Scopoletin Reactivates Latent HIV-1 by Inducing NF-κB Expression without Global T Cell Activation
Abstract
1. Introduction
2. Results
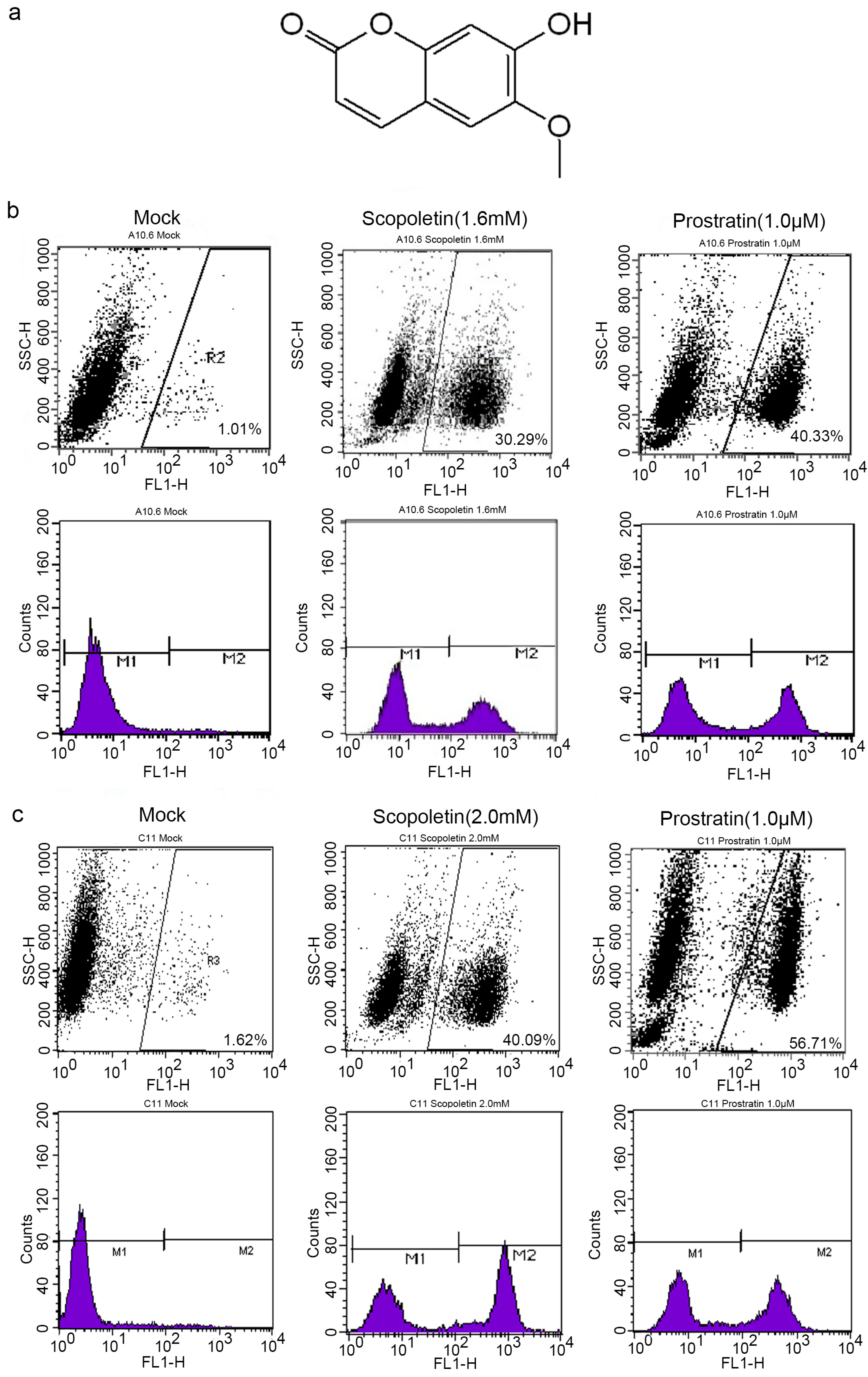
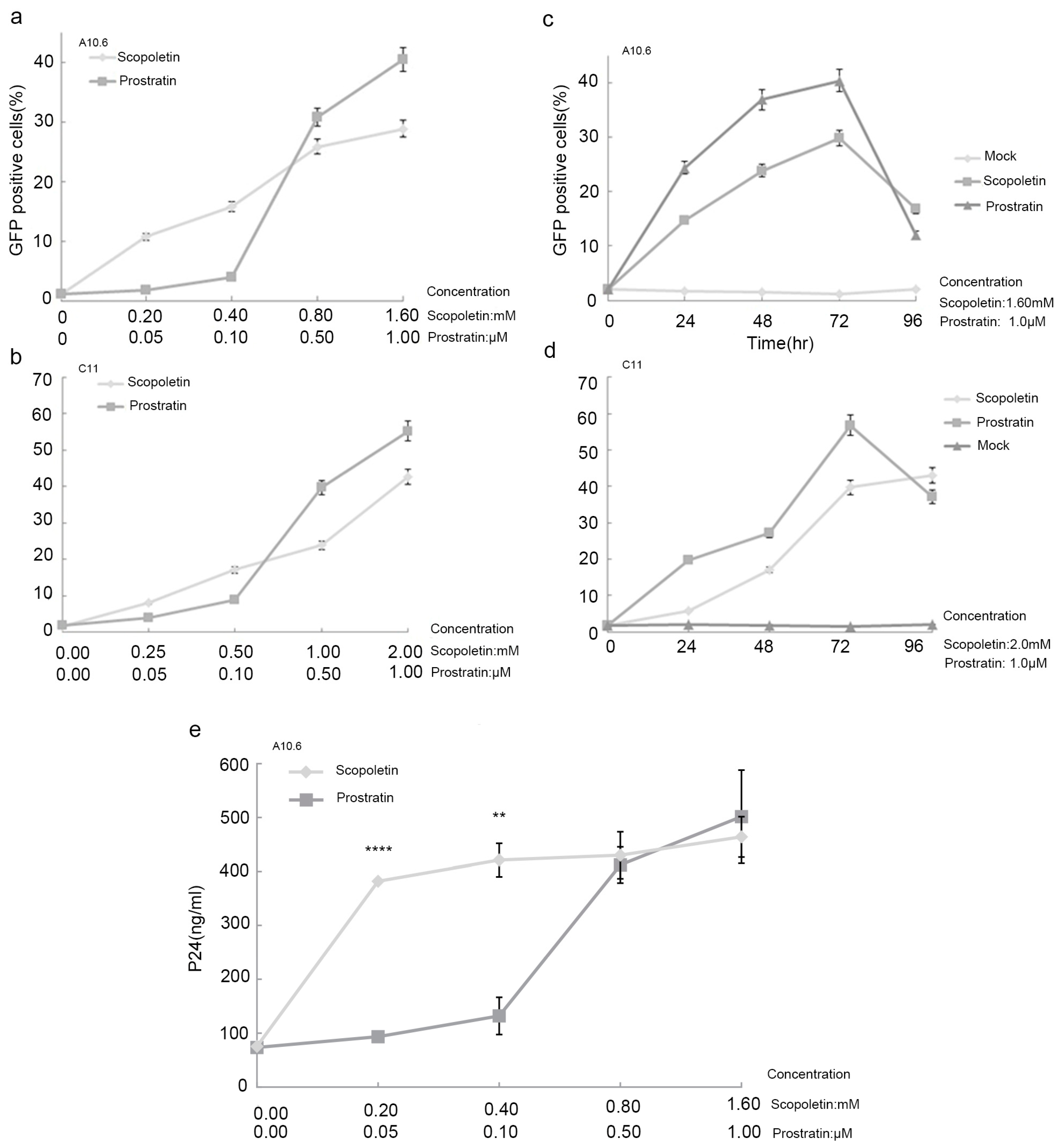
2.1. Scopoletin Activates Latent HIV-1 Replication
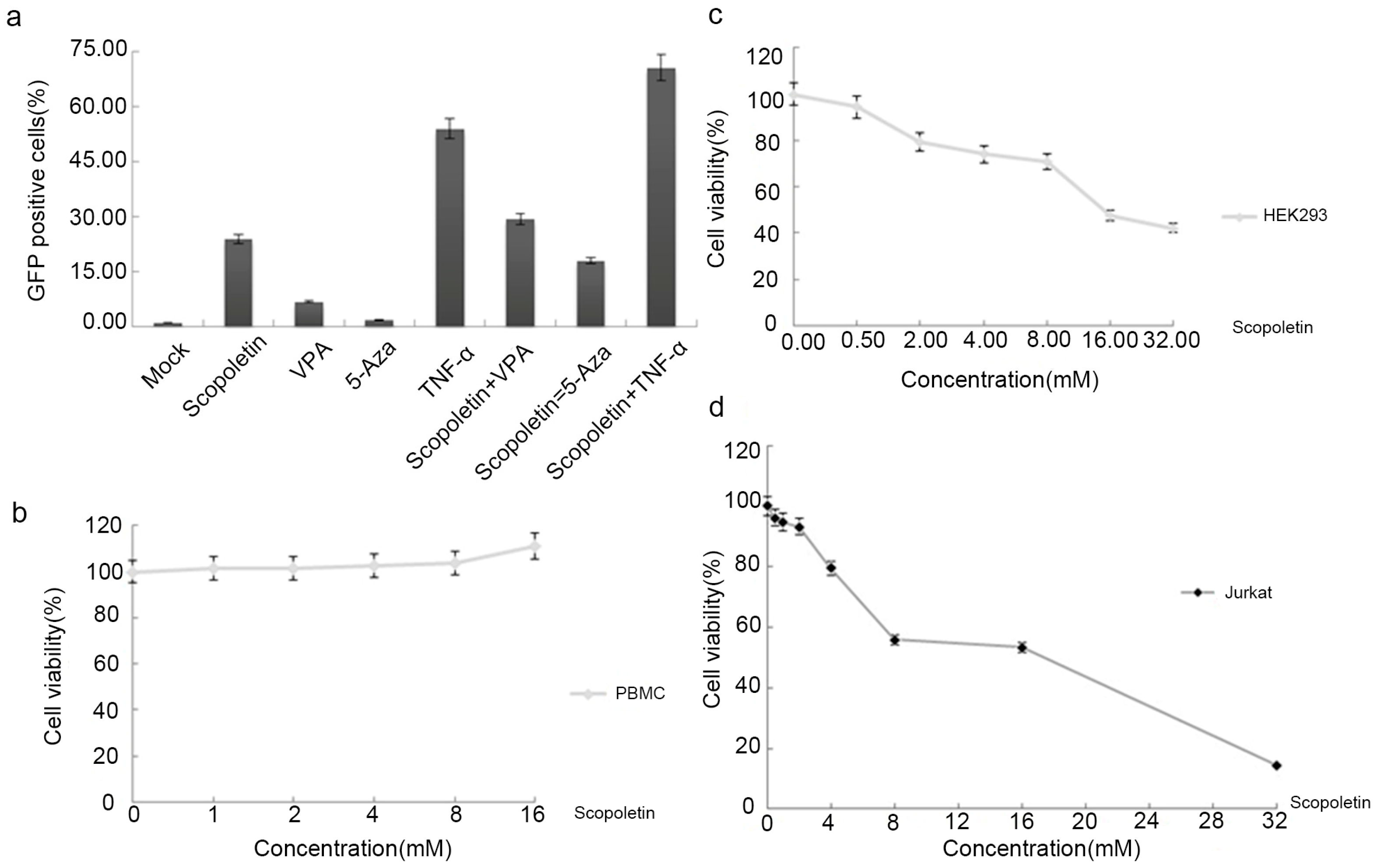
2.2. Testing the Synergistic Activation of HIV-1 Production by Scopoletin and Other Activators
2.3. Cytotoxicity Analysis of Scopoletin In Vitro
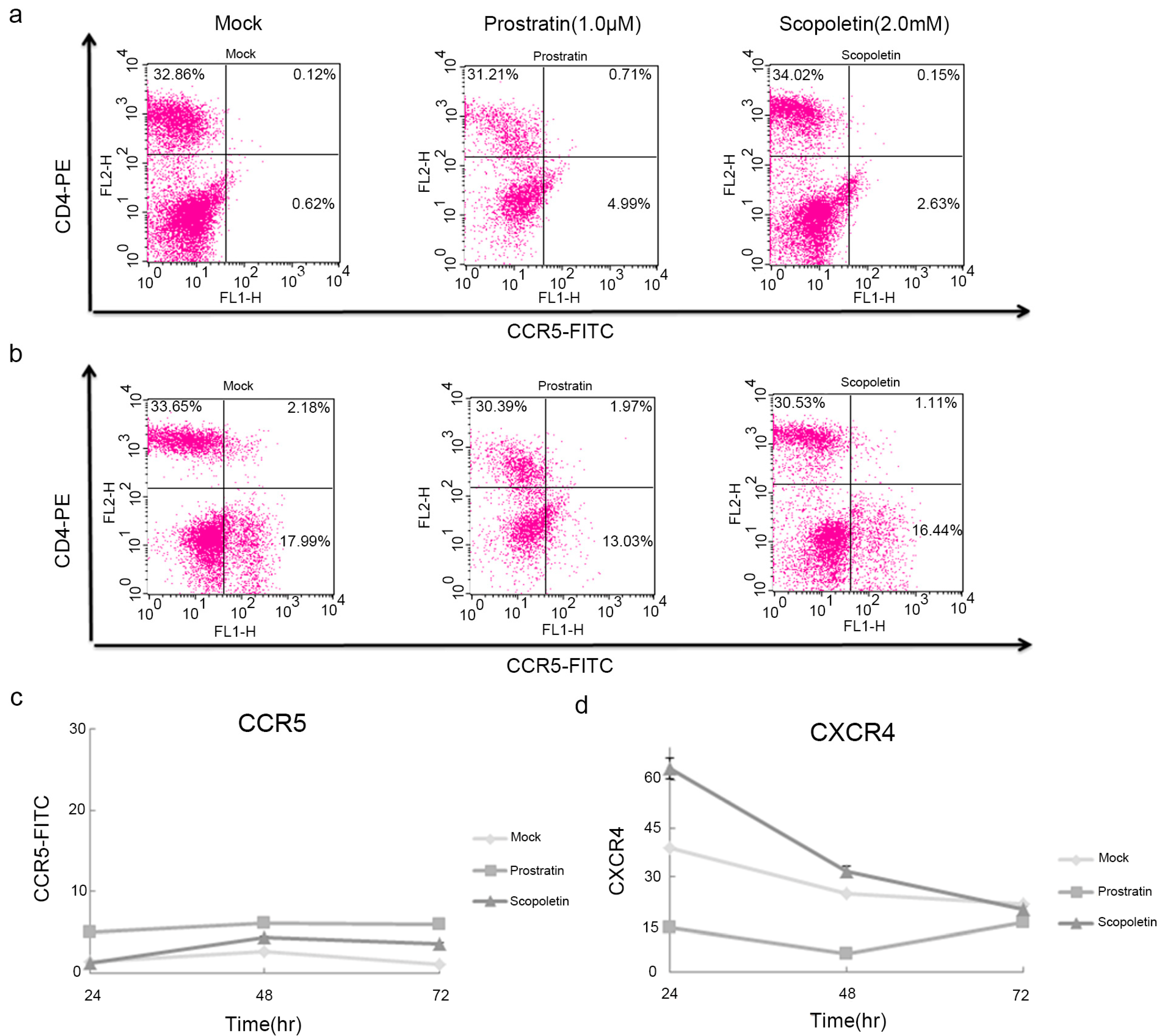
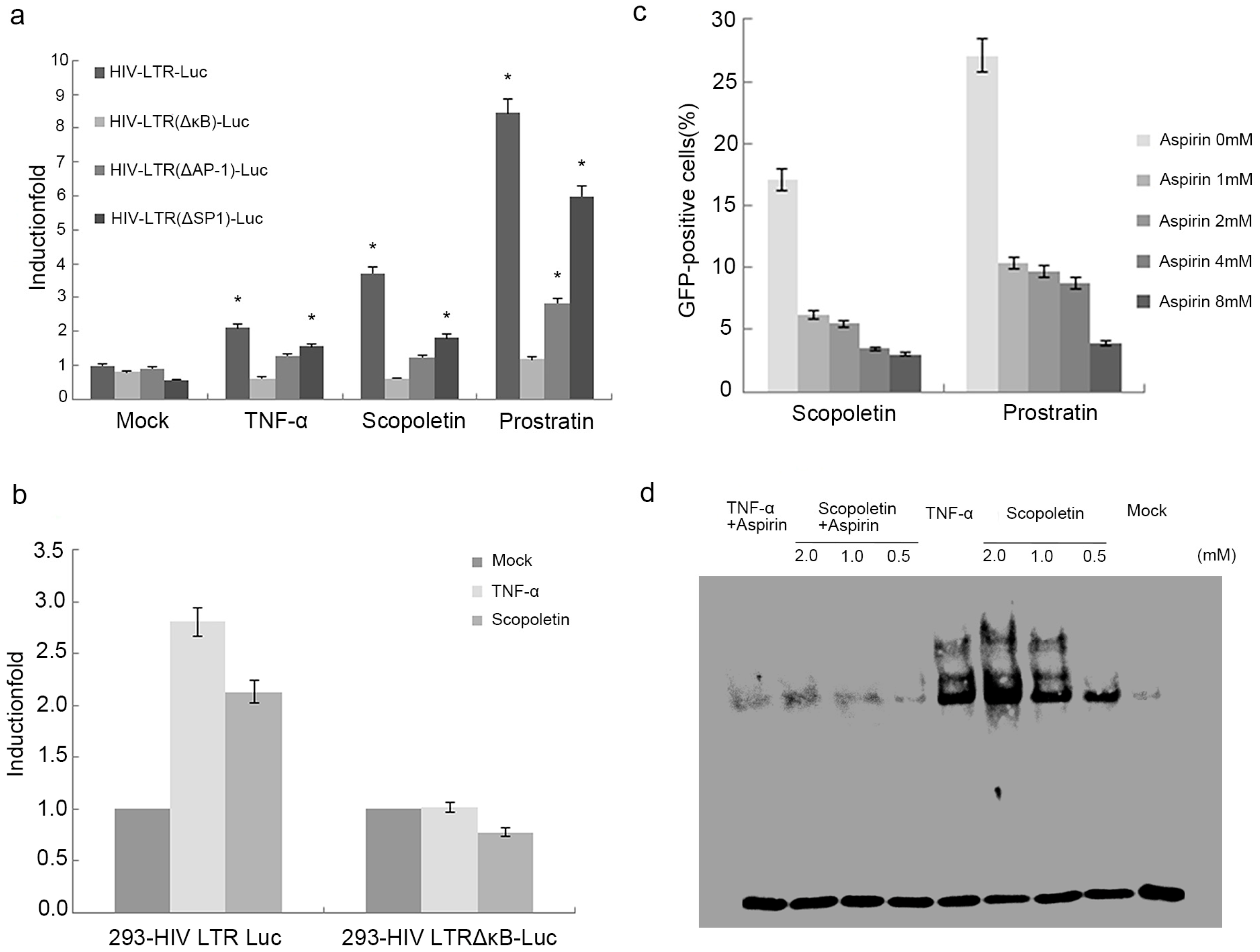
2.4. Scopoletin-Mediated Activation of HIV-1 Involves the Induction of NF-κB Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs)
4.3. Flow Cytometry
4.4. P24 Antigen ELISA Assay
4.5. Cytotoxicity Assay
4.6. Transient Transfection and Luciferase Assays
4.7. Cell Nuclear Protein Extraction and Electrophoretic Mobility Shift Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar] [CrossRef]
- Finzi, D.; Hermankova, M.; Pierson, T.; Carruth, L.M.; Buck, C.; Chaisson, R.E.; Quinn, T.C.; Chadwick, K.; Margolick, J.; Brookmeyer, R.; et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997, 278, 1295–1300. [Google Scholar] [CrossRef]
- Pitman, M.C.; Lau, J.S.Y.; McMahon, J.H.; Lewin, S.R. Barriers and strategies to achieve a cure for HIV. Lancet HIV 2018, 5, e317–e328. [Google Scholar] [CrossRef]
- Williams, S.A.; Kwon, H.; Chen, L.F.; Greene, W.C. Sustained induction of NF-kappa B is required for efficient expression of latent human immunodeficiency virus type 1. J. Virol. 2007, 81, 6043–6056. [Google Scholar] [CrossRef]
- Cary, D.C.; Fujinaga, K.; Peterlin, B.M. Molecular mechanisms of HIV latency. J. Clin. Investig. 2016, 126, 448–454. [Google Scholar] [CrossRef]
- Wong, L.M.; Jiang, G. NF-kappaB sub-pathways and HIV cure: A revisit. eBioMedicine 2021, 63, 103159. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Gabuzda, D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-κB. J. Biol. Chem. 1999, 274, 27981–27988. [Google Scholar] [CrossRef]
- Yang, X.Y.; Wang, Y.N.; Lu, P.P.; Shen, Y.Z.; Zhao, X.Y.; Zhu, Y.Q.; Jiang, Z.T.; Yang, H.; Pan, H.Y.; Zhao, L.; et al. PEBP1 suppresses HIV transcription and induces latency by inactivating MAPK/NF-κB signaling. EMBO Rep. 2020, 21, e49305. [Google Scholar] [CrossRef]
- Zhou, Q.; Yik, J.H. The Yin and Yang of P-TEFb regulation: Implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol. Mol. Biol. Rev. 2006, 70, 646–659. [Google Scholar] [CrossRef]
- Mbonye, U.; Kizito, F.; Karn, J. New insights into transcription elongation control of HIV-1 latency and rebound. Trends Immunol. 2023, 44, 60–71. [Google Scholar] [CrossRef]
- Li, R.; Caico, I.; Xu, Z.; Iqbal, M.S.; Romerio, F. Epigenetic Regulation of HIV-1 Sense and Antisense Transcription in Response to Latency-Reversing Agents. Noncoding RNA 2023, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.H.; Zhu, J.W.; Ni, R.Z.; Zheng, Y.T.; Chen, Y.Y.; Zheng, W.H.; Mu, D. TRIM5alpha recruits HDAC1 to p50 and Sp1 and promotes H3K9 deacetylation at the HIV-1 LTR. Nat. Commun. 2023, 14, 3343. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Zhao, X.Y.; Zhu, Y.Q.; Xun, J.N.; Wen, Q.; Pan, H.Y.; Yang, J.L.; Wang, J.; Liang, Z.M.; Shen, X.T.; et al. FBXO34 promotes latent HIV-1 activation by post-transcriptional modulation. Emerg. Microbes Infec. 2022, 11, 2785–2799. [Google Scholar] [CrossRef] [PubMed]
- Nathans, R.; Chu, C.Y.; Serquina, A.K.; Lu, C.C.; Cao, H.; Rana, T.M. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol. Cell 2009, 34, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qu, X.; Zhou, X.; Shen, Y.; Ji, H.; Fu, Z.; Deng, J.; Lu, P.; Yu, W.; Lu, H.; et al. Two cellular microRNAs, miR-196b and miR-1290, contribute to HIV-1 latency. Virology 2015, 486, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Wang, F.X.; Argyris, E.; Chen, K.Y.; Liang, Z.H.; Tian, H.; Huang, W.L.; Squires, K.; Verlinghieri, G.; Zhang, H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 2007, 13, 1241–1247. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV: Shock and kill. Nature 2012, 487, 439–440. [Google Scholar] [CrossRef]
- Board, N.L.; Moskovljevic, M.; Wu, F.; Siliciano, R.F.; Siliciano, J.D. Engaging innate immunity in HIV-1 cure strategies. Nat. Rev. Immunol. 2022, 22, 499–512. [Google Scholar] [CrossRef]
- Richman, D.D.; Margolis, D.M.; Delaney, M.; Greene, W.C.; Hazuda, D.; Pomerantz, R.J. The challenge of finding a cure for HIV infection. Science 2009, 323, 1304–1307. [Google Scholar] [CrossRef]
- Lu, W.; Yang, C.; Xu, X.; Chen, C.; Hou, X.; Fang, H.; Liu, S. A novel selective histone deacetylase I inhibitor CC-4a activates latent HIV-1 through NF-κB pathway. Life Sci. 2021, 267, 118427. [Google Scholar] [CrossRef]
- Larragoite, E.T.; Nell, R.A.; Martins, L.J.; Barrows, L.R.; Planelles, V.; Spivak, A.M. Histone deacetylase inhibition reduces deleterious cytokine release induced by ingenol stimulation. Biochem. Pharmacol. 2022, 195, 114844. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.A.; Tolstrup, M.; Brinkmann, C.R.; Olesen, R.; Erikstrup, C.; Solomon, A.; Winckelmann, A.; Palmer, S.; Dinarello, C.; Buzon, M.; et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: A phase 1/2, single group, clinical trial. Lancet HIV 2014, 1, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Kirchherr, J.L.; Sung, J.A.; Clutton, G.; Sholtis, K.; Xu, Y.; Allard, B.; Stuelke, E.; Kashuba, A.D.; Kuruc, J.D.; et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J. Clin. Investig. 2017, 127, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Das, B.; Dobrowolski, C.; Karn, J. Multiple Histone Lysine Methyltransferases Are Required for the Establishment and Maintenance of HIV-1 Latency. mBio 2017, 8, 1110–1128. [Google Scholar] [CrossRef]
- Li, Z.C.; Guo, J.; Wu, Y.T.; Zhou, Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 2013, 41, 277–287. [Google Scholar] [CrossRef]
- Lu, P.P.; Qu, X.Y.; Shen, Y.Z.; Jiang, Z.T.; Wang, P.F.; Zeng, H.X.; Ji, H.Y.; Deng, J.X.; Yang, X.Y.; Li, X.; et al. The BET inhibitor OTX015 reactivates latent HIV-1 through P-TEFb. Sci. Rep. 2016, 6, 24100. [Google Scholar] [CrossRef]
- Lu, P.P.; Shen, Y.Z.; Yang, H.; Wang, Y.N.; Jiang, Z.T.; Yang, X.Y.; Zhong, Y.C.; Pan, H.Y.; Xu, J.Q.; Lu, H.Z.; et al. BET inhibitors RVX-208 and PFI-1 reactivate HIV-1 from latency. Sci. Rep. 2017, 7, 16646. [Google Scholar] [CrossRef]
- Jiang, G.C.; Dandekar, S. Targeting NF-κB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res. Hum. Retrovir. 2015, 31, 4–12. [Google Scholar] [CrossRef]
- Margolis, D.M.; Archin, N.M.; Cohen, M.S.; Eron, J.J.; Ferrari, G.; Garcia, J.V.; Gay, C.L.; Goonetilleke, N.; Joseph, S.B.; Swanstrom, R.; et al. Curing HIV: Seeking to Target and Clear Persistent Infection. Cell 2020, 181, 189–206. [Google Scholar] [CrossRef]
- McBrien, J.B.; Mavigner, M.; Franchitti, L.; Smith, S.A.; White, E.; Tharp, G.K.; Walum, H.; Busman-Sahay, K.; Aguilera-Sandoval, C.R.; Thayer, W.O.; et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 2020, 578, 154–159. [Google Scholar] [CrossRef]
- Webb, G.M.; Li, S.B.; Mwakalundwa, G.; Folkvord, J.M.; Greene, J.M.; Reed, J.S.; Stanton, J.J.; Legasse, A.W.; Hobbs, T.; Martin, L.D.; et al. The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles. Blood Adv. 2018, 2, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Vibholm, L.; Schleimann, M.H.; Hojen, J.F.; Benfield, T.; Offersen, R.; Rasmussen, K.; Olesen, R.; Dige, A.; Agnholt, J.; Grau, J.; et al. Short-Course Toll-like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals with Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2017, 64, 1686–1695. [Google Scholar] [CrossRef]
- Macedo, A.B.; Novis, C.L.; Bosque, A. Targeting Cellular and Tissue HIV Reservoirs with Toll-like Receptor Agonists. Front. Immunol. 2019, 10, 2450. [Google Scholar] [CrossRef] [PubMed]
- Vibholm, L.K.; Konrad, C.V.; Schleimann, M.H.; Frattari, G.; Winckelmann, A.; Klastrup, V.; Jensen, N.M.; Jensen, S.S.; Schmidt, M.; Wittig, B.; et al. Effects of 24-week Toll-like receptor 9 agonist treatment in HIV type 1+ individuals. AIDS 2019, 33, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.C.; Mavigner, M.; Sampey, G.C.; Brooks, A.D.; Spagnuolo, R.A.; Irlbeck, D.M.; Mattingly, C.; Ho, P.T.; Schoof, N.; Cammon, C.G.; et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 2020, 578, 160–165. [Google Scholar] [CrossRef]
- Gay, C.L.; Bosch, R.J.; Ritz, J.; Hataye, J.M.; Aga, E.; Tressler, R.L.; Mason, S.W.; Hwang, C.K.; Grasela, D.M.; Ray, N.; et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 1725–1733. [Google Scholar] [CrossRef]
- Wightman, F.; Solomon, A.; Kumar, S.S.; Urriola, N.; Gallagher, K.; Hiener, B.; Palmer, S.; McNeil, C.; Garsia, R.; Lewin, S.R. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS 2015, 29, 504–506. [Google Scholar] [CrossRef]
- Archin, N.M.; Liberty, A.L.; Kashuba, A.D.; Choudhary, S.K.; Kuruc, J.D.; Crooks, A.M.; Parker, D.C.; Anderson, E.M.; Kearney, M.F.; Strain, M.C.; et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012, 487, 482–485. [Google Scholar] [CrossRef]
- Archin, N.M.; Bateson, R.; Tripathy, M.K.; Crooks, A.M.; Yang, K.H.; Dahl, N.P.; Kearney, M.F.; Anderson, E.M.; Coffin, J.M.; Strain, M.C.; et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J. Infect. Dis. 2014, 210, 728–735. [Google Scholar] [CrossRef]
- Elliott, J.H.; Wightman, F.; Solomon, A.; Ghneim, K.; Ahlers, J.; Cameron, M.J.; Smith, M.Z.; Spelman, T.; McMahon, J.; Velayudham, P.; et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014, 10, e1004473. [Google Scholar] [CrossRef]
- Fidler, S.; Stohr, W.; Pace, M.; Dorrell, L.; Lever, A.; Pett, S.; Kinloch-de Loes, S.; Fox, J.; Clarke, A.; Nelson, M.; et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): A phase 2, randomised trial. Lancet 2020, 395, 888–898. [Google Scholar] [CrossRef]
- Leth, S.; Schleimann, M.H.; Nissen, S.K.; Hojen, J.F.; Olesen, R.; Graversen, M.E.; Jorgensen, S.; Kjaer, A.S.; Denton, P.W.; Mork, A.; et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): A single-arm, phase 1B/2A trial. Lancet HIV 2016, 3, e463–e472. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Serrano-Villar, S.; Madrid-Elena, N.; Perez-Elias, M.J.; Martin, M.E.; Barbas, C.; Ruiperez, J.; Munoz, E.; Munoz-Fernandez, M.A.; Castor, T.; et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 2016, 30, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.M.; Andrade, A.; Eisele, E.; Hoh, R.; Bacchetti, P.; Bumpus, N.N.; Emad, F.; Buckheit, R., 3rd; McCance-Katz, E.F.; Lai, J.; et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin. Infect. Dis. 2014, 58, 883–890. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Cardellina, J.H., 2nd; McMahon, J.B.; Gulakowski, R.J.; Ishitoya, J.; Szallasi, Z.; Lewin, N.E.; Blumberg, P.M.; Weislow, O.S.; Beutler, J.A.; et al. A nonpromoting phorbol from the samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J. Med. Chem. 1992, 35, 1978–1986. [Google Scholar] [CrossRef]
- Rullas, J.; Bermejo, M.; Garcia-Perez, J.; Beltran, M.; Gonzalez, N.; Hezareh, M.; Brown, S.J.; Alcami, J. Prostratin induces HIV activation and downregulates HIV receptors in peripheral blood lymphocytes. Antivir. Ther. 2004, 9, 545–554. [Google Scholar] [CrossRef]
- Korin, Y.D.; Brooks, D.G.; Brown, S.; Korotzer, A.; Zack, J.A. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J. Virol. 2002, 76, 8118–8123. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Chen, L.F.; Kwon, H.; Fenard, D.; Bisgrove, D.; Verdin, E.; Greene, W.C. Prostratin antagonizes HIV latency by activating NF-kappaB. J. Biol. Chem. 2004, 279, 42008–42017. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.K.; Hua, J.; Zhou, J.P.; Zhang, H.B.; Zhu, H.Y.; Cheng, Y.H.; Gust, R. Synthesis and in vitro antitumor activity of novel scopoletin derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5008–5012. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Mizutani, M.; Sakata, K.; Shimizu, B. Molecular cloning and functional analysis of the ortho-hydroxylases of p-coumaroyl coenzyme A/feruloyl coenzyme A involved in formation of umbelliferone and scopoletin in sweet potato, Ipomoea batatas (L.) Lam. Phytochemistry 2012, 74, 49–57. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.H.; Zhou, X.; Qu, X.Y.; Wang, P.F.; Liu, S.J.; Lu, D.R.; Zhu, H.Z. Selective Histonedeacetylase Inhibitor M344 Intervenes in HIV-1 Latency through Increasing Histone Acetylation and Activation of NF-kappaB. PLoS ONE 2012, 7, e48832. [Google Scholar] [CrossRef] [PubMed]
- Reuse, S.; Calao, M.; Kabeya, K.; Guiguen, A.; Gatot, J.S.; Quivy, V.; Vanhulle, C.; Lamine, A.; Vaira, D.; Demonte, D.; et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: Implications for treatment of latent infection. PLoS ONE 2009, 4, e6093. [Google Scholar] [CrossRef] [PubMed]
- Kulkosky, J.; Culnan, D.M.; Roman, J.; Dornadula, G.; Schnell, M.; Boyd, M.R.; Pomerantz, R.J. Prostratin: Activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 2001, 98, 3006–3015. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, O.V.; Pfeifer, N.; Lengauer, T. Modelling binding between CCR5 and CXCR4 receptors and their ligands suggests the surface electrostatic potential of the co-receptor to be a key player in the HIV-1 tropism. Retrovirology 2013, 10, 130. [Google Scholar] [CrossRef]
- Lucera, M.B.; Tilton, C.A.; Mao, H.; Dobrowolski, C.; Tabler, C.O.; Haqqani, A.A.; Karn, J.; Tilton, J.C. The histone deacetylase inhibitor vorinostat (SAHA) increases the susceptibility of uninfected CD4+ T cells to HIV by increasing the kinetics and efficiency of postentry viral events. J. Virol. 2014, 88, 10803–10812. [Google Scholar] [CrossRef]
- Mehla, R.; Bivalkar-Mehla, S.; Zhang, R.; Handy, I.; Albrecht, H.; Giri, S.; Nagarkatti, P.; Nagarkatti, M.; Chauhan, A. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS ONE 2010, 5, e11160. [Google Scholar] [CrossRef]
- Victoriano, A.F.; Okamoto, T. Transcriptional control of HIV replication by multiple modulators and their implication for a novel antiviral therapy. AIDS Res. Hum. Retrovir. 2012, 28, 125–138. [Google Scholar] [CrossRef]
- Kutuk, O.; Basaga, H. Aspirin inhibits TNFalpha- and IL-1-induced NF-kappaB activation and sensitizes HeLa cells to apoptosis. Cytokine 2004, 25, 229–237. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, K.B.; Shin, B.C.; Seo, E.A.; Lee, Y.R.; Kim, J.S.; Park, J.W.; Park, B.H.; Ryu, D.G. Scopoletin induces apoptosis in human promyeloleukemic cells, accompanied by activations of nuclear factor kappaB and caspase-3. Life Sci. 2005, 77, 824–836. [Google Scholar] [CrossRef]
- Seo, E.J.; Saeed, M.; Law, B.Y.; Wu, A.G.; Kadioglu, O.; Greten, H.J.; Efferth, T. Pharmacogenomics of Scopoletin in Tumor Cells. Molecules 2016, 21, 496. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Naturforsch. C J. Biosci. 2022, 77, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Pereira Dos Santos Nascimento, M.V.; Arruda-Silva, F.; Gobbo Luz, A.B.; Baratto, B.; Venzke, D.; Mendes, B.G.; Fröde, T.S.; Geraldo Pizzolatti, M.; Dalmarco, E.M. Inhibition of the NF-kB and p38 MAPK pathways by scopoletin reduce the inflammation caused by carrageenan in the mouse model of pleurisy. Immunopharmacol. Immunotoxicol. 2016, 38, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tang, D.; Wu, Q.; Huang, Q.; Song, J.; Long, Q. Scopoletin inhibits PDGF-BB-induced proliferation and migration of airway smooth muscle cells by regulating NF-kB signaling pathway. Allergol. Immunopathol. 2022, 50, 92–98. [Google Scholar] [CrossRef]
- Gramatica, A.; Schwarzer, R.; Brantley, W.; Varco-Merth, B.; Sperber, H.S.; Hull, P.A.; Montano, M.; Migueles, S.A.; Rosenthal, D.; Hogan, L.E.; et al. Evaluating a New Class of AKT/mTOR Activators for HIV Latency-Reversing Activity Ex Vivo and In Vivo. J. Virol. 2021, 95, e02393-20. [Google Scholar] [CrossRef]
- Jordan, A.; Bisgrove, D.; Verdin, E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003, 22, 1868–1877. [Google Scholar] [CrossRef]
- Ding, D.L.; Qu, X.Y.; Li, L.; Zhou, X.; Liu, S.J.; Lin, S.G.; Wang, P.F.; Liu, S.H.; Kong, C.J.; Wang, X.H.; et al. Involvement of histone methyltransferase GLP in HIV-1 latency through catalysis of H3K9 dimethylation. Virology 2013, 440, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.Y.; Jiang, Z.T.; Lu, P.P.; Ma, L.; Li, C.; Pan, H.Y.; Fu, Z.; Qu, X.Y.; Wang, P.F.; Deng, J.X.; et al. Specific Reactivation of Latent HIV-1 by dCas9-SunTag-VP64-mediated Guide RNA Targeting the HIV-1 Promoter. Mol. Ther. 2016, 24, 508–521. [Google Scholar] [CrossRef]
- Wang, P.F.; Qu, X.Y.; Wang, X.H.; Liu, L.; Zhu, X.L.; Zeng, H.X.; Zhu, H.Z. As2O3 synergistically reactivate latent HIV-1 by induction of NF-kappaB. Antiviral. Res. 2013, 100, 688–697. [Google Scholar] [CrossRef]
- Wang, F.X.; Xu, Y.; Sullivan, J.; Souder, E.; Argyris, E.G.; Acheampong, E.A.; Fisher, J.; Sierra, M.; Thomson, M.M.; Najera, R.; et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J. Clin. Investig. 2005, 115, 128–137. [Google Scholar] [CrossRef]
- Bren, G.D.; Whitman, J.; Cummins, N.; Shepard, B.; Rizza, S.A.; Trushin, S.A.; Badley, A.D. Infected cell killing by HIV-1 protease promotes NF-kappaB dependent HIV-1 replication. PLoS ONE 2008, 3, e2112. [Google Scholar] [CrossRef] [PubMed]
- Lilling, G.; Elena, N.; Sidi, Y.; Bakhanashvili, M. p53-associated 3′–>5″ exonuclease activity in nuclear and cytoplasmic compartments of cells. Oncogene 2003, 22, 233–245. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Jiang, Z.; Liu, L.; Yang, X.; Li, M.; Cheng, Y.; Xu, J.; Yin, C.; Zhu, H. Scopoletin Reactivates Latent HIV-1 by Inducing NF-κB Expression without Global T Cell Activation. Int. J. Mol. Sci. 2023, 24, 12649. https://doi.org/10.3390/ijms241612649
Zhu Y, Jiang Z, Liu L, Yang X, Li M, Cheng Y, Xu J, Yin C, Zhu H. Scopoletin Reactivates Latent HIV-1 by Inducing NF-κB Expression without Global T Cell Activation. International Journal of Molecular Sciences. 2023; 24(16):12649. https://doi.org/10.3390/ijms241612649
Chicago/Turabian StyleZhu, Yuqi, Zhengtao Jiang, Lin Liu, Xinyi Yang, Min Li, Yipeng Cheng, Jianqing Xu, Chunhua Yin, and Huanzhang Zhu. 2023. "Scopoletin Reactivates Latent HIV-1 by Inducing NF-κB Expression without Global T Cell Activation" International Journal of Molecular Sciences 24, no. 16: 12649. https://doi.org/10.3390/ijms241612649
APA StyleZhu, Y., Jiang, Z., Liu, L., Yang, X., Li, M., Cheng, Y., Xu, J., Yin, C., & Zhu, H. (2023). Scopoletin Reactivates Latent HIV-1 by Inducing NF-κB Expression without Global T Cell Activation. International Journal of Molecular Sciences, 24(16), 12649. https://doi.org/10.3390/ijms241612649





