The Great Game between Plants and Viruses: A Focus on Protein Homeostasis
Abstract
1. Introduction
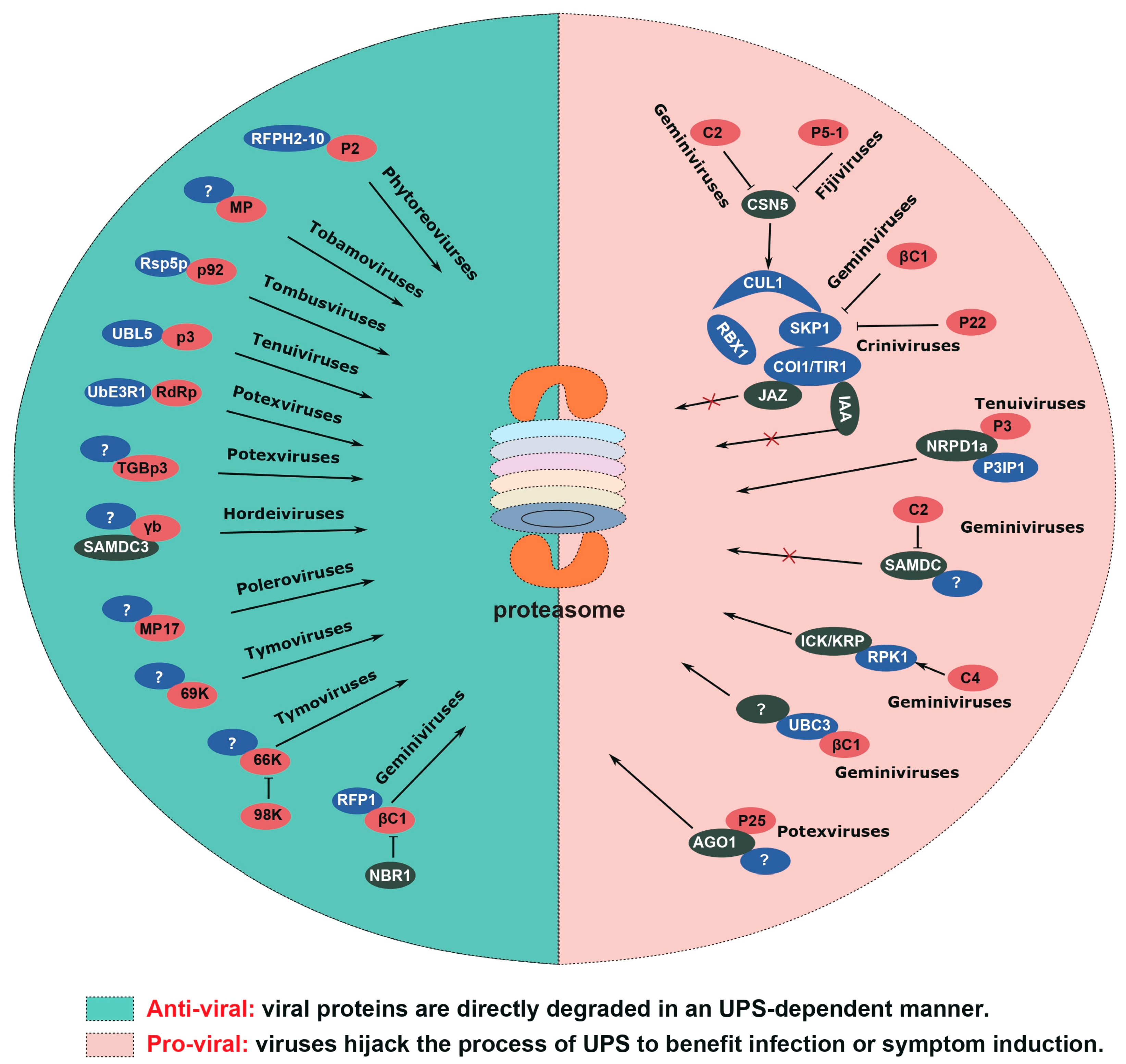
2. Friend or Foe: The Dual Role of UPS in Plant Virus Infection
2.1. UPS Directly Targets Viral Proteins for Degradation
2.2. Viruses Escape from UPS-Mediated Degradation of Viral Proteins
2.3. Viruses Hijack UPS for Targeting Host Factors Involved in Antiviral Responses
2.4. Viruses Induce Disease Symptoms by Exploiting UPS
2.5. The Role of Cell-Division-Cycle 48 in Maintaining Host and Viral Protein Homeostasis
3. Autophagy–Virus Interplay in Plants: From Antiviral Recognition to Proviral Manipulation
3.1. Viral Proteins Are Directly Targeted for Degradation through Autophagy
3.2. Viral Proteins Interfere with the Autophagy-Mediated Antiviral Responses
3.3. Viruses Exploit the Autophagy to Suppress Antiviral Signaling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicaise, V. Crop immunity against viruses: Outcomes and future challenges. Front. Plant Sci. 2014, 5, 660. [Google Scholar] [CrossRef] [PubMed]
- Mandadi, K.K.; Scholthof, K.B. Plant immune responses against viruses: How does a virus cause disease? Plant Cell 2013, 25, 1489–1505. [Google Scholar] [CrossRef] [PubMed]
- Calil, I.P.; Fontes, E.P.B. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2017, 119, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, L. Crosstalk between Ubiquitination and Other Post-translational Protein Modifications in Plant Immunity. Plant Commun. 2020, 1, 100041. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ismayil, A.; Liu, Y. Autophagy in Plant–virus Interactions. Annu. Rev. Virol. 2020, 7, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Dubiella, U.; Serrano, I. The Ubiquitin Proteasome System as a Double Agent in Plant–virus Interactions. Plants 2021, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.; Dikic, I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 2019, 366, 818–822. [Google Scholar] [CrossRef]
- Alcaide-Loridan, C.; Jupin, I. Ubiquitin and plant viruses, let’s play together! Plant Physiol. 2012, 160, 72–82. [Google Scholar] [CrossRef]
- Komander, D.; Clague, M.J.; Urbé, S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef]
- Lobaina, D.P.; Tarazi, R.; Castorino, T.; Vaslin, M.F.S. The Ubiquitin-Proteasome System (UPS) and Viral Infection in Plants. Plants 2022, 11, 2476. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, X.; Otegui, M.S. Plant autophagy: New flavors on the menu. Curr. Opin. Plant Biol. 2018, 46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Y. Autophagy in plant viral infection. FEBS Lett. 2022, 596, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, S.; Yang, X.; Yang, X.; Zhang, T.; Zhou, G. Friend or Enemy: A Dual Role of Autophagy in Plant Virus Infection. Front. Microbiol. 2020, 11, 736. [Google Scholar] [CrossRef]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, M.; Goto, A.; Watanabe, Y. The tobacco ubiquitin-activating enzymes NtE1A and NtE1B are induced by tobacco mosaic virus, wounding and stress hormones. Mol. Cells 2005, 19, 228–231. [Google Scholar] [PubMed]
- Becker, F.; Buschfeld, E.; Schell, J.; Bachmair, A. Altered response to viral infection by tobacco plants perturbed in ubiquitin system. Plant J. 1993, 3, 875–881. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Serino, G.; Deng, X.W.; Dinesh-Kumar, S.P. Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. Plant Cell 2002, 14, 1483–1496. [Google Scholar] [CrossRef]
- Ballut, L.; Petit, F.; Mouzeyar, S.; Le Gall, O.; Candresse, T.; Schmid, P.; Nicolas, P.; Badaoui, S. Biochemical identification of proteasome-associated endonuclease activity in sunflower. Biochim. Biophys. Acta 2003, 1645, 30–39. [Google Scholar] [CrossRef]
- Shirasu, K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 2009, 60, 139–164. [Google Scholar] [CrossRef]
- Verchot, J. Plant Virus Infection and the Ubiquitin Proteasome Machinery: Arms Race along the Endoplasmic Reticulum. Viruses 2016, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Dunigan, D.D.; Dietzgen, R.G.; Schoelz, J.E.; Zaitlin, M. Tobacco mosaic virus particles contain ubiquitinated coat protein subunits. Virology 1988, 165, 310–312. [Google Scholar] [CrossRef]
- Reichel, C.; Beachy, R.N. Degradation of tobacco mosaic virus movement protein by the 26S proteasome. J. Virol. 2000, 74, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, T.; Boevink, P.; Haupt, S.; Roberts, A.G.; Toth, R.; Valentine, T.; Chapman, S.; Oparka, K.J. Functional analysis of a DNA-shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of tobacco mosaic virus. Plant Cell 2002, 14, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Drugeon, G.; Jupin, I. Stability in vitro of the 69K movement protein of Turnip yellow mosaic virus is regulated by the ubiquitin-mediated proteasome pathway. J. Gen Virol. 2002, 83, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.; Hofius, D.; Sonnewald, U. Intracellular trafficking of Potato leafroll virus movement protein in transgenic Arabidopsis. Traffic 2007, 8, 1205–1214. [Google Scholar] [CrossRef]
- Ju, H.J.; Ye, C.M.; Verchot-Lubicz, J. Mutational analysis of PVX TGBp3 links subcellular accumulation and protein turnover. Virology 2008, 375, 103–117. [Google Scholar] [CrossRef]
- Camborde, L.; Planchais, S.; Tournier, V.; Jakubiec, A.; Drugeon, G.; Lacassagne, E.; Pflieger, S.; Chenon, M.; Jupin, I. The ubiquitin-proteasome system regulates the accumulation of Turnip yellow mosaic virus RNA-dependent RNA polymerase during viral infection. Plant Cell 2010, 22, 3142–3152. [Google Scholar] [CrossRef]
- Liu, L.; Jin, L.; Huang, X.; Geng, Y.; Li, F.; Qin, Q.; Wang, R.; Ji, S.; Zhao, S.; Xie, Q.I.; et al. OsRFPH2-10, a ring-H2 finger E3 ubiquitin ligase, is involved in rice antiviral defense in the early stages of rice dwarf virus infection. Mol. Plant 2014, 7, 1057–1060. [Google Scholar] [CrossRef]
- Barajas, D.; Li, Z.; Nagy, P.D. The Nedd4-type Rsp5p ubiquitin ligase inhibits tombusvirus replication by regulating degradation of the p92 replication protein and decreasing the activity of the tombusvirus replicase. J. Virol. 2009, 83, 11751–11764. [Google Scholar] [CrossRef]
- Barajas, D.; Kovalev, N.; Qin, J.; Nagy, P.D. Novel mechanism of regulation of tomato bushy stunt virus replication by cellular WW-domain proteins. J. Virol. 2015, 89, 2064–2079. [Google Scholar] [CrossRef][Green Version]
- Qin, J.; Barajas, D.; Nagy, P.D. An inhibitory function of WW domain-containing host proteins in RNA virus replication. Virology 2012, 426, 106–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, I.H.; Chang, J.E.; Wu, C.Y.; Huang, Y.P.; Hsu, Y.H.; Tsai, C.H. An E3 ubiquitin ligase from Nicotiana benthamiana targets the replicase of Bamboo mosaic virus and restricts its replication. Mol. Plant Pathol. 2019, 20, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, L.; Lu, Y.; Peng, J.; Zheng, H.; Yang, Q.; Rao, S.; Wu, G.; Li, J.; Chen, Z.; et al. Ubiquitin-Like protein 5 interacts with the silencing suppressor P3 of rice stripe virus and mediates its degradation through the 26S proteasome pathway. PLoS Pathog. 2020, 16, e1008780. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Li, W.; Wen, Z.; Duan, J.; Jiang, Z.; Zhang, D.; Xie, X.; Wang, X.; Li, F.; et al. SAMDC3 enhances resistance to Barley stripe mosaic virus by promoting the ubiquitination and proteasomal degradation of viral γb protein. New Phytol. 2022, 234, 618–633. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Hu, T.; Bao, M.; Cao, L.; Zhang, H.; Song, F.; Xie, Q.; Zhou, X. Tobacco RING E3 Ligase NtRFP1 Mediates Ubiquitination and Proteasomal Degradation of a Geminivirus-Encoded βC1. Mol. Plant 2016, 9, 911–925. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, M.; Gong, P.; Li, F.; Zhou, X. Selective autophagic receptor NbNBR1 prevents NbRFP1-mediated UPS-dependent degradation of βC1 to promote geminivirus infection. PLoS Pathog. 2021, 17, e1009956. [Google Scholar] [CrossRef]
- Nair, A.; Chatterjee, K.S.; Jha, V.; Das, R.; Shivaprasad, P.V. Stability of Begomoviral pathogenicity determinant βC1 is modulated by mutually antagonistic SUMOylation and SIM interactions. BMC Biol. 2020, 18, 110. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q.; Zhao, T.; Xiang, H.; Zhang, X.; Wu, Z.; Zhou, C.; Zhang, X.; Wang, Y.; Zhang, Y.; et al. Interaction between Brassica yellows virus silencing suppressor P0 and plant SKP1 facilitates stability of P0 in vivo against degradation by proteasome and autophagy pathways. New Phytol. 2019, 222, 1458–1473. [Google Scholar] [CrossRef]
- Chenon, M.; Camborde, L.; Cheminant, S.; Jupin, I. A viral deubiquitylating enzyme targets viral RNA-dependent RNA polymerase and affects viral infectivity. EMBO J. 2012, 31, 741–753. [Google Scholar] [CrossRef]
- Dreher, K.; Callis, J. Ubiquitin, hormones and biotic stress in plants. Ann. Bot. 2007, 99, 787–822. [Google Scholar] [CrossRef] [PubMed]
- Saxena, H.; Negi, H.; Sharma, B. Role of F-box E3-ubiquitin ligases in plant development and stress responses. Plant Cell Rep. 2023, 42, 1133–1146. [Google Scholar] [CrossRef]
- He, L.; Chen, X.; Yang, J.; Zhang, T.; Li, J.; Zhang, S.; Zhong, K.; Zhang, H.; Chen, J.; Yang, J. Rice black-streaked dwarf virus-encoded P5-1 regulates the ubiquitination activity of SCF E3 ligases and inhibits jasmonate signaling to benefit its infection in rice. New Phytol. 2020, 225, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Durán, R.; Rosas-Díaz, T.; Gusmaroli, G.; Luna, A.P.; Taconnat, L.; Deng, X.W.; Bejarano, E.R. Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell 2011, 23, 1014–1032. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Liu, N.; Xie, K.; Dai, Y.; Han, S.; Zhao, X.; Qian, L.; Wang, Y.; Zhao, J.; Gorovits, R.; et al. CLCuMuB βC1 Subverts Ubiquitination by Interacting with NbSKP1s to Enhance Geminivirus Infection in Nicotiana benthamiana. PLoS Pathog. 2016, 12, e1005668. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Liu, X.; Navas-Castillo, J.; Zang, L.; Fan, Z.; Zhu, X.; Zhou, T. Tomato chlorosis virus-encoded P22 suppresses auxin signalling to promote infection via interference with SKP1-Cullin-F-box(TIR1) complex assembly. Plant Cell Environ. 2021, 44, 3155–3172. [Google Scholar] [CrossRef]
- Lai, J.; Chen, H.; Teng, K.; Zhao, Q.; Zhang, Z.; Li, Y.; Liang, L.; Xia, R.; Wu, Y.; Guo, H.; et al. RKP, a RING finger E3 ligase induced by BSCTV C4 protein, affects geminivirus infection by regulation of the plant cell cycle. Plant J. 2009, 57, 905–917. [Google Scholar] [CrossRef]
- Chiu, M.H.; Chen, I.H.; Baulcombe, D.C.; Tsai, C.H. The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol. Plant Pathol. 2010, 11, 641–649. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Huang, X.; Xia, R.; Zhao, Q.; Lai, J.; Teng, K.; Li, Y.; Liang, L.; Du, Q.; et al. BSCTV C2 attenuates the degradation of SAMDC1 to suppress DNA methylation-mediated gene silencing in Arabidopsis. Plant Cell 2011, 23, 273–288. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, Y.; Xu, L.; Wu, K.C.; Yang, L.; Shi, C.N.; Yang, G.Y.; Chen, D.; Yu, F.F.; Xie, Q.; et al. A Bunyavirus-Inducible Ubiquitin Ligase Targets RNA Polymerase IV for Degradation during Viral Pathogenesis in Rice. Mol. Plant 2020, 13, 836–850. [Google Scholar] [CrossRef]
- Eini, O.; Dogra, S.; Selth, L.A.; Dry, I.B.; Randles, J.W.; Rezaian, M.A. Interaction with a host ubiquitin-conjugating enzyme is required for the pathogenicity of a geminiviral DNA beta satellite. Mol. Plant-Microbe Interact. MPMI 2009, 22, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J. Endoplasmic reticulum-mediated protein quality control in arabidopsis. Front. Plant Sci. 2014, 5, 162. [Google Scholar] [CrossRef] [PubMed]
- Copeland, C.; Woloshen, V.; Huang, Y.; Li, X. Atcdc48a is involved in the turnover of an nlr immune receptor. Plant J. 2016, 88, 294–305. [Google Scholar] [CrossRef]
- Feng, Z.; Kovalev, N.; Nagy, P.D. Multifunctional role of the co-opted Cdc48 AAA+ ATPase in tombusvirus replication. Virology 2022, 576, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Niehl, A.; Amari, K.; Gereige, D.; Brandner, K.; Mély, Y.; Heinlein, M. Control of Tobacco mosaic virus movement protein fate by CELL-DIVISION-CYCLE protein48. Plant Physiol. 2012, 160, 2093–2108. [Google Scholar] [CrossRef] [PubMed]
- Niehl, A.; Amari, K.; Heinlein, M. CDC48 function during TMV infection: Regulation of virus movement and replication by degradation? Plant Signal. Behav. 2013, 8, e22865. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Czymmek, K.; Tallóczy, Z.; Levine, B.; Dinesh-Kumar, S.P. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005, 121, 567–577. [Google Scholar] [CrossRef]
- Ismayil, A.; Yang, M.; Liu, Y. Role of autophagy during plant–virus interactions. Semin. Cell Dev. Biol. 2020, 101, 36–40. [Google Scholar] [CrossRef]
- Nakahara, K.S.; Masuta, C.; Yamada, S.; Shimura, H.; Kashihara, Y.; Wada, T.S.; Meguro, A.; Goto, K.; Tadamura, K.; Sueda, K.; et al. Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc. Natl. Acad. Sci. USA 2012, 109, 10113–10118. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, G.; Sheng, L.; Chen, M.; Hu, C.; Ye, Y.; Yue, X.; Chen, S.; OuYang, W.; Xia, Z. Regulatory roles of selective autophagy through targeting of native proteins in plant adaptive responses. Plant Cell Rep. 2022, 41, 2125–2138. [Google Scholar] [CrossRef]
- Hafrén, A.; Macia, J.L.; Love, A.J.; Milner, J.J.; Drucker, M.; Hofius, D. Selective autophagy limits cauliflower mosaic virus infection by NBR1-mediated targeting of viral capsid protein and particles. Proc. Natl. Acad. Sci. USA 2017, 114, E2026–E2035. [Google Scholar] [CrossRef]
- Hafrén, A.; Üstün, S.; Hochmuth, A.; Svenning, S.; Johansen, T.; Hofius, D. Turnip Mosaic Virus Counteracts Selective Autophagy of the Viral Silencing Suppressor HCpro. Plant Physiol. 2018, 176, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lu, Y.; Zheng, X.; Yang, X.; Chen, Y.; Zhang, T.; Zhao, X.; Wang, S.; Zhao, X.; Song, X.; et al. The plant protein NbP3IP directs degradation of Rice stripe virus P3 silencing suppressor protein to limit virus infection through interaction with the autophagy-related protein NbATG8. New Phytol. 2021, 229, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, C.; Li, Y.; Wu, G.; Hou, X.; Zhou, X.; Wang, A. Beclin1 restricts RNA virus infection in plants through suppression and degradation of the viral polymerase. Nat. Commun. 2018, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhao, J.; Feng, Y.; Zou, J.; Ye, J.; Liu, J.; Han, C.; Li, D.; Wang, X. A selective autophagy receptor VISP1 induces symptom recovery by targeting viral silencing suppressors. Nat. Commun. 2023, 14, 3852. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, Q.; Rui, P.; Lu, Y.; Sun, Z.; Chen, J.; Wang, Y.; Yan, F. Rice stripe virus P2 protein interacts with ATG5 and is targeted for degradation by autophagy. Front. Microbiol. 2023, 14, 1191403. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Zhang, C.; Zhou, X. Nuclear autophagy degrades a geminivirus nuclear protein to restrict viral infection in solanaceous plants. New Phytol. 2020, 225, 1746–1761. [Google Scholar] [CrossRef]
- Haxim, Y.; Ismayil, A.; Jia, Q.; Wang, Y.; Zheng, X.; Chen, T.; Qian, L.; Liu, N.; Wang, Y.; Han, S.; et al. Autophagy functions as an antiviral mechanism against geminiviruses in plants. eLife 2017, 6, e23897. [Google Scholar] [CrossRef]
- Prasad, A.; Prasad, M. Interaction of ToLCNDV TrAP with SlATG8f marks it susceptible to degradation by autophagy. Cell. Mol. Life Sci. 2022, 79, 241. [Google Scholar] [CrossRef]
- Ismayil, A.; Yang, M.; Haxim, Y.; Wang, Y.; Li, J.; Han, L.; Wang, Y.; Zheng, X.; Wei, X.; Nagalakshmi, U.; et al. Cotton leaf curl Multan virus βC1 Protein Induces Autophagy by Disrupting the Interaction of Autophagy-Related Protein 3 with Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Cell 2020, 32, 1124–1135. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Xie, X.; Yue, N.; Li, J.; Wang, X.B.; Han, C.; Yu, J.; Liu, Y.; Li, D. Barley stripe mosaic virus γb Protein Subverts Autophagy to Promote Viral Infection by Disrupting the ATG7-ATG8 Interaction. Plant Cell 2018, 30, 1582–1595. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Ge, L.; Zhang, M.; Li, F.; Zhou, X. Geminiviral C2 proteins inhibit active autophagy to facilitate virus infection by impairing the interaction of ATG7 and ATG8. J. Integra Plant Biol. 2023, 65, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Niu, E.; Ye, C.; Zhao, W.; Kondo, H.; Wu, Y.; Chen, J.; Andika, I.B.; Sun, L. Coat protein of Chinese wheat mosaic virus upregulates and interacts with cytosolic glyceraldehyde-3-phosphate dehydrogenase, a negative regulator of plant autophagy, to promote virus infection. J. Integr. Plant Biol. 2022, 64, 1631–1645. [Google Scholar] [CrossRef]
- Shang, K.; Xiao, L.; Zhang, X.; Zang, L.; Zhao, D.; Wang, C.; Wang, X.; Zhou, T.; Zhu, C.; Zhu, X. Tomato chlorosis virus P22 interacts with NbBAG5 to inhibit autophagy and regulate virus infection. Mol. Plant Pathol. 2023, 24, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Ahmad, S.; Chen, Y.; Liang, Q.; Zhang, L.; Chen, B.; Wen, R. P3/P3N-PIPO of PVY interacting with BI-1 inhibits the degradation of NIb by ATG6 to facilitate virus replication in N. benthamiana. Front. Plant Sci. 2023, 14, 1183144. [Google Scholar] [CrossRef]
- Baulcombe, D.C. The Role of Viruses in Identifying and Analyzing RNA Silencing. Annu. Rev. Virol. 2022, 9, 353–373. [Google Scholar] [CrossRef]
- Vaucheret, H. Plant ARGONAUTES. Trends Plant Sci. 2008, 13, 350–358. [Google Scholar] [CrossRef]
- Derrien, B.; Baumberger, N.; Schepetilnikov, M.; Viotti, C.; De Cillia, J.; Ziegler-Graff, V.; Isono, E.; Schumacher, K.; Genschik, P. Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 15942–15946. [Google Scholar] [CrossRef]
- Bortolamiol, D.; Pazhouhandeh, M.; Marrocco, K.; Genschik, P.; Ziegler-Graff, V. The Polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr. Biol. 2007, 17, 1615–1621. [Google Scholar] [CrossRef]
- Pazhouhandeh, M.; Dieterle, M.; Marrocco, K.; Lechner, E.; Berry, B.; Brault, V.; Hemmer, O.; Kretsch, T.; Richards, K.E.; Genschik, P.; et al. F-box-like domain in the polerovirus protein P0 is required for silencing suppressor function. Proc. Natl. Acad. Sci. USA 2006, 103, 1994–1999. [Google Scholar] [CrossRef]
- Baumberger, N.; Tsai, C.H.; Lie, M.; Havecker, E.; Baulcombe, D.C. The Polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr. Biol. 2007, 17, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Liu, S.; Zou, J.; Zhao, J.; Zhu, F.; Chai, L.; Wang, Y.; Han, C.; Wang, X. A small peptide inhibits siRNA amplification in plants by mediating autophagic degradation of SGS3/RDR6 bodies. EMBO J. 2021, 40, e108050. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, N.; Li, Z.; Xu, X.; Wang, Y.; Yang, X.; Liu, S.S.; Wang, A.; Zhou, X. A calmodulin-like protein suppresses RNA silencing and promotes geminivirus infection by degrading SGS3 via the autophagy pathway in Nicotiana benthamiana. PLoS Pathog. 2017, 13, e1006213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, A. The Potyvirus Silencing Suppressor Protein VPg Mediates Degradation of SGS3 via Ubiquitination and Autophagy Pathways. J. Virol. 2017, 91, e01478-16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, D.; Yang, G.; Yu, X.; Wu, J. Rice Stripe Mosaic Virus-Encoded P4 Is a Weak Suppressor of Viral RNA Silencing and Is Required for Disease Symptom Development. Mol. Plant-Microbe Interact. MPMI 2020, 33, 412–422. [Google Scholar] [CrossRef]
- Fu, S.; Xu, Y.; Li, C.; Li, Y.; Wu, J.; Zhou, X. Rice Stripe Virus Interferes with S-acylation of Remorin and Induces Its Autophagic Degradation to Facilitate Virus Infection. Mol. Plant 2018, 11, 269–287. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Tang, Z.; Zhang, L.; Dai, Z.; Lyu, S.; Li, Y.; Hou, X.; Bernards, M.; Wang, A. A plant RNA virus activates selective autophagy in a UPR-dependent manner to promote virus infection. New Phytol. 2020, 228, 522–639. [Google Scholar] [CrossRef]
- Wu, H.; Qu, X.; Dong, Z.; Luo, L.; Shao, C.; Forner, J.; Lohmann, J.; Su, M.; Xu, M.; Liu, X.; et al. WUSCHEL triggers innate antiviral immunity in plant stem cells. Science 2020, 370, 227–231. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Jing, X.; Wang, C.; Wang, P.; Huang, Z.; Sun, B.; Li, P.; Li, H.; Zhang, C. The Great Game between Plants and Viruses: A Focus on Protein Homeostasis. Int. J. Mol. Sci. 2023, 24, 12582. https://doi.org/10.3390/ijms241612582
Sun H, Jing X, Wang C, Wang P, Huang Z, Sun B, Li P, Li H, Zhang C. The Great Game between Plants and Viruses: A Focus on Protein Homeostasis. International Journal of Molecular Sciences. 2023; 24(16):12582. https://doi.org/10.3390/ijms241612582
Chicago/Turabian StyleSun, Hangjun, Xinxin Jing, Chaonan Wang, Pengyue Wang, Ziting Huang, Bingjian Sun, Pengbai Li, Honglian Li, and Chao Zhang. 2023. "The Great Game between Plants and Viruses: A Focus on Protein Homeostasis" International Journal of Molecular Sciences 24, no. 16: 12582. https://doi.org/10.3390/ijms241612582
APA StyleSun, H., Jing, X., Wang, C., Wang, P., Huang, Z., Sun, B., Li, P., Li, H., & Zhang, C. (2023). The Great Game between Plants and Viruses: A Focus on Protein Homeostasis. International Journal of Molecular Sciences, 24(16), 12582. https://doi.org/10.3390/ijms241612582




