PAX2 Gene Mutation in Pediatric Renal Disorders—A Narrative Review
Abstract
1. Introduction
2. Literature Review for PAX2-Related Kidney Disorders
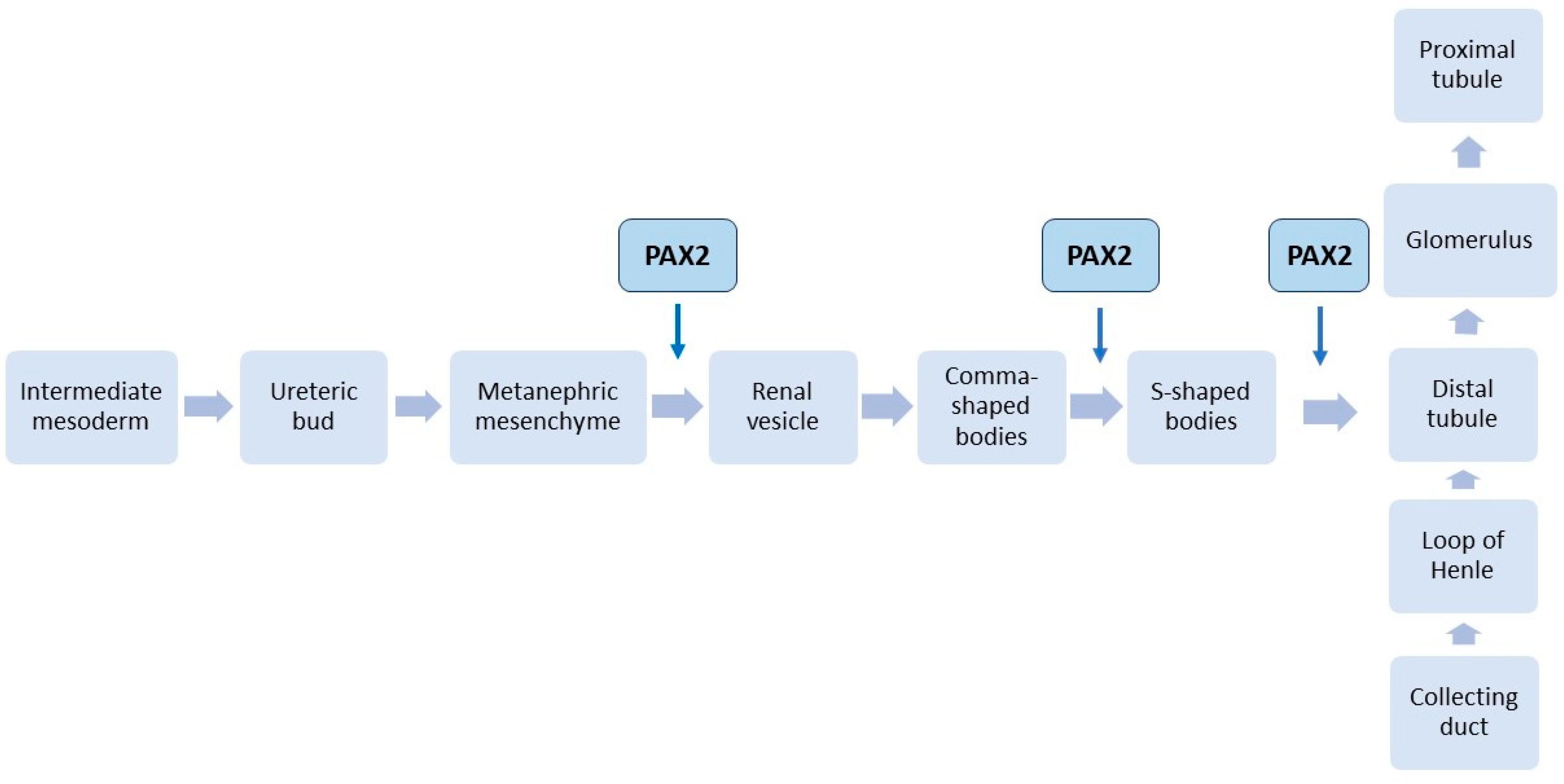
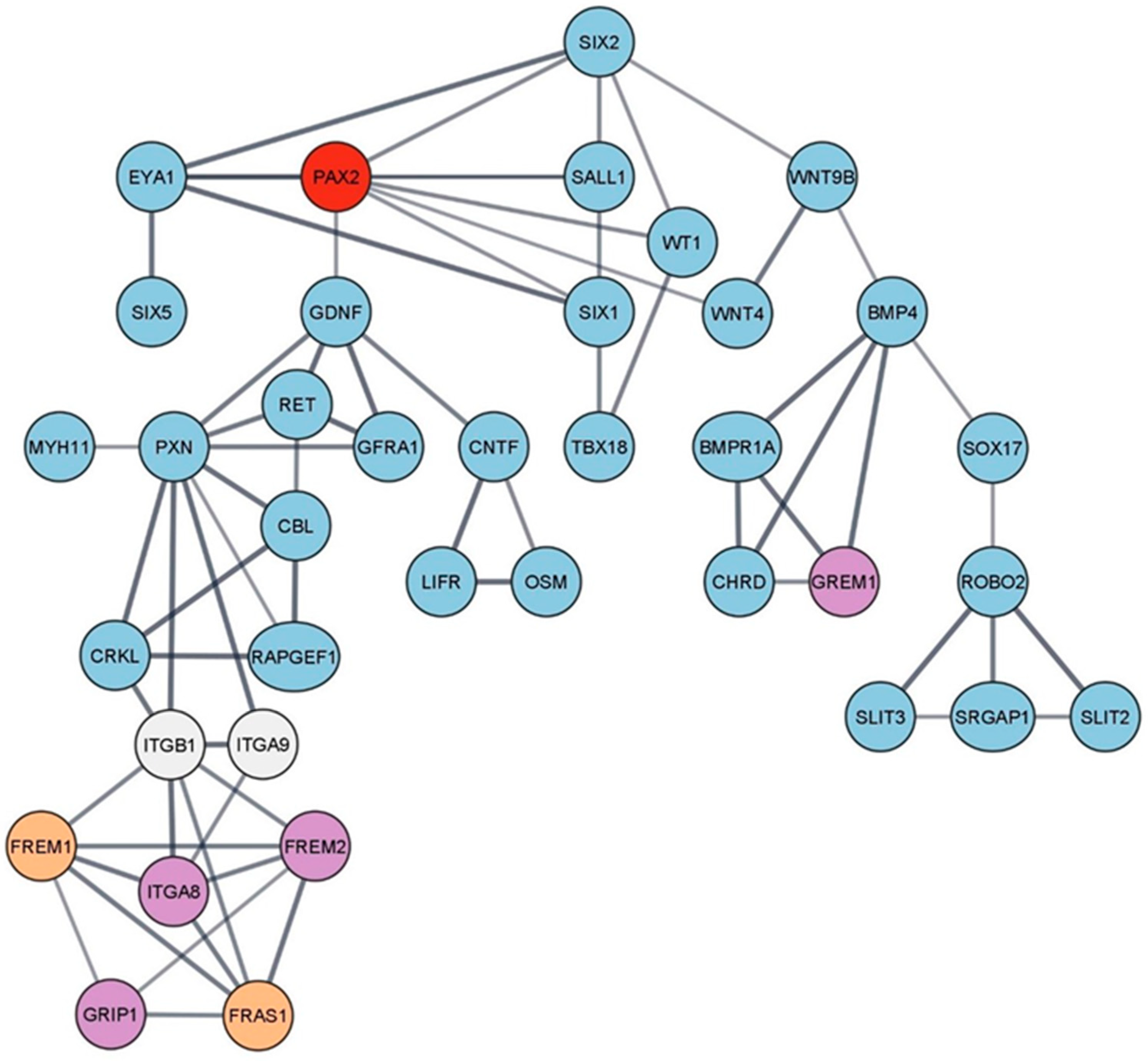
3. PAX2 Gene and Kidneys
3.1. PAX2 Gene Mutations
3.2. PAX2 Mutations’ Consequences
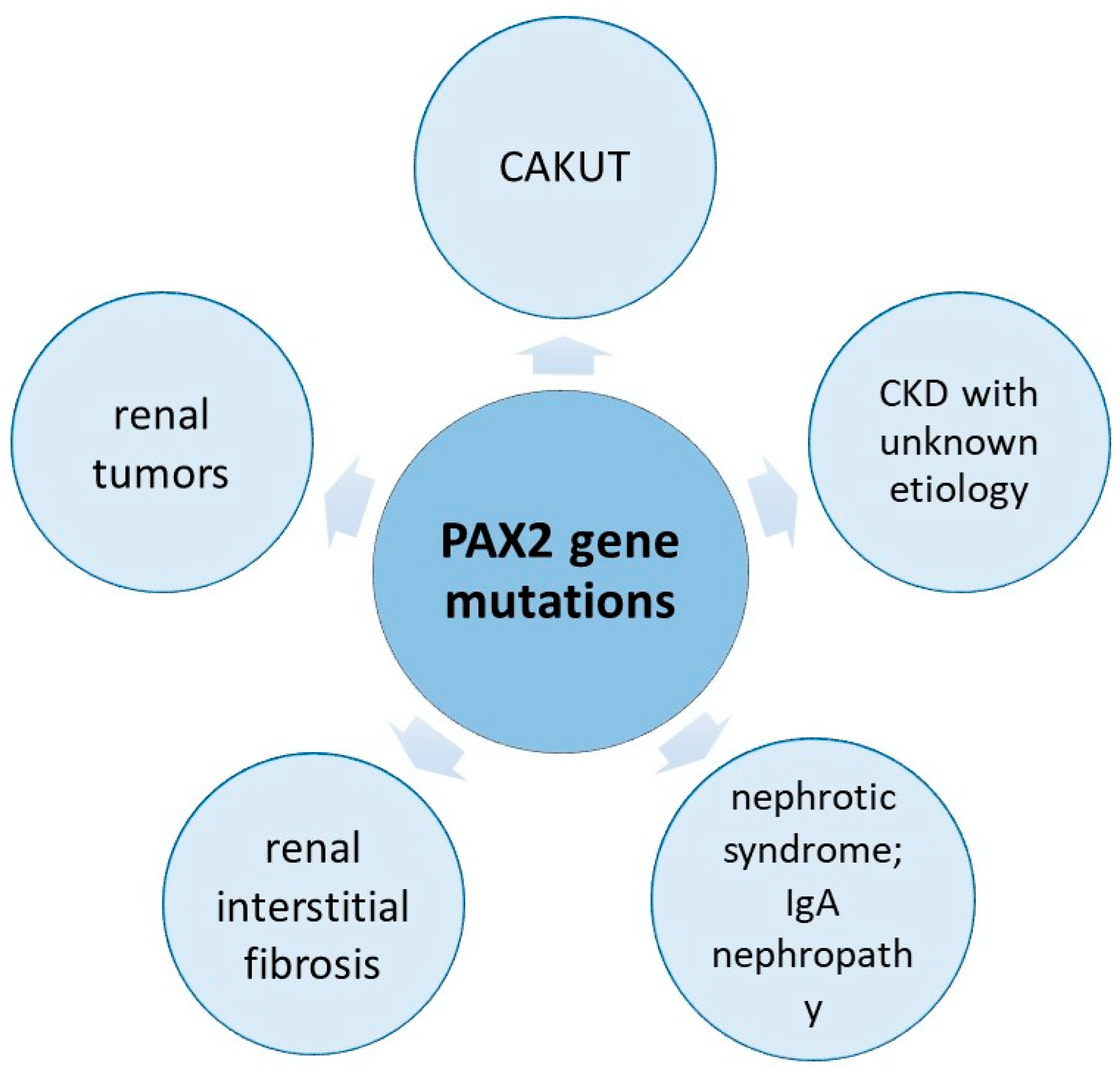
3.3. PAX2 Mutations and Their Role in Children with Renal Pathology
3.3.1. PAX2 Mutations in Children with CAKUTs
3.3.2. PAX2 in Children with Other Renal Disorders
Renal Interstitial Fibrosis
Renal Hypoplasia
Cystic Disorders
Nephrotic Syndrome
| PAX2 Gene Mutation | Molecular Consequence | Clinical Significance 1 | Renal/Urinary Malformation | Reference |
|---|---|---|---|---|
| c.43+5G>A | Intron variant/splice donor 5th base variant | LP | Focal segmental glomerulosclerosis 7 | [53] |
| c.76dup (p.Val26fs) | Frameshift | P | Unspecified cystic kidney disease | [54] |
| c.76dupG,(p.Val26Glyfs*28) | Frameshift | P | Focal segmental glomerulosclerosis 7/severe renal hypoplasia with end-stage renal disease | [43] |
| c.418C>G (p.Arg140Trp) | Missense | P | Renal hypoplasia | [14] |
| c.119–120del (p.Arg40fs) | Frameshift | P | Glomerulomegaly, Focal segmental glomerulosclerosis 7 | [55] |
| c.187G>A (p.Gly63Ser) | Missense | P | Focal segmental glomerulosclerosis 7 | [55] |
| c.576del (p.Ile193fs) | Frameshift | LP | Renal hypoplasia | [56] |
| c.68T>C (p.Leu23Pro) | Missense | P | Bilateral kidney agenesis | [44] |
| c.254G>T (p.Gly85Val) | Missense | LP | Focal segmental glomerulosclerosis 7, Steroid-resistant nephrotic syndrome | [40] |
| c.275C>T (p.Thr92Met) | Missense | LP | Focal segmental glomerulosclerosis 7, Steroid-resistant nephrotic syndrome | [40] |
| c.576del (p.Ile193fs) | Frameshift | LP | Renal hypoplasia | [56] |
| c.153_155delCTGinsTT | Frameshift | LP | Renal cystic hypodysplasia | [2] |
| c.155G>A | Frameshift | LP | Dysplastic multicystic kidney | [40] |
| c.69delC | Frameshift | P | Bilateral renal hypodysplasia, Vesicoureteral reflux and hearing loss | [2] |
| c.419G>T (p.Arg140Leu) | Missense | LP | Focal segmental glomerulosclerosis 7 | [50] |
| c.69delC (p.Val26CysfsX2) | Frameshift | P | ESRD due to Focal segmental glomerulosclerosis 7 | [50] |
| c.1178G>C (p.Arg393Pro) | Missense | P | Focal segmental glomerulosclerosis 7 | [50] |
Urogenital Cancers
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| CAKUT | Congenital anomalies of the kidney and urinary tract |
| CCAM | Congenital Cystic Adenomatoid Malformation |
| ESRD | End-stage renal disease |
| FSGS | Focal segmental glomerulosclerosis |
| GDNF | Glial-cell-derived neurotrophic factor |
| Grg4 | Groucho-related Protein 4 |
| HNF1b | HNF1-homeobox B |
| MM | Metanephric mesenchyme |
| MCDK | Multicystic dysplastic kidney |
| PAX2 | Paired box protein 2 |
| PHB | Prohibitin |
| RCC | Renal cell carcinoma |
| RCS | Renal coloboma syndrome |
| RIF | Renal interstitial fibrosis |
| SRNS | Steroid-resistant nephrotic syndrome |
| VUR | Vesicoureteral reflux |
| WT1 | Wilms tumor |
References
- Capone, V.P.; Morello, W.; Taroni, F.; Montini, G. Genetics of Congenital Anomalies of the Kidney and Urinary Tract: The Current State of Play. Int. J. Mol. Sci. 2017, 18, 796. [Google Scholar] [CrossRef] [PubMed]
- Negrisolo, S.; Benetti, E. PAX2 and CAKUT Phenotypes: Report on Two New Variants and a Review of Mutations from the Leiden Open Variation Database. Int. J. Mol. Sci. 2023, 24, 4165. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ahmed, D. Embryology, Kidney, Bladder, and Ureter; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547747/ (accessed on 10 July 2023).
- Harshman, L.A.; Brophy, P.D. PAX2 in human kidney malformations and disease. Pediatr. Nephrol. 2012, 27, 1265–1275. [Google Scholar] [CrossRef]
- Grimley, E.; Dressler, G.R. Are Pax proteins potential therapeutic targets in kidney disease and cancer? Kidney Int. 2018, 94, 259–267. [Google Scholar] [CrossRef]
- Woroniecki, R.; Gaikwad, A.B.; Susztak, K. Fetal environment, epigenetics, and pediatric renal disease. Pediatr. Nephrol. 2011, 26, 705–711. [Google Scholar] [CrossRef]
- Patel, S.R.; Ranghini, E.; Dressler, G.R. Mechanisms of gene activation and repression by Pax proteins in the developing kidney. Pediatr. Nephrol. 2013, 29, 589–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fletcher, J.; Hu, M.; Berman, Y.; Collins, F.; Grigg, J.; McIver, M.; Ju, H.; Alexander, S.I. Multicystic Dysplastic Kidney and Variable Phenotype in a Family with a Novel Deletion Mutation of PAX2. J. Am. Soc. Nephrol. 2005, 16, 2754–2761. [Google Scholar] [CrossRef]
- Winyard, P.J.; Risdon, R.A.; Sams, V.R.; Dressler, G.R.; Woolf, A.S. The PAX2 tanscription factor is expressed in cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J. Clin. Investig. 1996, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Rooney, K.M.; Woolf, A.S.; Kimber, S.J. Towards Modelling Genetic Kidney Diseases with Human Pluripotent Stem Cells. Nephron Physiol. 2021, 145, 285–296. [Google Scholar] [CrossRef]
- Chan, K.; Li, X. Current Epigenetic Insights in Kidney Development. Genes 2021, 12, 1281. [Google Scholar] [CrossRef]
- Kaku, Y.; Taguchi, A.; Tanigawa, S.; Haque, F.; Sakuma, T.; Yamamoto, T.; Nishinakamura, R. PAX2 is dispensable for in vitro nephron formation from human induced pluripotent stem cells. Sci. Rep. 2017, 7, 4554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, L.; Yang, H.; Bai, Y.; Jiang, H.; Li, Y. Pax2 may play a role in kidney development by regulating the expression of TBX1. Mol. Biol. Rep. 2014, 41, 7491–7498. [Google Scholar] [CrossRef]
- Zhang, L.; Zhai, S.-B.; Zhao, L.-Y.; Zhang, Y.; Sun, B.-C.; Ma, Q.-S. New PAX2 heterozygous mutation in a child with chronic kidney disease: A case report and review of the literature. BMC Nephrol. 2018, 19, 245. [Google Scholar] [CrossRef]
- Narlis, M.; Grote, D.; Gaitan, Y.; Boualia, S.K.; Bouchard, M. Pax2 and Pax8 Regulate Branching Morphogenesis and Nephron Differentiation in the Developing Kidney. J. Am. Soc. Nephrol. 2007, 18, 1121–1129. [Google Scholar] [CrossRef]
- Torres, M.; Gómez-Pardo, E.; Dressler, G.R.; Gruss, P. Pax-2 controls multiple steps of urogenital development. Development 1995, 121, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Longaretti, L.; Trionfini, P.; Brizi, V.; Xinaris, C.; Mele, C.; Breno, M.; Romano, E.; Giampietro, R.; Remuzzi, G.; Benigni, A.; et al. Unravelling the Role of PAX2 Mutation in Human Focal Segmental Glomerulosclerosis. Biomedicines 2021, 9, 1808. [Google Scholar] [CrossRef]
- Hays, T.; Thompson, M.V.; Bateman, D.A.; Sahni, R.; Tolia, V.N.; Clark, R.H.; Gharavi, A.G. The Prevalence and Clinical Significance of Congenital Anomalies of the Kidney and Urinary Tract in Preterm Infants. JAMA Netw. Open 2022, 5, e2231626. [Google Scholar] [CrossRef]
- Radhakrishna, V.; Kumaravel, S.; Priyamvada, P.; Hanumanthappa, N.; Jindal, B.; Govindarajan, K.; Naredi, B.K.; Rijhwani, A. Clinico-Biochemical Profile of Children with Congenital Anomalies of the Kidney and Urinary Tract: A Cross-Sectional Study. Kidney Dis. 2019, 5, 51–57. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wang, X.-W.; Liu, C.-H.; Gao, D.M.X.-J.; Jiang, X.-Y.; Mao, J.-H.; Zhu, G.-H.; Zhang, A.-H.; Wang, M.; Dang, X.-Q.; et al. Genetic spectrum of CAKUT and risk factors for kidney failure: A pediatric multicenter cohort study. Nephrol. Dial. Transplant. 2022, gfac338. [Google Scholar] [CrossRef]
- Harada, R.; Hamasaki, Y.; Okuda, Y.; Hamada, R.; Ishikura, K. Epidemiology of pediatric chronic kidney disease/kidney failure: Learning from registries and cohort studies. Pediatr. Nephrol. 2022, 37, 1215–1229. [Google Scholar] [CrossRef]
- Sanna-Cherchi, S.; Westland, R.; Ghiggeri, G.M.; Gharavi, A.G. Genetic basis of human congenital anomalies of the kidney and urinary tract. J. Clin. Investig. 2018, 128, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Kagan, M.; Pleniceanu, O.; Vivante, A. The genetic basis of congenital anomalies of the kidney and urinary tract. Pediatr. Nephrol. 2022, 37, 2231–2243. [Google Scholar] [CrossRef]
- Weber, S. Novel genetic aspects of congenital anomalies of kidney and urinary tract. Curr. Opin. Pediatr. 2012, 24, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sanchez-Ferras, O.; Bouchard, M. Pax genes in renal development, disease and regeneration. Semin. Cell Dev. Biol. 2015, 44, 97–106. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Chen, C.-C.; Lee, N.-C.; Sung, J.-M.; Chou, Y.-Y.; Chiou, Y.-Y. PAX2 Mutation-Related Renal Hypodysplasia: Review of the Literature and Three Case Reports. Front. Pediatr. 2022, 9, 765929. [Google Scholar] [CrossRef] [PubMed]
- Bonsib, S.M. Renal Hypoplasia, From Grossly Insufficient to Not Quite Enough: Consideration for Expanded Concepts Based Upon the Author’s Perspective with Historical Review. Adv. Anat. Pathol. 2020, 27, 311–330. [Google Scholar] [CrossRef]
- Porteous, S.; Torban, E.; Cho, N.-P.; Cunliffe, H.; Chua, L.; McNoe, L.; Ward, T.; Souza, C.; Gus, P.; Giugliani, R.; et al. Primary renal hypoplasia in humans and mice with PAX2 mutations: Evidence of increased apoptosis in fetal kidneys of Pax21Neu +/− mutant mice. Hum. Mol. Genet. 2000, 9, 1–11. [Google Scholar] [CrossRef]
- Aier, I.; Semwal, R.; Raj, U.; Varadwaj, P.K. Comparative modeling and structure based drug repurposing of PAX2 transcription factor for targeting acquired chemoresistance in pancreatic ductal adenocarcinoma. J. Biomol. Struct. Dyn. 2020, 39, 2071–2078. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Yamamura, Y.; Furuichi, K.; Murakawa, Y.; Hirabayashi, S.; Yoshihara, M.; Sako, K.; Kitajima, S.; Toyama, T.; Iwata, Y.; Sakai, N.; et al. Identification of candidate PAX2-regulated genes implicated in human kidney development. Sci. Rep. 2021, 11, 9123. [Google Scholar] [CrossRef]
- Negrisolo, S.; Benetti, E.; Centi, S.; Della Vella, M.; Ghirardo, G.; Zanon, G.; Murer, L.; Artifoni, L. PAX2 gene mutations in pediatric and young adult transplant recipients: Kidney and urinary tract malformations without ocular anomalies. Clin. Genet. 2011, 80, 581–585. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Y.; Xiao, H.; Yao, Y.; Liu, X.; Su, B.; Zhang, H.; Xu, K.; Wang, S.; Wang, F.; et al. Diverse phenotypes in children with PAX2-related disorder. Mol. Genet. Genom. Med. 2019, 7, e701. [Google Scholar] [CrossRef]
- Forero-Delgadillo, J.M.; Ochoa, V.; Duque, N.; Restrepo, J.M.; Londoño, H.; Nastasi-Catanese, J.A.; Pachajoa, H. New PAX2 Mutation Associated with Polycystic Kidney Disease: A Case Report. Clin. Med. Insights Pediatr. 2021, 15, 1179556521992354. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, P.; Wu, J.; Chang, Q. A novel PAX2 heterozygous mutation in a family with Papillorenal syndrome: A case report and review of the literature. Am. J. Ophthalmol. Case Rep. 2021, 22, 101091. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Fang, Y.; Shi, H.; Xiang, T.; Liu, J.; Liu, J.; Tang, X.; Fang, X.; Chen, J.; et al. Phenotypic spectrum and genetics of PAX2-related disorder in the Chinese cohort. BMC Med. Genom. 2021, 14, 250. [Google Scholar] [CrossRef]
- Rossanti, R.; Morisada, N.; Nozu, K.; Kamei, K.; Horinouchi, T.; Yamamura, T.; Minamikawa, S.; Fujimura, J.; Nagano, C.; Sakakibara, N.; et al. Clinical and genetic variability of PAX2-related disorder in the Japanese population. J. Hum. Genet. 2020, 65, 541–549. [Google Scholar] [CrossRef]
- Hu, R.; Wen, Y.; Ye, W.; Zhang, L.; Si, N.; Zheng, K. FSGS in Chinese twins with a de novo PAX2 mutation: A case report and review of the literature. J. Nephrol. 2021, 34, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Barua, M.; Stellacci, E.; Stella, L.; Weins, A.; Genovese, G.; Muto, V.; Caputo, V.; Toka, H.R.; Charoonratana, V.T.; Tartaglia, M.; et al. Mutations in PAX2 Associate with Adult-Onset FSGS. J. Am. Soc. Nephrol. 2014, 25, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Iatropoulos, P.; Daina, E.; Mele, C.; Maranta, R.; Remuzzi, G.; Noris, M. Discordant phenotype in monozygotic twins with renal coloboma syndrome and a PAX2 mutation. Pediatr. Nephrol. 2012, 27, 1989–1993. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, Y.H.; Jang, Y.; Park, E.; Lee, H.; Kim, S.H.; Cheong, H.I. Genotype-phenotype analysis in patients with PAX2 mutations: Beyond renal coloboma syndrome. Res. Squre 2023, preprint. [Google Scholar] [CrossRef]
- Zhou, T.-B.; Zeng, Z.-Y.; Qin, Y.-H.; Zhao, Y.-J. Less Expression of Prohibitin Is Associated with Increased Paired Box 2 (PAX2) in Renal Interstitial Fibrosis Rats. Int. J. Mol. Sci. 2012, 13, 9808–9825. [Google Scholar] [CrossRef] [PubMed]
- Iwafuchi, Y.; Morioka, T.; Morita, T.; Yanagihara, T.; Oyama, Y.; Morisada, N.; Iijima, K.; Narita, I. Diverse Renal Phenotypes Observed in a Single Family with a Genetic Mutation in Paired Box Protein 2. Case Rep. Nephrol. Dial. 2016, 6, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, K.; Iijima, K.; Shirakawa, T.; Kitagawa, K.; Satomura, K.; Nakamura, H.; Yoshikawa, N. PAX2 Gene Mutation in a Family with Isolated Renal Hypoplasia. J. Am. Soc. Nephrol. 2001, 12, 1769–1772. [Google Scholar] [CrossRef]
- Benetti, E.; Artifoni, L.; Salviati, L.; Pinello, L.; Perrotta, S.; Zuffardi, O.; Zacchello, G.; Murer, L. Renal hypoplasia without optic coloboma associated with PAX2 gene deletion. Nephrol. Dial. Transplant. 2007, 22, 2076–2078. [Google Scholar] [CrossRef]
- Salomon, R.; Tellier, A.-L.; Attie-Bitach, T.; Amiel, J.; Vekemans, M.; Lyonnet, S.; Dureau, P.; Niaudet, P.; Gubler, M.-C.; Broyer, M. PAX2 mutations in oligomeganephronia. Enetic Disord. Dev. 2001, 59, 457–462. [Google Scholar] [CrossRef]
- Rasmussen, M.; Nielsen, M.L.; Manak, J.R.; Mogensen, H.; Lildballe, D.L. PAX2 variant associated with bilateral kidney agenesis and broad intrafamilial disease variability. Clin. Kidney J. 2020, 14, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Vivante, A.; Chacham, O.S.; Shril, S.; Schreiber, R.; Mane, S.M.; Pode-Shakked, B.; Hildebrandt, F. Dominant PAX2 mutations may cause steroid-resistant nephrotic syndrome and FSGS in children. Pediatr Nephrol. 2019, 34, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Ben Moshe, Y.; Bekheirnia, N.; Smith, R.J.H.; Hicks, J.; Braun, M.C.; Bekheirnia, M.R. Genetic diagnosis and renal biopsy findings in the setting of a renal genetics clinic. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, M.A.; Sompallae, R.R.; Nishimura, C.J.; Kwitek, A.E.; Kimble, M.J.; Freese, M.E.; Campbell, C.A.; Smith, R.J.; Thomas, C.P. Targeted broad-based genetic testing by next-generation sequencing informs diagnosis and facilitates management in patients with kidney diseases. Nephrol. Dial. Transplant. 2021, 36, 295–305. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, L.; Ni, F.; Wang, M.; Feng, S.; Gao, X.; Chan, H.; Yang, X.; Lee, H.; Chi, H.; et al. Whole-exome sequencing of a multicenter cohort identifies genetic changes associated with clinical phenotypes in pediatric nephrotic syndrome. Genes Dis. 2022, 9, 1662–1673. [Google Scholar] [CrossRef]
- Grossman, O.K.; Schretlen, C.F.; Nield, L.S. Concordant nephrotic syndrome in twins with PAX2 and MYO1E mutations. Clin. Nephrol. Case Stud. 2022, 10, 37–41. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. ClinVar; [VCV000807455.2]. Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000807455.2 (accessed on 12 April 2023).
- Domingo-Gallego, A.; Pybus, M.; Bullich, G.; Furlano, M.; Ejarque-Vila, L.; Lorente-Grandoso, L.; Ruiz, P.; Fraga, G.; González, M.L.; Piñero-Fernández, J.A.; et al. Clinical utility of genetic testing in early-onset kidney disease: Seven genes are the main players. Nephrol. Dial. Transplant. 2021, 37, 687–696. [Google Scholar] [CrossRef]
- Okumura, T.; Furuichi, K.; Higashide, T.; Sakurai, M.; Hashimoto, S.-I.; Shinozaki, Y.; Hara, A.; Iwata, Y.; Sakai, N.; Sugiyama, K.; et al. Association of PAX2 and Other Gene Mutations with the Clinical Manifestations of Renal Coloboma Syndrome. PLoS ONE 2015, 10, e0142843. [Google Scholar] [CrossRef]
- Jordan, P.; Dorval, G.; Arrondel, C.; Morinière, V.; Tournant, C.; Audrezet, M.; Michel-Calemard, L.; Putoux, A.; Lesca, G.; Labalme, A.; et al. Targeted next-generation sequencing in a large series of fetuses with severe renal diseases. Hum. Mutat. 2022, 43, 347–361. [Google Scholar] [CrossRef]
- Li, L.; Li, C.G.; Almomani, S.N.; Hossain, S.M.; Eccles, M.R. Co-Expression of Multiple PAX Genes in Renal Cell Carcinoma (RCC) and Correlation of High PAX Expression with Favorable Clinical Outcome in RCC Patients. Int. J. Mol. Sci. 2023, 24, 11432. [Google Scholar] [CrossRef]
- Sefidbakht, S.; Khorsand-Rahimzadeh, A.; Omidi, S.; Mohsenpourian, S.; Mirzaian, E. Expression of PAX2 and PAX8 in Wilms Tumor: A Tissue Microarray-based Immunohistochemical Study. Iran. J. Pathol. 2021, 16, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Eccles, M.R. PAX Genes in Cancer; Friends or Foes? Front. Genet. 2012, 3, 6. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Al-Hujaily, E.M.; Alamri, S.; Ghandorah, S.; Garson, K.; Vanderhyden, B.C. PAX2 induces vascular-like structures in normal ovarian cells and ovarian cancer. Exp. Ther. Med. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Zhu, Q.-C.; Sun, J.-H.; Liang, M.-J.; Zhang, Z.-Y.; Xia, Y. PHF20L1 mediates PAX2 expression to promote angiogenesis and liver metastasis in colorectal cancer through regulating HIC1. Biol. Chem. 2022, 403, 917–928. [Google Scholar] [CrossRef]
- Dressler, G.R. Patterning and early cell lineage decisions in the developing kidney: The role of Pax genes. Pediatr. Nephrol. 2011, 26, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Gnarra, J.R.; Dressler, G.R. Expression of Pax-2 in human renal cell carcinoma and growth inhibition by antisense oligonucleo-tides. Cancer Res. 1995, 55, 4092–4098. [Google Scholar] [PubMed]
- Hueber, P.-A.; Iglesias, D.; Chu, L.L.; Eccles, M.; Goodyer, P. In vivo validation of PAX2 as a target for renal cancer therapy. Cancer Lett. 2008, 265, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hueber, P.-A.; Waters, P.; Clarke, P.; Eccles, M.; Goodyer, P. PAX2 inactivation enhances cisplatin-induced apoptosis in renal carcinoma cells. Kidney Int. 2006, 69, 1139–1145, Erratum in: Kidney Int. 2006, 70, 1666. [Google Scholar] [CrossRef] [PubMed]
- Grimley, E.; Liao, C.; Ranghini, E.J.; Nikolovska-Coleska, Z.; Dressler, G.R. Inhibition of Pax2 Transcription Activation with a Small Molecule that Targets the DNA Binding Domain. ACS Chem. Biol. 2017, 12, 724–734. [Google Scholar] [CrossRef]
- Bradford, S.T.; Grimley, E.; Laszczyk, A.M.; Lee, P.H.; Patel, S.R.; Dressler, G.R. Identification of Pax protein inhibitors that suppress target gene expression and cancer cell proliferation. Cell Chem. Biol. 2022, 29, 412–422.e4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, C.; Chirtes, C.; Baczoni, B.; Banescu, C. PAX2 Gene Mutation in Pediatric Renal Disorders—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 12737. https://doi.org/10.3390/ijms241612737
Muntean C, Chirtes C, Baczoni B, Banescu C. PAX2 Gene Mutation in Pediatric Renal Disorders—A Narrative Review. International Journal of Molecular Sciences. 2023; 24(16):12737. https://doi.org/10.3390/ijms241612737
Chicago/Turabian StyleMuntean, Carmen, Camelia Chirtes, Balazs Baczoni, and Claudia Banescu. 2023. "PAX2 Gene Mutation in Pediatric Renal Disorders—A Narrative Review" International Journal of Molecular Sciences 24, no. 16: 12737. https://doi.org/10.3390/ijms241612737
APA StyleMuntean, C., Chirtes, C., Baczoni, B., & Banescu, C. (2023). PAX2 Gene Mutation in Pediatric Renal Disorders—A Narrative Review. International Journal of Molecular Sciences, 24(16), 12737. https://doi.org/10.3390/ijms241612737






