | ACTAGGGTCTCGCACCATGGCTCTTCCTATAACAGGCGAT |
| OE-SsNRT1.1A-R | ACTAGGGTCTCTACCGTCAATGGCAGGCTGGGGT |
| OE-SsNRT1.1B-F | ACTAGGGTCTCGCACCATGGCTCATAATATTACTAGTATGG |
| OE-SsNRT1.1B-R | ACTAGGGTCTCTACCGTTAGTGACAAGCCGGACCATCGTC |
| OE-SsNRT1.1C-F | ACTAGGGTCTCGCACCATGGAGGGGAAGATGAGTTGGG |
| OE-SsNRT1.1C-R | ACTAGGGTCTCTACCGCTAGCATTTTTCATCAGCACCATTA |
| OE-SsNRT1.1D-F | ACTAGGGTCTCGCACCATGGTTTTAGTTGGAGAGTTTGA |
| OE-SsNRT1.1D-R | ACTAGGGTCTCTACCGTTAGGCAACTCCATTGCCTTTTTCCC |
| OE-SsHINT1-F | ACTAGGGTCTCGCACCATGAGCAATGACAATCTTATAA |
| OE-SsHINT1-R | ACTAGGGTCTCTACCCAAATGAACTGGCCCCCTGGCT |
| CDS III/3′ PCR Primer | AAGCAGTGGTATCAACGCAGAGTGGCCATTATGGCCGGG |
| SMART IV Oligonucleotide | ATTCTAGAGGCCGAGGCGGCCGACATGTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTVN |
| P1-F | TACGATGTTCCAGATTACGCTGGATCCAAGCAGTGGTATCAACGCAGAGTGG |
| P2-R | TACGATGTTCCAGATTACGCTGGATCCAAAGCAGTGGTATCAACGCAGAGTGG |
| P3-F | TACGATGTTCCAGATTACGCTGGATCCAAAAGCAGTGGTATCAACGCAGAGTGG |
| P4-R | GGTATCGATAAGCTTGATATCGAATTCCTAGAGGCCGAGGCGGCCGACATG |
| Prime M1 | AAGCAGTGGTATCAACGCAGAGT |
| pPR3-N-F | CGGTAAAACCGGAACATTGGA |
| pPR3-N-R | ACTTCAGGTTGTCTAACTCCT |
| GFP-SsNRT1.1A-F | ACTAGGGTCTCGCTCCATGGCTCTTCCTATAACAGGCGAT |
| GFP-SsNRT1.1A-R | ACTAGGGTCTCTACCGTCAATGGCAGGCTGGGGT |
| GFP-SsNRT1.1B-F | ACTAGGGTCTCGCTCCATGGCTCATAATATTACTAGTATGG |
| GFP-SsNRT1.1B-R | ACTAGGGTCTCTTTAGTGACAAGCCGGACCATCGTC |
| GFP-SsNRT1.1C-F | ACTAGGGTCTCGCTCCATGGAGGGGAAGATGAGTTGGG |
| GFP-SsNRT1.1C-R | ACTAGGGTCTCTACCGCTAGCATTTTTCATCAGCACCATTA |
| GFP-SsNRT1.1D-F | ACTAGGGTCTCGCTCCATGGTTTTAGTTGGAGAGTTTGATAAACATGCT |
| GFP-SsNRT1.1D-R | ACTAGGGTCTCTACCGTTAGGCAACTCCATTGCCTTTTTCCC |
| ACTIN-F | TGGTGTCATGGTTGGGATG |
| ACTIN-R | CACCACTGAGCACAATGTTAC |
| qRT-SsNRT1.1A-F | AAGCTTGGGGTCACTAGCAG |
| qRT-SsNRT1.1A-R | CTGCTGTTTTTGTCGCTGCT |
| qRT-SsNRT1.1B-F | AGGTTTTCCGGCTAATCGCA |
| qRT-SsNRT1.1B-R | AGGTTTTCCGGCTAATCGCA |
| qRT-SsNRT1.1C-F | GCTTCGACCACCACCATGTA |
| qRT-SsNRT1.1C-R | GCTTCGACCACCACCATGTA |
| qRT-SsNRT1.1D-F | TACTGGTGGATGGCTTGCTG |
| qRT-SsNRT1.1D-R | TACTGGTGGATGGCTTGCTG |
| qRT-SsHINT1-F | ACAGGAAGGCCTTGACGATG |
| qRT-SsHINT1-R | TTTGGCGTCCTCCAAGAAGG |
| pBT3-SsNRT1.1C-F | AATTCCTGCAGGGCCATTACGGCCATGGAGGGGAAGATGAGTTG |
| pBT3-SsNRT1.1C-R | CTACTTACCATGGGGCCGAGGCGGCCATGGTGCTGATGAAAAATGC |
| BiFC-SsNRT1.1C-F | ACTAGGGTCTCGCACCATGGAGGGGAAGATGAGTTGGGC |
| BiFC-SsNRT1.1C-R | ACTAGGGTCTCTCGGAGCATTTTTCATCAGCACCATTAGCACTAGC |
| BiFC-SsHINT1-F | ACTAGGGTCTCGCACCATGGCTCAATCAGCGAAACCAATCAC |
| BiFC-SsHINT1- R | ACTAGGGTCTCTCGGAGACATAAACACCGGTTGATAGGAGTAAAAATAAGGAT |
| At SOS1- F | CCTCGAGAAGGTTGGCTTGT |
| At SOS1-R | ATGCAGGAGGAAGAGCAACC |
| At SOS2-F | GCTCATTGTCACTGCAAGGG |
| At SOS2-R | ACTCCTTCCTGAGGCAATGC |
| At SOS3-F | GTCACGCCATTCACGGTAGA |
| At SOS3-R | GTCACGCCATTCACGGTAGA |
| AtRD22-F | TTTGGAATACGCGGGACACA |
| AtRD22-R | AAGGAACCATCGTGCAGCTT |
| AtRD29A-F | TGAAGCCAGAATCGCCACAT |
| AtRD29A-R | TGAAGCCAGAATCGCCACAT |
| Component | Volume (µL) |
|---|---|
| cDNA | 3–5 |
| 2 × qPCR SuperMix | 10 |
| Primer-F (10 μM) | 1 |
| Primer-R (10 μM) | 1 |
| ddH2O | 3–5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Wang, S.; Cui, C.; Wu, X.; Zhu, J. Suaeda salsa NRT1.1 Is Involved in the Regulation of Tolerance to Salt Stress in Transgenic Arabidopsis. Int. J. Mol. Sci. 2023, 24, 12761. https://doi.org/10.3390/ijms241612761
Xiong Y, Wang S, Cui C, Wu X, Zhu J. Suaeda salsa NRT1.1 Is Involved in the Regulation of Tolerance to Salt Stress in Transgenic Arabidopsis. International Journal of Molecular Sciences. 2023; 24(16):12761. https://doi.org/10.3390/ijms241612761
Chicago/Turabian StyleXiong, Yi, Saisai Wang, Cuijie Cui, Xiaoyan Wu, and Jianbo Zhu. 2023. "Suaeda salsa NRT1.1 Is Involved in the Regulation of Tolerance to Salt Stress in Transgenic Arabidopsis" International Journal of Molecular Sciences 24, no. 16: 12761. https://doi.org/10.3390/ijms241612761
APA StyleXiong, Y., Wang, S., Cui, C., Wu, X., & Zhu, J. (2023). Suaeda salsa NRT1.1 Is Involved in the Regulation of Tolerance to Salt Stress in Transgenic Arabidopsis. International Journal of Molecular Sciences, 24(16), 12761. https://doi.org/10.3390/ijms241612761





