Purine Metabolism and Pyrimidine Metabolism Alteration Is a Potential Mechanism of BDE-47-Induced Apoptosis in Marine Rotifer Brachionus plicatilis
Abstract
1. Introduction
2. Results
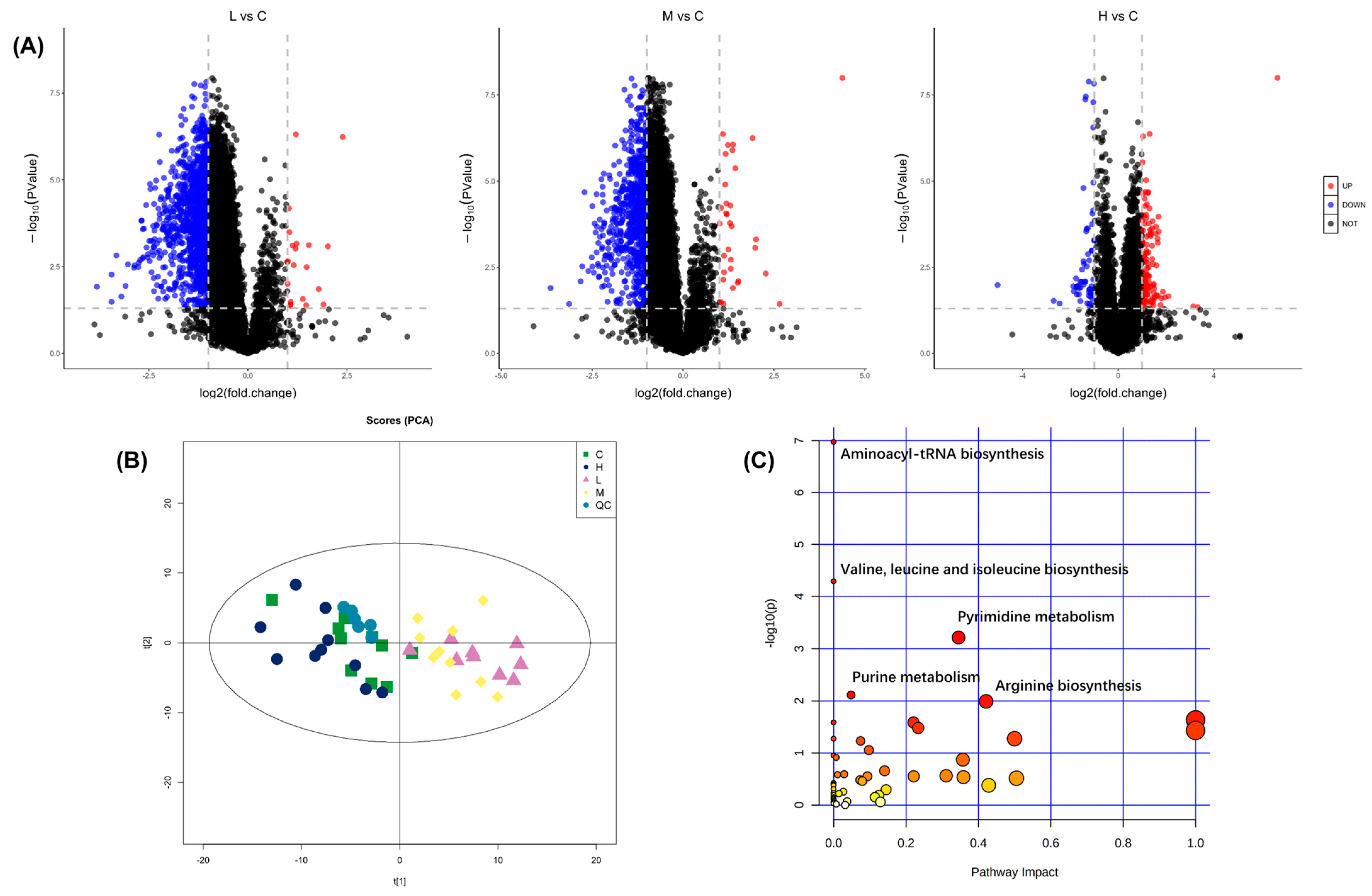
2.1. Metabolomics Analysis of B. plicatilis with BDE-47 Stress
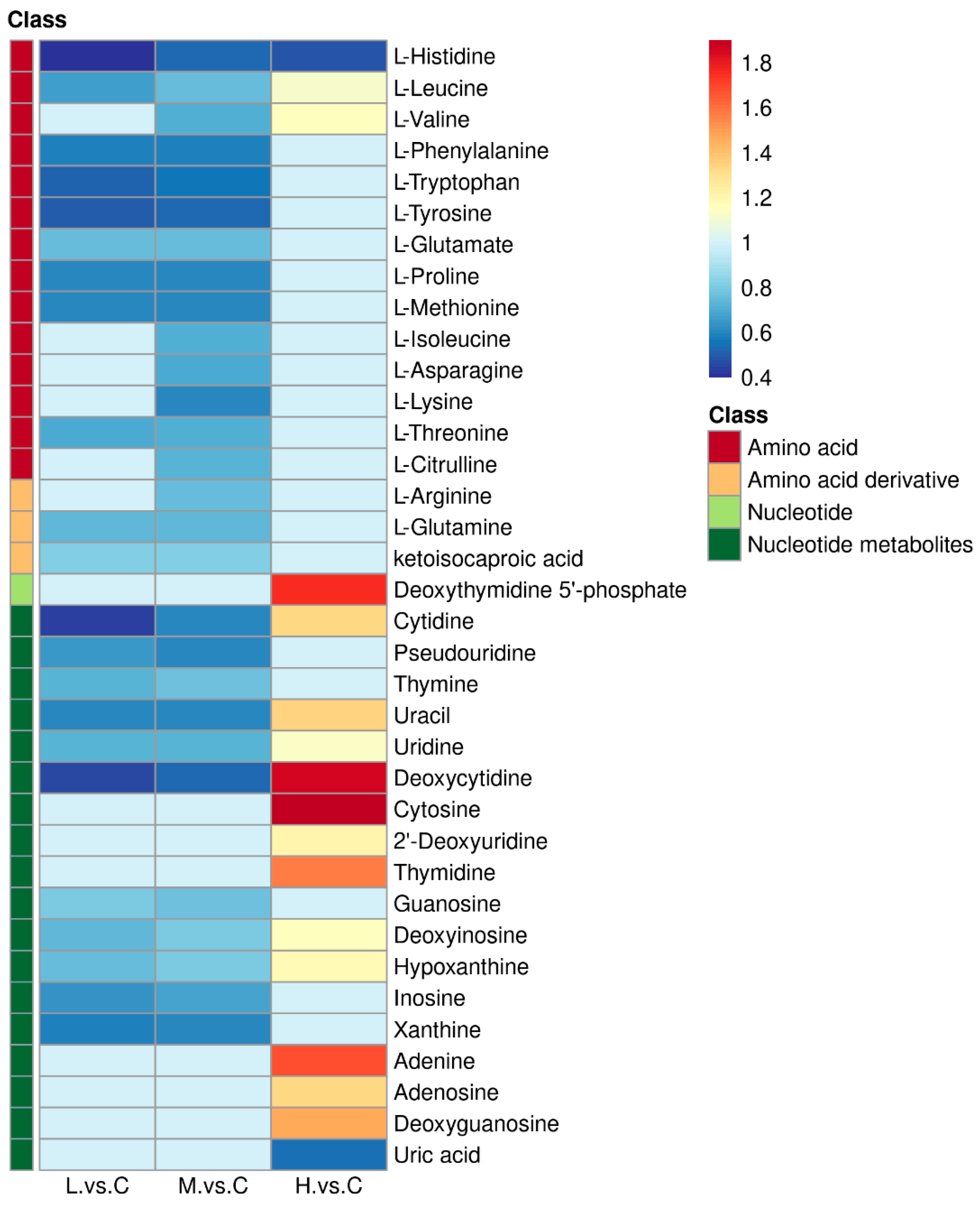
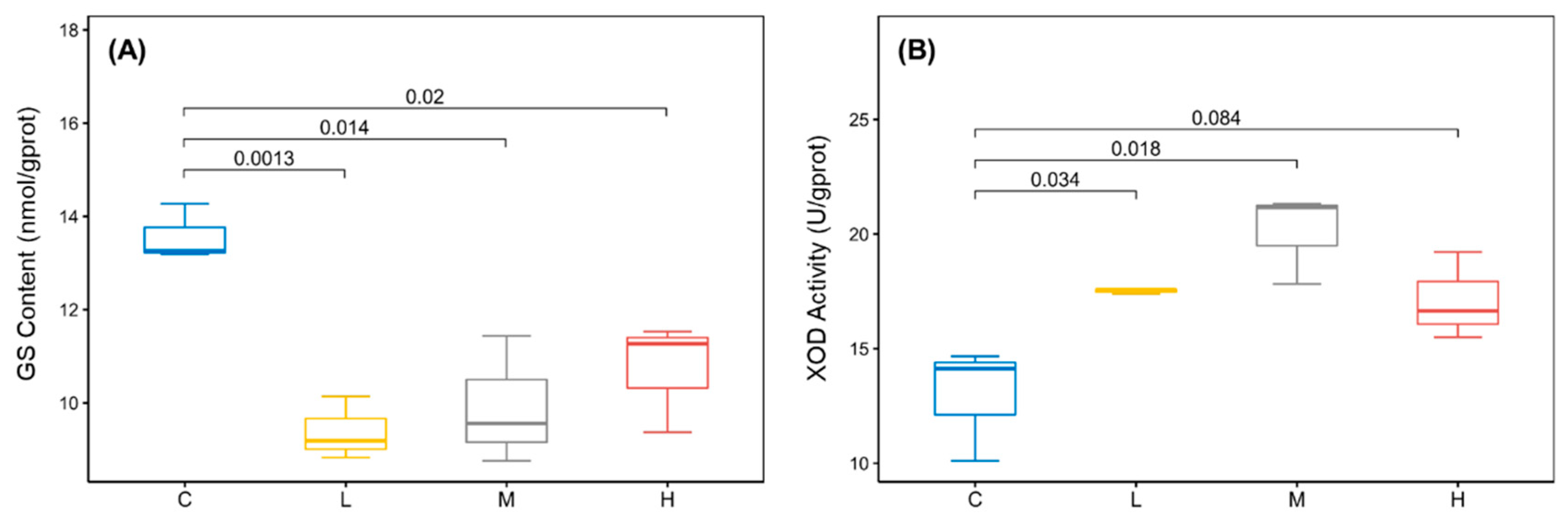
2.2. BDE-47 Impacted the Purine Metabolism and Pyrimidine Metabolism Pathways of B. plicatilis
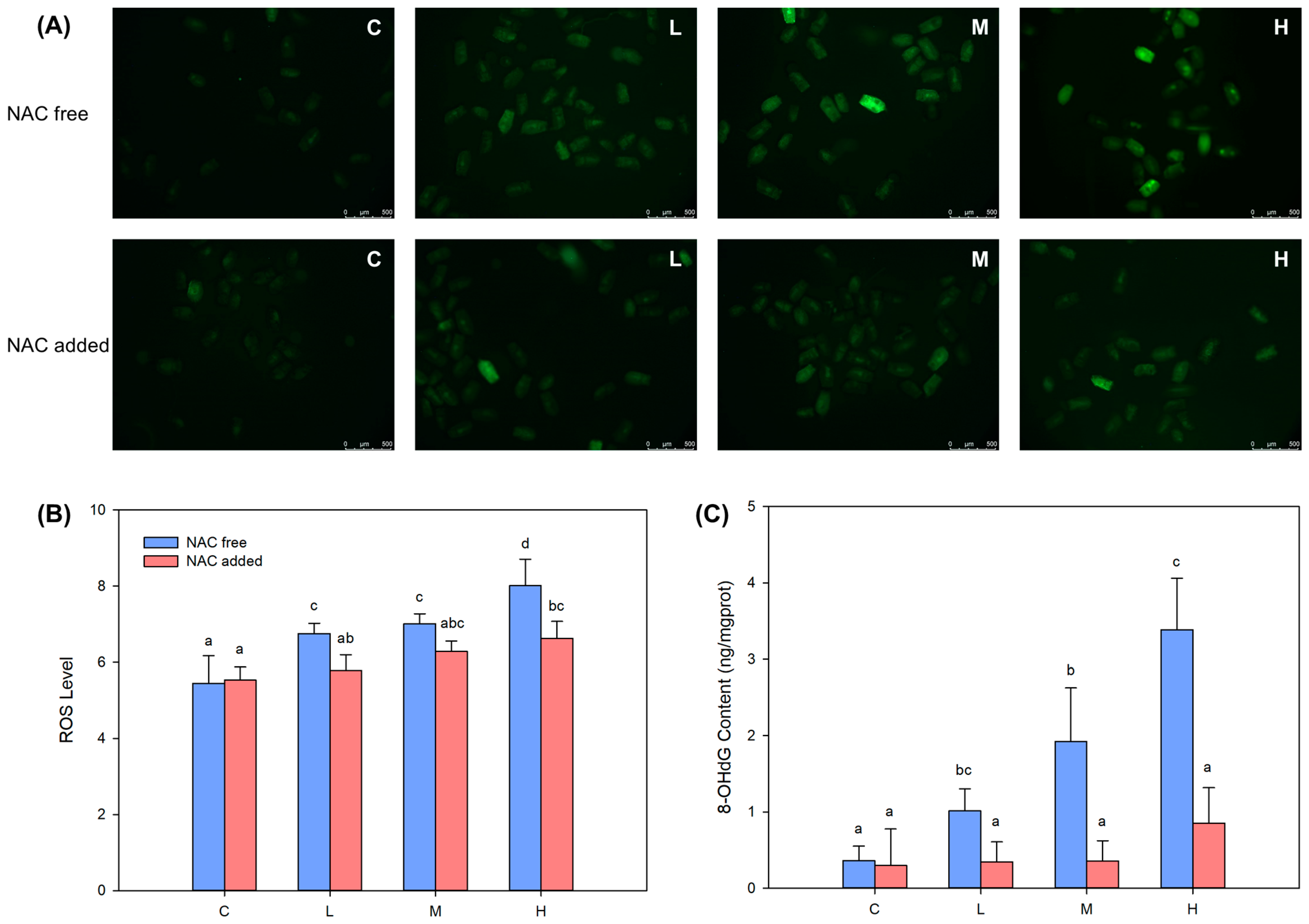
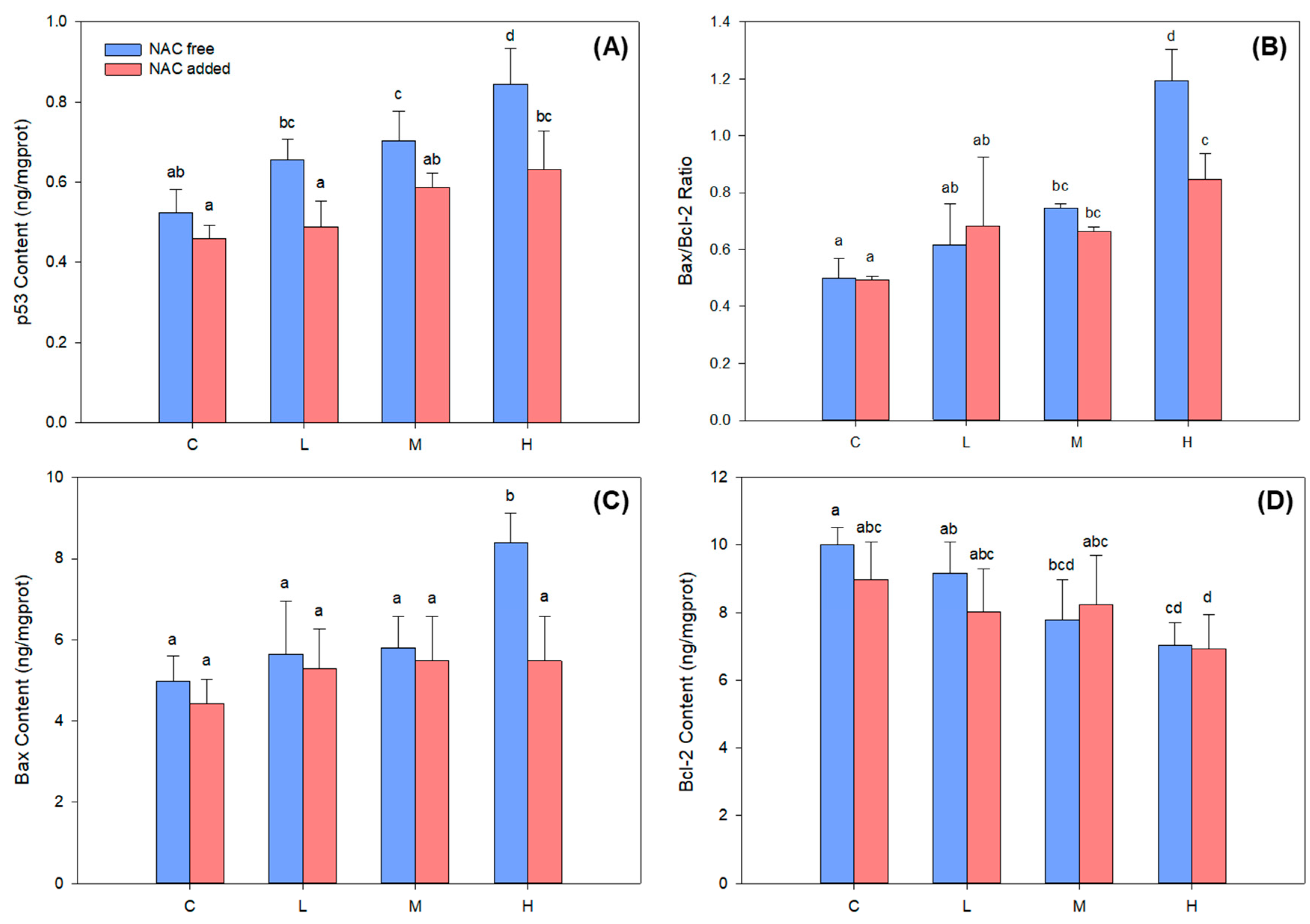
2.3. BDE-47-Induced ROS Overproduction Caused DNA Damage and p53 Signaling Pathway Activation in B. plicatilis
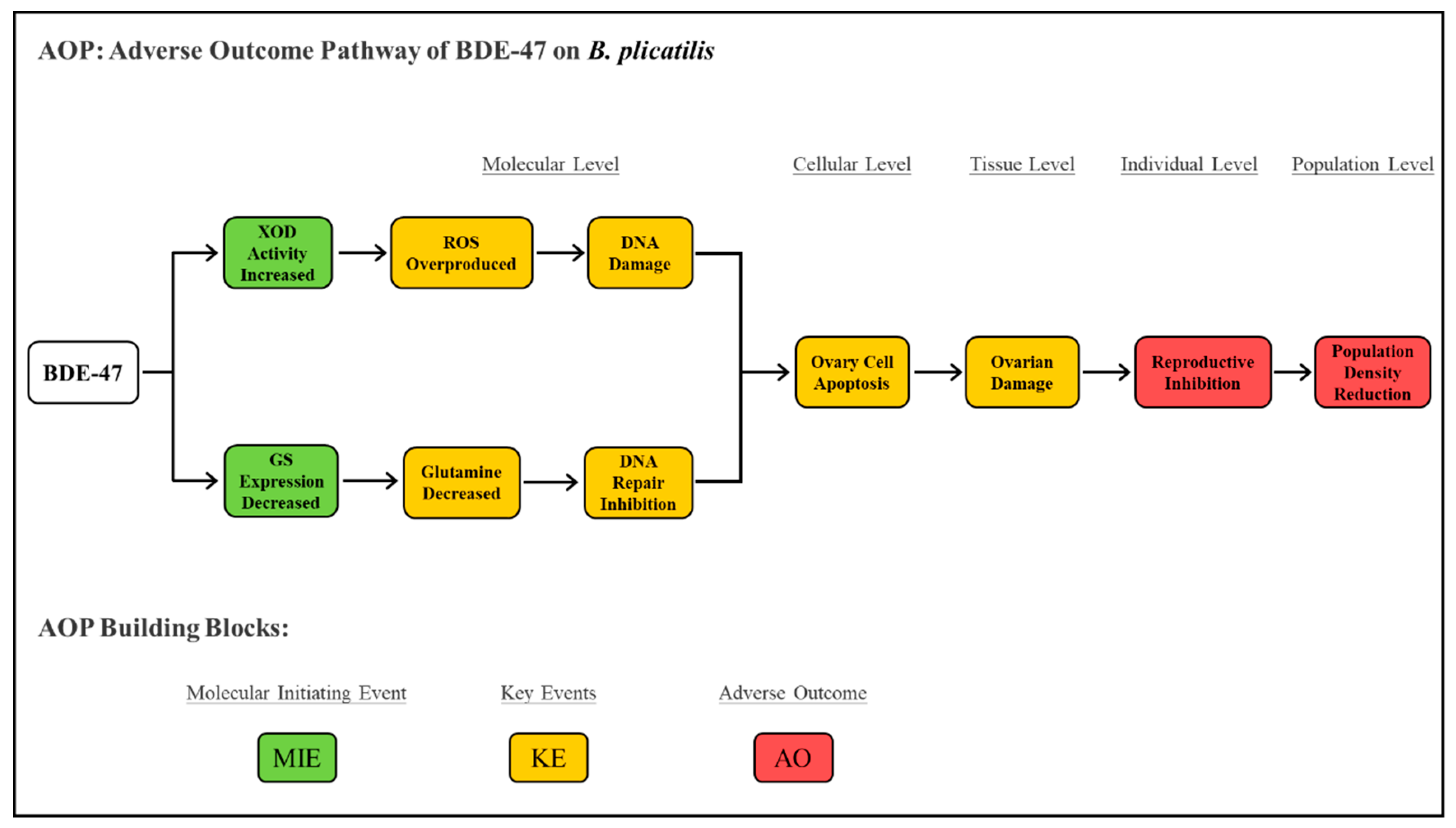
3. Discussion
4. Materials and Methods
4.1. Organism and Chemicals
4.2. Experimental System
4.3. Metabolomics Analysis
4.4. Determination of Key Enzyme in Purine Metabolism and Pyrimidine Metabolism
4.5. Determination of ROS, 8-OHdG Content, and p53 Signaling Pathway Related Proteins Expression with and without NAC Addition
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, A. PBDEs in the marine environment: Sources, pathways and the role of microplastics. Environ. Pollut. 2022, 301, 118943. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, G.B. An overview of polybrominated diphenyl ethers (PBDEs) in the marine environment. Ocean Sci. J. 2015, 50, 119–142. [Google Scholar] [CrossRef]
- Li, X.D.; Wang, X.Y.; Xu, M.E.; Jiang, Y.; Yan, T.; Wang, X.C. Progress on the usage of the rotifer Brachionus plicatilis in marine ecotoxicology: A review. Aquat. Toxicol. 2020, 229, 105678. [Google Scholar] [CrossRef]
- Sha, J.; Wang, Y.; Chen, H.; Wang, M.; Wang, H.; Li, X.; Qi, L.; Tang, X. Using population demographic parameters to assess impacts of two polybrominated diphenyl ethers (BDE-47, BDE-209) on the rotifer Brachionus plicatilis. Ecotoxicol. Env. Saf. 2015, 119, 106–115. [Google Scholar] [CrossRef]
- Liu, C.; Tang, X.; Zhou, B.; Jiang, Y.; Lv, M.; Zang, Y.; Wang, Y. Is it photosensitization or photodegradation when UV-B irradiation is combined with BDE-47? Evidence from the growth and reproduction changes of rotifer Brachionus plicatilis. Sci. Total Environ. 2018, 628–629, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Sha, J.; Chen, H.; Sun, T.; Wang, Y. The reproductive toxicity on the rotifer Brachionus plicatilis induced by BDE-47 and studies on the effective mechanism based on antioxidant defense system changes. Chemosphere 2015, 135, 129–137. [Google Scholar] [CrossRef]
- Wang, B.Y.; Guo, Y.; Zhou, B.; Zhang, H.; Cui, X.Y.; Sun, Y.; Wang, Y. A possible speculation on the involvement of ROS and lysosomes mediated mitochondrial pathway in apoptosis of rotifer Brachionus plicatilis with BDE-47 exposure. Sci. Total Environ. 2021, 787, 147315. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, N.; He, Q.; Wang, B.Y.; Cao, S.; Wang, Y. Autophagy: A newly discovered protective mechanism in the marine rotifer Brachionus plicatilis in response to BDE-47 exposure. Aquat. Toxicol. 2023, 259, 106536. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Q.C.; Miao, X.Y.; Fan, T.L.; Meng, Z.Y.; Chen, X.J.; Zhu, W.T. Study on toxicity effects of environmental pollutants based on metabolomics: A review. Chemosphere 2022, 286, 131815. [Google Scholar] [CrossRef]
- Fu, Q.G.; Scheidegger, A.; Laczko, E.; Hollender, J. Metabolomic Profiling and Toxicokinetics Modeling to Assess the Effects of the Pharmaceutical Diclofenac in the Aquatic Invertebrate Hyalella azteca. Environ. Sci. Technol. 2021, 55, 7920–7929. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.H.; Chen, D.Y.; Xu, E.G.; Luo, X.; Zeng, J.N.; Huan, T.; Li, L.; Wang, Y.J. Toxicity mechanisms of polystyrene microplastics in marine mussels revealed by high-coverage quantitative metabolomics using chemical isotope labeling liquid chromatography mass spectrometry. J. Hazard. Mater. 2021, 417, 126003. [Google Scholar] [CrossRef]
- Ji, F.F.; Wei, J.T.; Luan, H.M.; Li, M.; Cai, Z.W. Study of metabolic disorders associated with BDE-47 exposure in Drosophila model by MS-based metabolomics. Ecotoxicol. Environ. Saf. 2019, 184, 109606. [Google Scholar] [CrossRef]
- Yang, C.X.; Wei, J.T.; Cao, G.D.; Cai, Z.W. Lipid metabolism dysfunction and toxicity of BDE-47 exposure in white adipose tissue revealed by the integration of lipidomics and metabolomics. Sci. Total Environ. 2022, 806, 150350. [Google Scholar] [CrossRef]
- Wei, J.T.; Xiang, L.; Yuan, Z.G.; Li, S.F.; Yang, C.X.; Liu, H.X.; Jiang, Y.Y.; Cai, Z.W. Metabolic profiling on the effect of 2,2 ‘,4,4 ‘-tetrabromodiphenyl ether (BDE-47) in MCF-7 cells. Chemosphere 2018, 192, 297–304. [Google Scholar] [CrossRef]
- Robinson, A.D.; Eich, M.L.; Varambally, S. Dysregulation of de novo nucleotide biosynthetic pathway enzymes in cancer and targeting opportunities. Cancer Lett. 2020, 470, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.J.; Li, Z.; Xiao, L.B.; Hu, W.F.; Zhang, L.; Xie, B.W.; Zhou, Q.; He, J.J.; Qiu, Y.F.; Wen, M.; et al. Glutamine Synthetase Promotes Radiation Resistance via Facilitating Nucleotide Metabolism and Subsequent DNA Damage Repair. Cell Rep. 2019, 28, 1136–1143.e4. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Fan, T.W.M. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Sun, T.; Lu, K.; Zhao, X.; Zhang, Z.; Lv, M.; Liu, C.; Zhou, B. Depicting an energetic chain involved in physiological responses of blue mussel Mytilus edulis coping with BDE-47 exposure. Chemosphere 2021, 269, 128736. [Google Scholar] [CrossRef]
- Yang, C.X.; Wong, C.M.; Wei, J.T.; Chung, A.C.K.; Cai, Z.W. The brominated flame retardant BDE 47 upregulates purine metabolism and mitochondrial respiration to promote adipocyte differentiation. Sci. Total Environ. 2018, 644, 1312–1322. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, X.; Sun, T.; Wang, Y. BDE-47 exposure changed the immune function of haemocytes in Mytilus edulis: An explanation based on ROS-mediated pathway. Aquat. Toxicol. 2017, 182, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Pan, Z.J.; Mengqiu, -L.; Hong, F.S.; Zhu, C.K.; Wu, N.; Chang, G.L.; Wang, H.; Zhao, X.X. BDE-47 induced apoptosis in zebrafish embryos through mitochondrial ROS-mediated JNK signaling. Chemosphere 2020, 258, 127385. [Google Scholar] [CrossRef]
- Shaoyong, W.K.; Liu, Y.L.; Xu, B.C.; Pan, B.; Xianmi, X.; Wang, Y.Z.; Jin, M.L. Exposure to BDE-47 causes female infertility risk and induces oxidative stress and lipotoxicity-mediated ovarian hormone secretion disruption in mice. Sci. Total Environ. 2022, 842, 156885. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M.; Knapen, D.; Vergauwen, L.; Hengstler, J.G.; Angrish, M.; Whelan, M. Adverse outcome pathways: A concise introduction for toxicologists. Arch. Toxicol. 2017, 91, 3697–3707. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Env. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Env. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Jian, X.Y.; Tang, X.X.; Xu, N.N.; Sha, J.J.; Wang, Y. Responses of the rotifer Brachionus plicatilis to flame retardant (BDE-47) stress. Mar. Pollut. Bull. 2017, 116, 298–306. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Wang, J.; You, X.; Zhou, B.; Wang, Y.; Zhou, Z. Purine Metabolism and Pyrimidine Metabolism Alteration Is a Potential Mechanism of BDE-47-Induced Apoptosis in Marine Rotifer Brachionus plicatilis. Int. J. Mol. Sci. 2023, 24, 12726. https://doi.org/10.3390/ijms241612726
Cao S, Wang J, You X, Zhou B, Wang Y, Zhou Z. Purine Metabolism and Pyrimidine Metabolism Alteration Is a Potential Mechanism of BDE-47-Induced Apoptosis in Marine Rotifer Brachionus plicatilis. International Journal of Molecular Sciences. 2023; 24(16):12726. https://doi.org/10.3390/ijms241612726
Chicago/Turabian StyleCao, Sai, Jiayi Wang, Xinye You, Bin Zhou, You Wang, and Zhongyuan Zhou. 2023. "Purine Metabolism and Pyrimidine Metabolism Alteration Is a Potential Mechanism of BDE-47-Induced Apoptosis in Marine Rotifer Brachionus plicatilis" International Journal of Molecular Sciences 24, no. 16: 12726. https://doi.org/10.3390/ijms241612726
APA StyleCao, S., Wang, J., You, X., Zhou, B., Wang, Y., & Zhou, Z. (2023). Purine Metabolism and Pyrimidine Metabolism Alteration Is a Potential Mechanism of BDE-47-Induced Apoptosis in Marine Rotifer Brachionus plicatilis. International Journal of Molecular Sciences, 24(16), 12726. https://doi.org/10.3390/ijms241612726





