Downregulation of Swine Leukocyte Antigen Expression Decreases the Strength of Xenogeneic Immune Responses towards Renal Proximal Tubular Epithelial Cells
Abstract
1. Introduction
2. Results
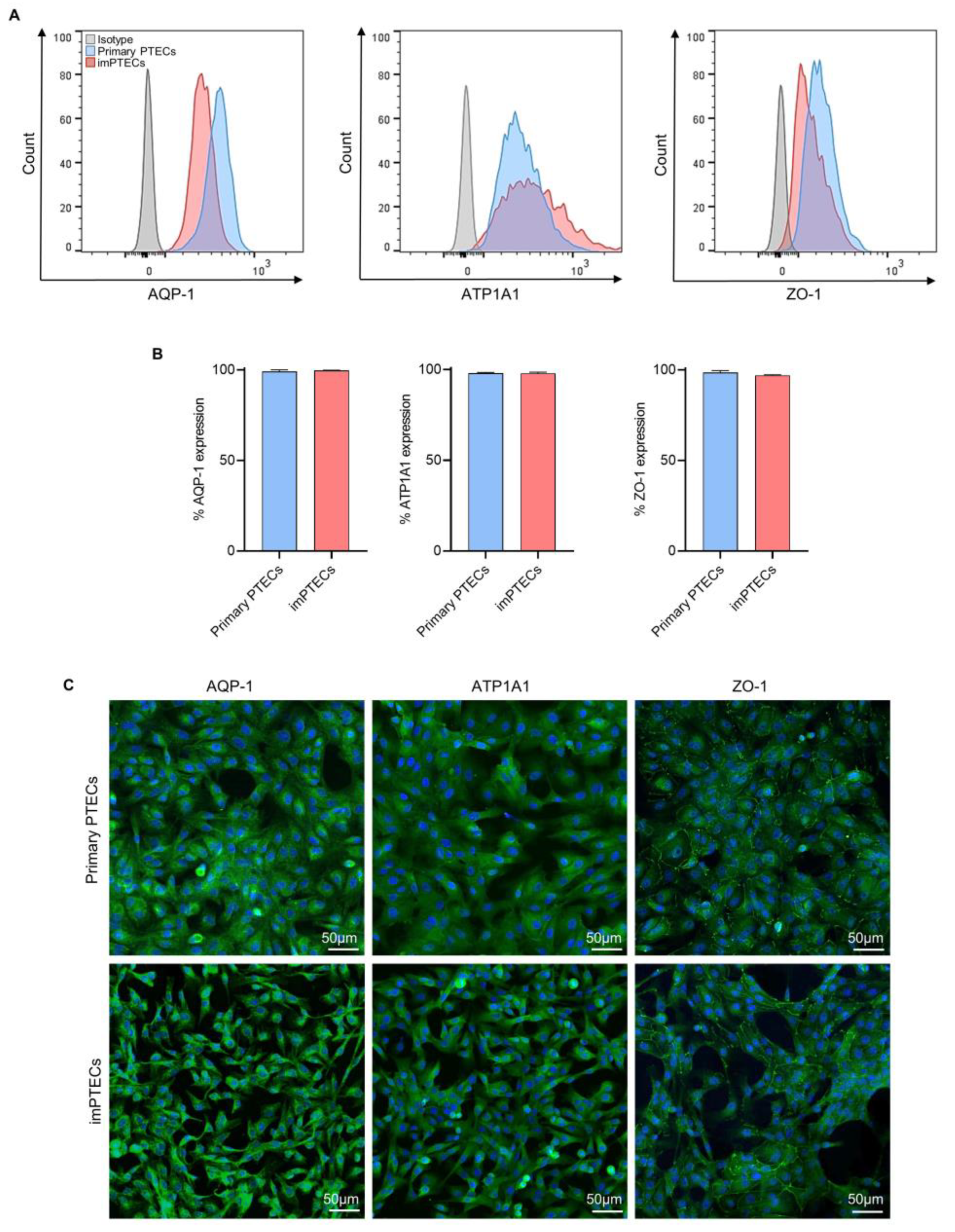
2.1. Immortalized PTECs Maintain Typical Cell Morphology
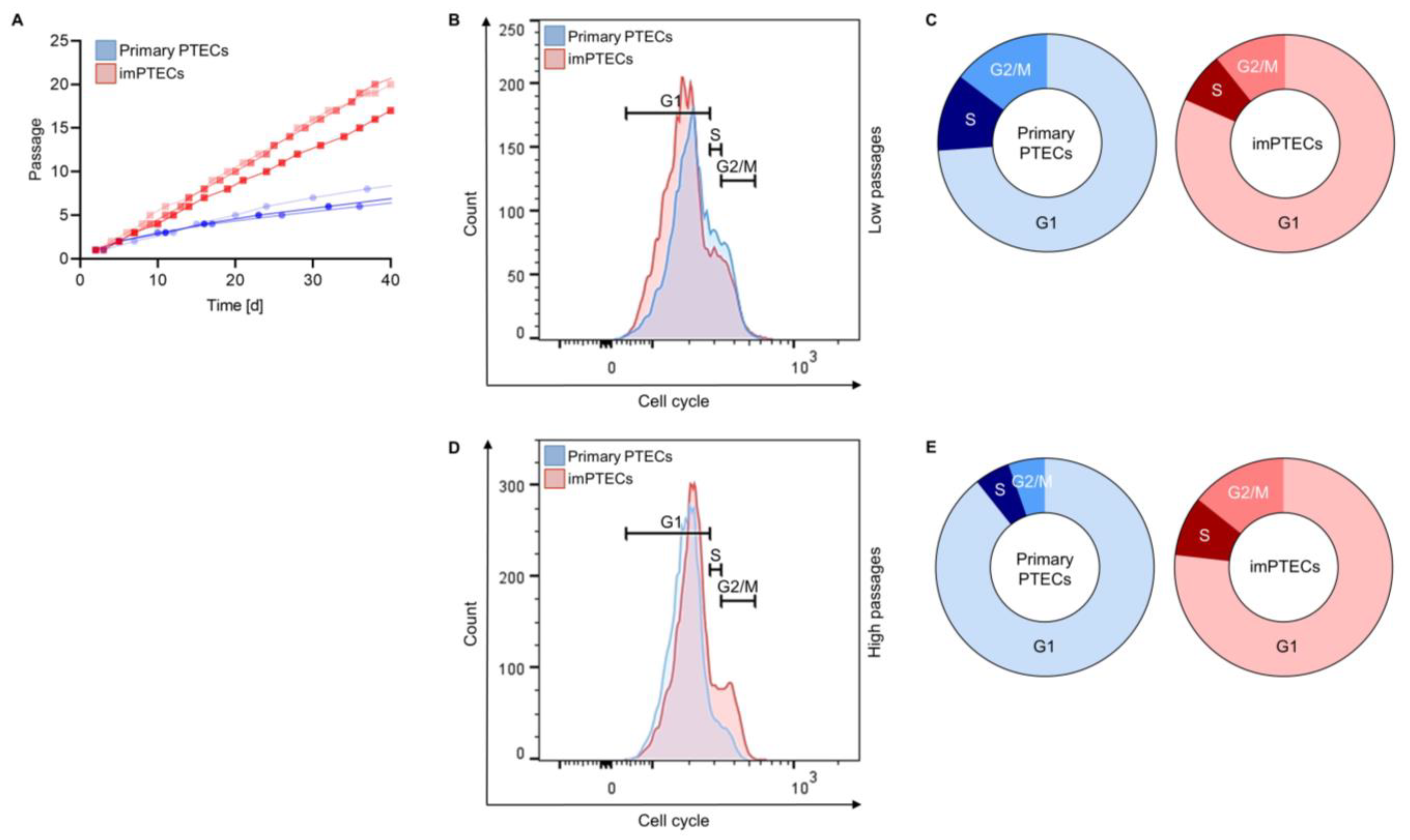
2.2. ImPTECs Exhibit Altered Growth Dynamics
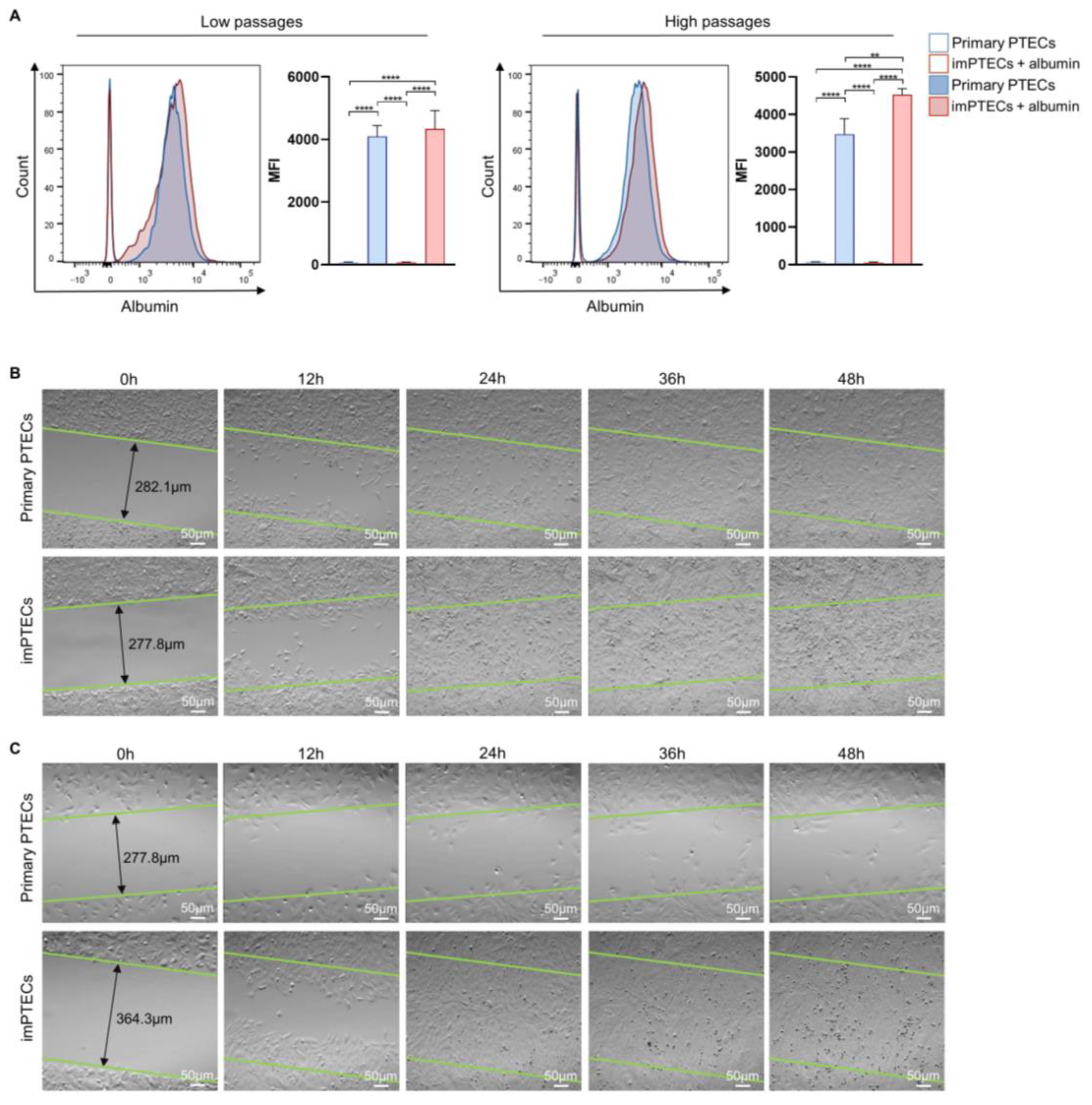
2.3. Immortalization Does Not Affect Cell Function
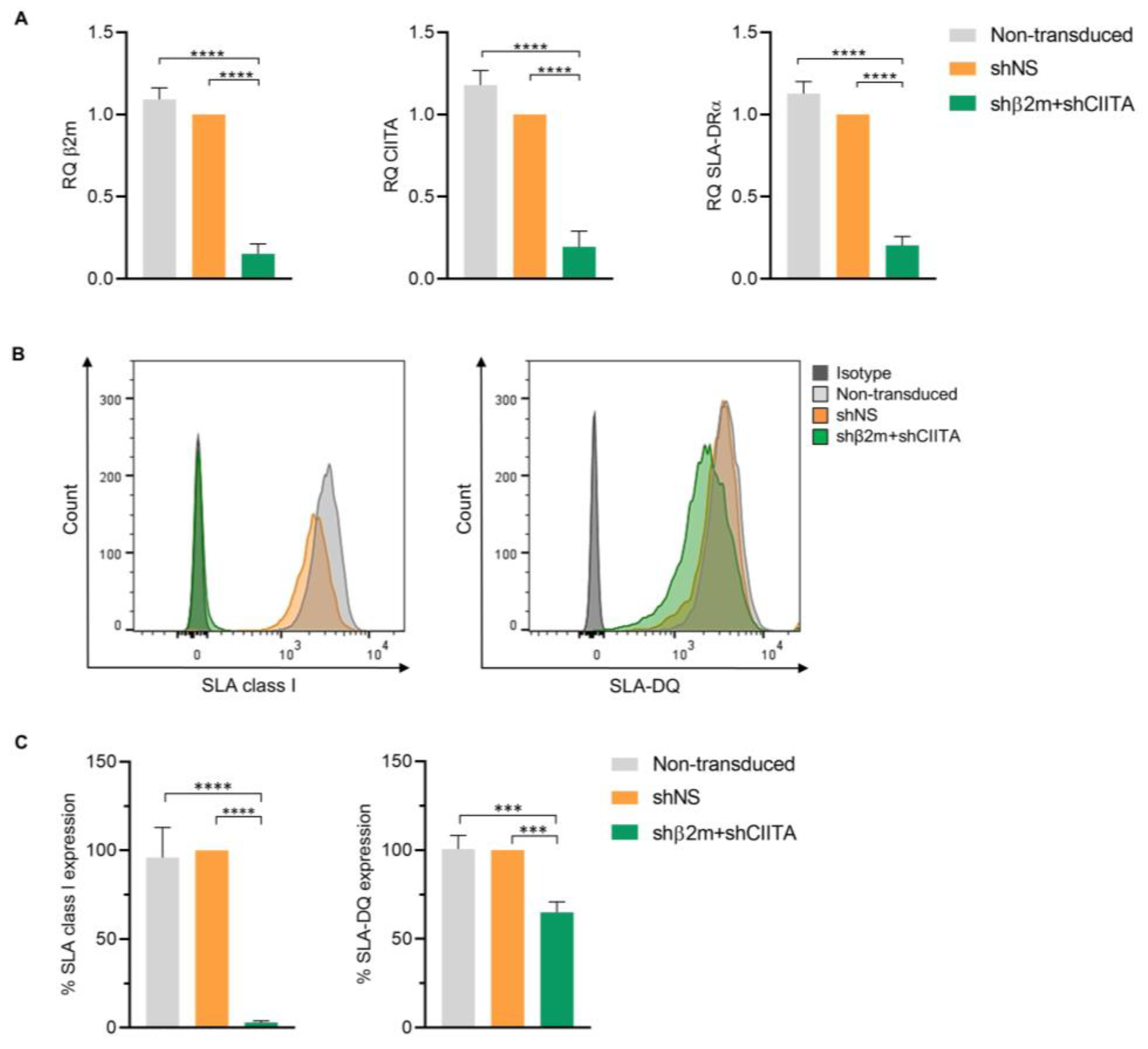
2.4. Silencing SLA Class I and Class II Expression by Genetic Engineering of imPTECs
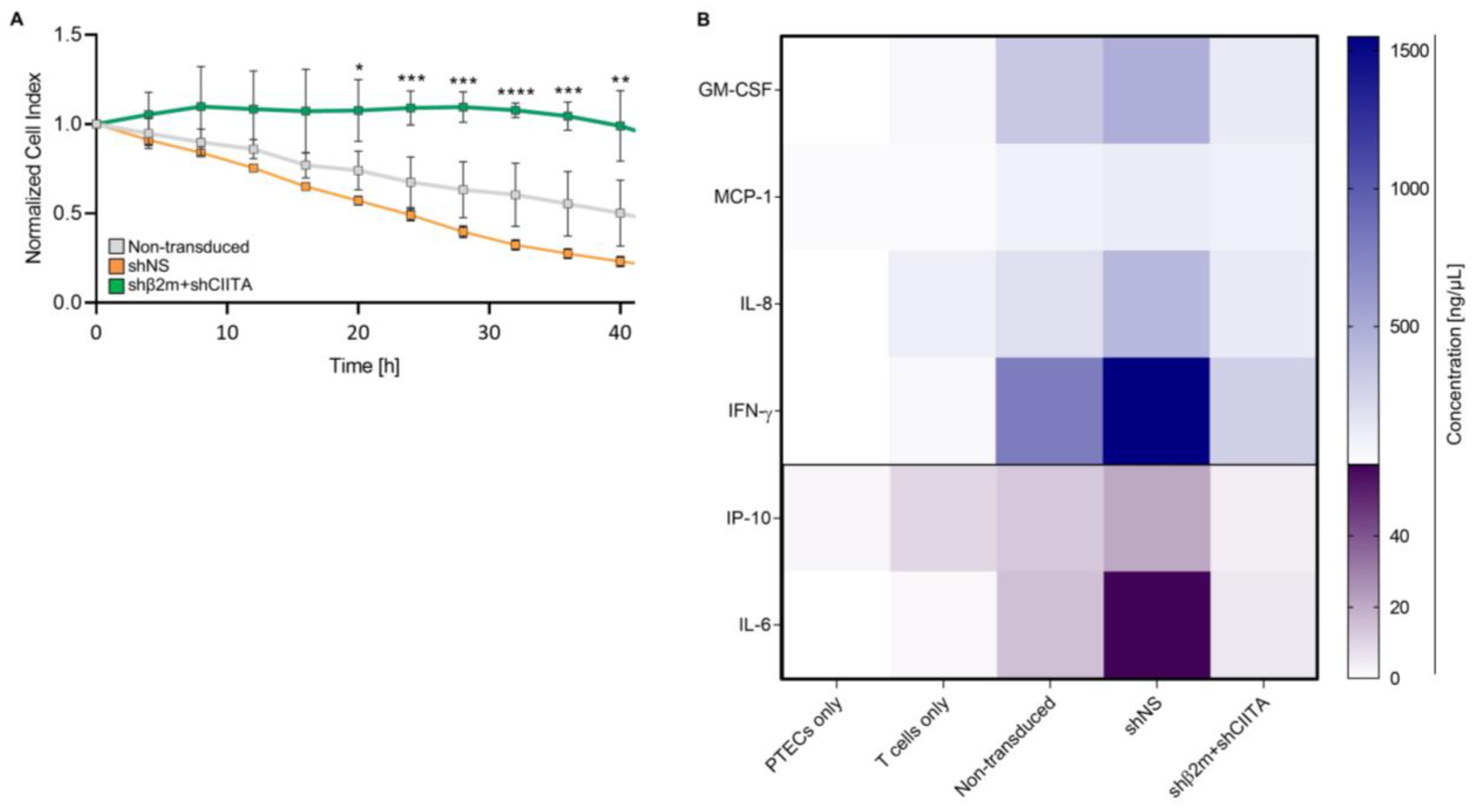
2.5. SLA-Silenced PTECs Induce Weaker Xenogeneic T-Cell Responses
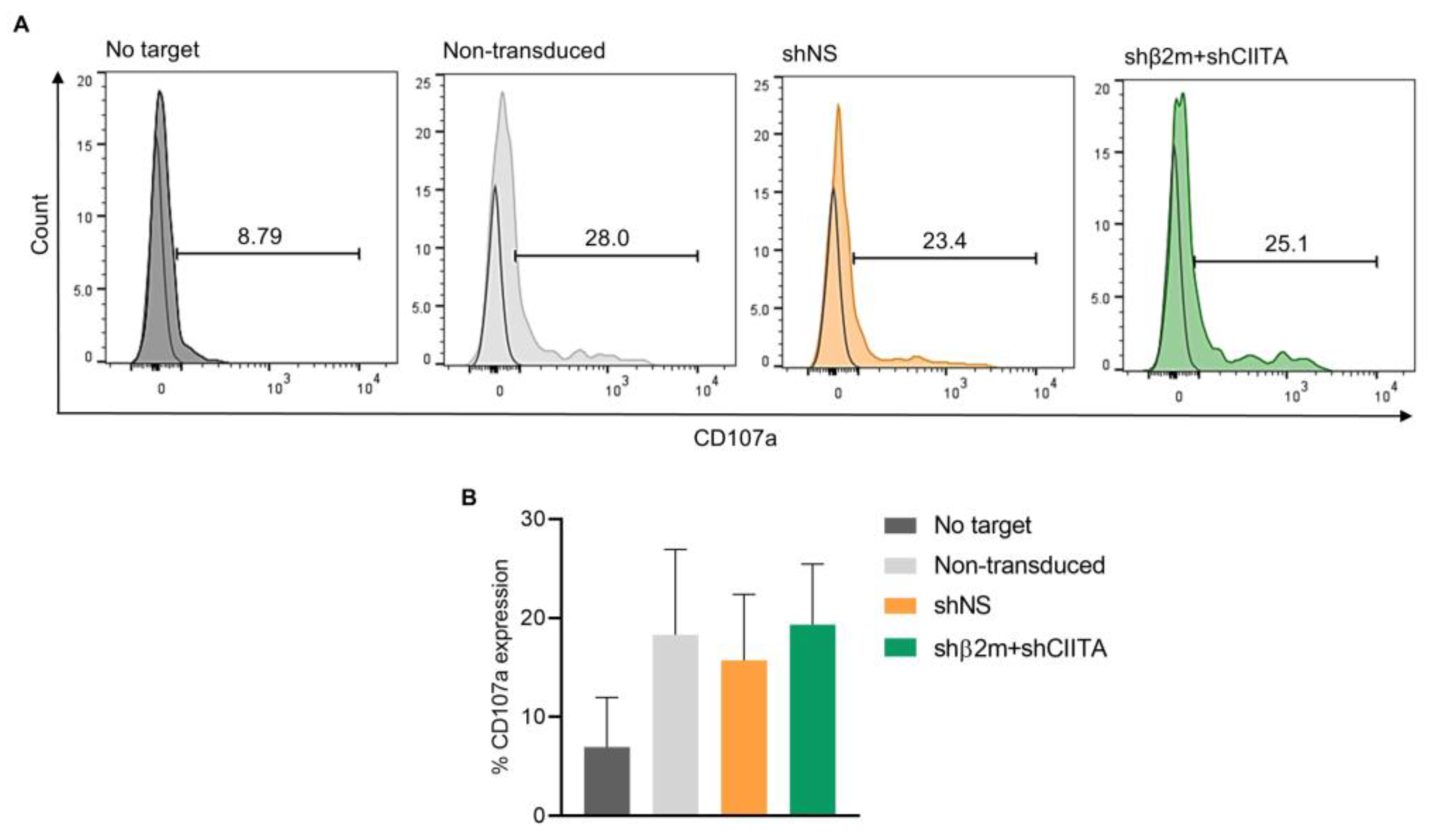
2.6. Silencing SLA Class I Expression on ImPTECs Does Not Trigger NK Cell Cytotoxicity
3. Discussion
4. Material and Methods
4.1. Isolation of PTECs
4.2. Phenotypical Characterization of PTECs
4.3. Lentiviral Vector Production
4.4. Generation of imPTEC Lines
4.5. Evaluation of Immortalization Efficiency
4.6. Assessment of Proliferation and Cell Cycle Analysis
4.7. Albumin Uptake Assay by Endocytosis
4.8. Migration Assay
4.9. Assessment of Silencing SLA Class I and Class II Expression
4.10. T-Cell Cytotoxicity Assay
4.11. NK Cell Degranulation Assay
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Rijt, S.; Leemans, J.C.; Florquin, S.; Houtkooper, R.H.; Tammaro, A. Immunometabolic rewiring of tubular epithelial cells in kidney disease. Nat. Rev. Nephrol. 2022, 18, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Glorie, K.M.; de Klerk, M.; Wagelmans, A.P.; van de Klundert, J.J.; Zuidema, W.C.; Claas, F.H.; Weimar, W. Coordinating unspecified living kidney donation and transplantation across the blood-type barrier in kidney exchange. Transplantation 2013, 96, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Johnson, D.W.; Melk, A.; Foster, B.J.; Blazek, K.; Craig, J.C.; Wong, G. Survival after Kidney Transplantation during Childhood and Adolescence. Clin. J. Am. Soc. Nephrol. 2020, 15, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Melandro, F.; Del Turco, S.; Ghinolfi, D. Age disparities in transplantation. Curr. Opin. Organ Transplant. 2021, 26, 536–541. [Google Scholar] [CrossRef]
- Mustian, M.N.; Locke, J.E. Positive Crossmatch Kidney Transplantation: State of the Art and Future Perspectives. Curr. Pharm. Des. 2020, 26, 3460–3467. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Hara, H.; Iwase, H.; Yamamoto, T.; Jagdale, A.; Kumar, V.; Mannon, R.B.; Hanaway, M.J.; Anderson, D.J.; Eckhoff, D.E. Clinical Pig Kidney Xenotransplantation: How Close Are We? J. Am. Soc. Nephrol. 2020, 31, 12–21. [Google Scholar] [CrossRef]
- Meier, R.P.H.; Longchamp, A.; Mohiuddin, M.; Manuel, O.; Vrakas, G.; Maluf, D.G.; Buhler, L.H.; Muller, Y.D.; Pascual, M. Recent progress and remaining hurdles toward clinical xenotransplantation. Xenotransplantation 2021, 28, e12681. [Google Scholar] [CrossRef]
- Reardon, S. First pig-to-human heart transplant: What can scientists learn? Nature 2022, 601, 305–306. [Google Scholar] [CrossRef]
- Reardon, S. First pig kidneys transplanted into people: What scientists think. Nature 2022, 605, 597–598. [Google Scholar] [CrossRef]
- Smith, P.L.; Buffington, D.A.; Humes, H.D. Kidney epithelial cells. Methods Enzymol. 2006, 419, 194–207. [Google Scholar]
- Stamellou, E.; Leuchtle, K.; Moeller, M.J. Regenerating tubular epithelial cells of the kidney. Nephrol. Dial. Transplant. 2021, 36, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Marquez-Expósito, L.; Rodrigues-Diez, R.; Sanz, A.B.; Guiteras, R.; Doladé, N.; Rubio-Soto, I.; Manonelles, A.; Codina, S.; Ortiz, A.; et al. Molecular Mechanisms of Kidney Injury and Repair. Int. J. Mol. Sci. 2022, 23, 1542. [Google Scholar] [CrossRef] [PubMed]
- Nguan, C.Y.; Du, C. Renal tubular epithelial cells as immunoregulatory cells in renal allograft rejection. Transplant. Rev. Orlando 2009, 23, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Silva-Aguiar, R.P.; Peruchetti, D.B.; Florentino, L.S.; Takiya, C.M.; Marzolo, M.P.; Dias, W.B.; Pinheiro, A.A.S.; Caruso-Neves, C. Albumin Expands Albumin Reabsorption Capacity in Proximal Tubule Epithelial Cells through a Positive Feedback Loop between AKT and Megalin. Int. J. Mol. Sci. 2022, 23, 848. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Gradisnik, L. Tissue Augmentation in Wound Healing: The Role of Endothelial and Epithelial Cells. Med. Arch. 2018, 72, 444–448. [Google Scholar] [CrossRef]
- Figueiredo, C.; Carvalho Oliveira, M.; Chen-Wacker, C.; Jansson, K.; Hoffler, K.; Yuzefovych, Y.; Pogozhykh, O.; Jin, Z.; Kuhnel, M.; Jonigk, D.; et al. Immunoengineering of the Vascular Endothelium to Silence MHC Expression During Normothermic ex vivo Lung Perfusion. Hum. Gene Ther. 2019, 30, 485–496. [Google Scholar] [CrossRef]
- Sake, H.J.; Frenzel, A.; Lucas-Hahn, A.; Nowak-Imialek, M.; Hassel, P.; Hadeler, K.G.; Hermann, D.; Becker, R.; Eylers, H.; Hein, R.; et al. Possible detrimental effects of beta-2-microglobulin knockout in pigs. Xenotransplantation 2019, 26, e12525. [Google Scholar] [CrossRef]
- Yuzefovych, Y.; Valdivia, E.; Rong, S.; Hack, F.; Rother, T.; Schmitz, J.; Brasen, J.H.; Wedekind, D.; Moers, C.; Wenzel, N.; et al. Genetic Engineering of the Kidney to Permanently Silence MHC Transcripts During ex vivo Organ Perfusion. Front. Immunol. 2020, 11, 265. [Google Scholar] [CrossRef]
- Valdivia, E.; Bertolin, M.; Breda, C.; Carvalho Oliveira, M.; Salz, A.K.; Hofmann, N.; Börgel, M.; Blasczyk, R.; Ferrari, S.; Figueiredo, C. Genetic Modification of Limbal Stem Cells to Decrease Allogeneic Immune Responses. Front. Immunol. 2021, 12, 747357. [Google Scholar] [CrossRef]
- Scalea, J.; Hanecamp, I.; Robson, S.C.; Yamada, K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation 2012, 19, 23–30. [Google Scholar] [CrossRef]
- Siu, J.H.Y.; Surendrakumar, V.; Richards, J.A.; Pettigrew, G.J. T cell Allorecognition Pathways in Solid Organ Transplantation. Front. Immunol. 2018, 9, 2548. [Google Scholar] [CrossRef] [PubMed]
- Crane, A.T.; Aravalli, R.N.; Asakura, A.; Grande, A.W.; Krishna, V.D.; Carlson, D.F.; Cheeran, M.C.; Danczyk, G.; Dutton, J.R.; Hackett, P.B.; et al. Interspecies Organogenesis for Human Transplantation. Cell. Transplant. 2019, 28, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Puga Yung, G.; Schneider, M.K.J.; Seebach, J.D. The Role of NK Cells in Pig-to-Human Xenotransplantation. J. Immunol. Res. 2017, 2017, 4627384. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, M.; Rapak, A. Effect of Natural Compounds on NK Cell Activation. J. Immunol. Res. 2018, 2018, 4868417. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Gaston, R.; Eckhoff, D.; Ladowski, J.; Yamamoto, T.; Wang, L.; Iwase, H.; Hara, H.; Tector, M.; Tector, A.J. Xenotransplantation-the current status and prospects. Br. Med. Bull. 2018, 125, 5–14. [Google Scholar] [CrossRef]
- Sykes, M.; Sachs, D.H. Transplanting organs from pigs to humans. Sci. Immunol. 2019, 4, eaau6298. [Google Scholar] [CrossRef]
- Langley, G.; Evans, T.; Holgate, S.T.; Jones, A. Replacing animal experiments: Choices, chances and challenges. Bioessays 2007, 29, 918–926. [Google Scholar] [CrossRef]
- Lewis, A.; Koukoura, A.; Tsianos, G.I.; Gargavanis, A.A.; Nielsen, A.A.; Vassiliadis, E. Organ donation in the US and Europe: The supply vs demand imbalance. Transplant. Rev. Orlando 2021, 35, 100585. [Google Scholar] [CrossRef]
- Banga, S.S.; Kim, S.; Hubbard, K.; Dasgupta, T.; Jha, K.K.; Patsalis, P.; Hauptschein, R.; Gamberi, B.; Dalla-Favera, R.; Kraemer, P.; et al. SEN6, a locus for SV40-mediated immortalization of human cells, maps to 6q26-27. Oncogene 1997, 14, 313–321. [Google Scholar] [CrossRef][Green Version]
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef]
- Golias, C.H.; Charalabopoulos, A.; Charalabopoulos, K. Cell proliferation and cell cycle control: A mini review. Int. J. Clin. Pract. 2004, 58, 1134–1141. [Google Scholar] [CrossRef]
- Maqsood, M.I.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of cell lines: Challenges and advantages of establishment. Cell. Biol. Int. 2013, 37, 1038–1045. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.K.; Cantley, L.G. Cellular maintenance and repair of the kidney. Annu. Rev. Physiol. 2010, 72, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.M.; Estrada, J.L.; Wang, Z.Y.; Blosser, R.J.; Smith, R.F.; Sidner, R.A.; Paris, L.L.; Blankenship, R.L.; Ray, C.N.; Miner, A.C.; et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J. Immunol. 2014, 193, 5751–5757. [Google Scholar] [CrossRef]
- Vormann, M.K.; Vriend, J.; Lanz, H.L.; Gijzen, L.; van den Heuvel, A.; Hutter, S.; Joore, J.; Trietsch, S.J.; Stuut, C.; Nieskens, T.T.G.; et al. Implementation of a Human Renal Proximal Tubule on a Chip for Nephrotoxicity and Drug Interaction Studies. J. Pharm. Sci. 2021, 110, 1601–1614. [Google Scholar] [CrossRef]
- Valdivia, E.; Rother, T.; Yuzefovych, Y.; Hack, F.; Wenzel, N.; Blasczyk, R.; Krezdorn, N.; Figueiredo, C. Genetic Modification of Limbs Using Ex Vivo Machine Perfusion. Hum. Gene Ther. 2022, 33, 460–471. [Google Scholar] [CrossRef]
- Börger, A.K.; Eicke, D.; Wolf, C.; Gras, C.; Aufderbeck, S.; Schulze, K.; Engels, L.; Eiz-Vesper, B.; Schambach, A.; Guzman, C.A.; et al. Generation of HLA-Universal iPSC-Derived Megakaryocytes and Platelets for Survival Under Refractoriness Conditions. Mol. Med. 2016, 22, 274–285. [Google Scholar] [CrossRef]
- Jordan, S.C.; Choi, J.; Kim, I.; Wu, G.; Toyoda, M.; Shin, B.; Vo, A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Hidalgo, L.G.; Halloran, P.F. Role of IFN-gamma in allograft rejection. Crit. Rev. Immunol. 2002, 22, 317–349. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Oliveira, M.; Valdivia, E.; Verboom, M.; Yuzefovych, Y.; Sake, H.J.; Pogozhykh, O.; Niemann, H.; Schwinzer, R.; Petersen, B.; Seissler, J.; et al. Generating low immunogenic pig pancreatic islet cell clusters for xenotransplantation. J. Cell. Mol. Med. 2020, 24, 5070–5081. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Lo, P.C.; Sakai, R.; Noguchi, Y.; Kodama, T.; Yoneyama, T.; Toyama, C.; Wang, H.T.; Esquivel, E.; Jiaravuthisan, P.; et al. A Strategy for Suppressing Macrophage-mediated Rejection in Xenotransplantation. Transplantation 2020, 104, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Benichou, G.; Yamada, Y.; Aoyama, A.; Madsen, J.C. Natural killer cells in rejection and tolerance of solid organ allografts. Curr. Opin. Organ Transplant. 2011, 16, 47–53. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmalkuche, K.; Schwinzer, R.; Wenzel, N.; Valdivia, E.; Petersen, B.; Blasczyk, R.; Figueiredo, C. Downregulation of Swine Leukocyte Antigen Expression Decreases the Strength of Xenogeneic Immune Responses towards Renal Proximal Tubular Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 12711. https://doi.org/10.3390/ijms241612711
Schmalkuche K, Schwinzer R, Wenzel N, Valdivia E, Petersen B, Blasczyk R, Figueiredo C. Downregulation of Swine Leukocyte Antigen Expression Decreases the Strength of Xenogeneic Immune Responses towards Renal Proximal Tubular Epithelial Cells. International Journal of Molecular Sciences. 2023; 24(16):12711. https://doi.org/10.3390/ijms241612711
Chicago/Turabian StyleSchmalkuche, Katharina, Reinhard Schwinzer, Nadine Wenzel, Emilio Valdivia, Björn Petersen, Rainer Blasczyk, and Constanca Figueiredo. 2023. "Downregulation of Swine Leukocyte Antigen Expression Decreases the Strength of Xenogeneic Immune Responses towards Renal Proximal Tubular Epithelial Cells" International Journal of Molecular Sciences 24, no. 16: 12711. https://doi.org/10.3390/ijms241612711
APA StyleSchmalkuche, K., Schwinzer, R., Wenzel, N., Valdivia, E., Petersen, B., Blasczyk, R., & Figueiredo, C. (2023). Downregulation of Swine Leukocyte Antigen Expression Decreases the Strength of Xenogeneic Immune Responses towards Renal Proximal Tubular Epithelial Cells. International Journal of Molecular Sciences, 24(16), 12711. https://doi.org/10.3390/ijms241612711







