Tools for Etiologic Diagnosis of Drug-Induced Allergic Conditions
Abstract
1. Introduction
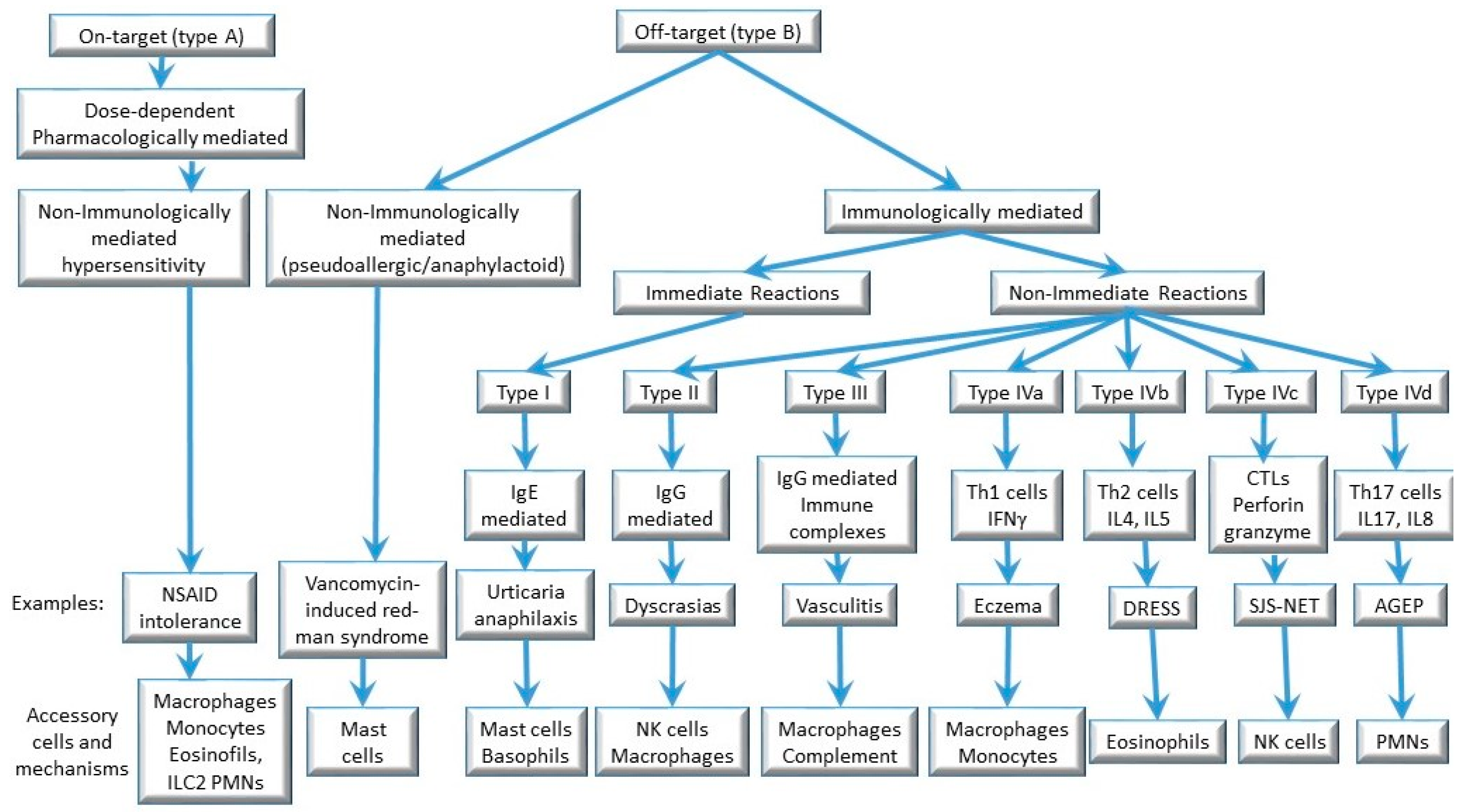
2. Drug Hypersensitivity Reactions: Clinical Classification and Phenotypes
2.1. Immediate Drug Hypersensitivity Reactions
2.2. Non-Immediate/Delayed Allergic Drug Hypersensitivity Reactions
3. Pathogenesis and Pathophysiology
3.1. Immediate IgE-Mediated Allergic Reactions (Type I Reactions)
3.2. Non-Immediate T Cell-Mediated Allergic Reactions (Type IV Reactions)
4. Etiologic Diagnosis: Identification of the Culprit Drug
4.1. Clinical History
4.2. Pharmacovigilance Algorithms
4.3. Value and Limitations of In Vivo Tests
4.3.1. Skin Tests: Prick and Intradermal Tests
- Technique and interpretation of the results
- Non-irritant drug concentrations
- Time to perform skin tests
- Value of skin tests according to the drug and clinical entity
- Safety of skin testing in immediate and non-immediate reactions
4.3.2. Epicutaneous Tests
- Technique and interpretation
| Time | 3–6 weeks to 3–6 months after the resolution of the reaction [116,117] |
| Vehicles [57,118,120,121] | Liquid drugs: placed directly in the chamber Petrolatum (10–30% concentration): Lesser concentrations to prevent false positive result: celecoxib, some formulations of colchicine, valproate, misoprostol, diltiazem, or chloroquine pills Ethyl alcohol: corticosteroids, cotrimoxazole Dimethylsulfoxide: benznidazole |
| Reading | Application: day 1 (D1) 1st reading: day 2 (D2)—(30 min after removing the test material) Last reading: day 5 to 7 (corticosteroids and aminoglycosides) [118] |
| Localization | Upper back (preferred) Outer surface of the forearm Postlesional skin (FDE) |
| Drugs to be suspended before the test | Topical corticosteroids: 7 days before Systemic corticosteroids, ultraviolet (UV) exposure and immunosuppressive therapies: one month before Antihistamines: do not interfere |
| Results | Erythema and papules (+) Erythema, papules, and vesicles (++) Erythema, papules, and numerous confluent vesicles/blisters (+++) [57] |
| Photopatch | Patch test in duplicate One area exposed to 5 joules/cm2 of UVA irradiation for 20–30 min Readings: at 48 h (before irradiation) and 72–96 h Result: comparison between both areas |
| ROAT | FDE or high clinical suspicion with negative results in the regular patch test Applied in post-lesioned skin sometimes following local tape stripping Same or higher allergen concentration than previous negative test Application every 24–48 h until a positive result is obtained |
- Value of epicutaneous tests according to the drug and clinical entity
- Safety of epicutaneous tests
4.3.3. Drug Challenge Tests
4.4. Value and Limitations of In Vitro Tests
4.4.1. In Vitro Assay for Detection of Specific IgE
- Procedure
- Value and utility
- Limitations
4.4.2. Serum Tryptase Measurement
- Principle of the procedure
- Value and utility
- Limitations
4.4.3. In Vitro Histamine Release
- Procedure
- Value and utility
- Limitations
4.4.4. Basophil Activation Test
- Procedure
- Value and utility
- Limitations
4.4.5. Lymphocyte Transformation Test
- Definition
- Procedure
- Value and Utility
- Limitations
4.4.6. Enzyme-Linked Immunosorbent Spot Assay
- Definition
- Procedure
- Value, Utility
- Limitations
4.4.7. Cyto-Lymphocyte Transformation Test
- Definition
- Procedure
- Value and utility
- Limitations
4.4.8. Human Leukocyte Antigen Determinations
- Definition
- Procedure
- Value and utility
- Limitations
5. Algorithms for Diagnosis of Immediate and Non-Immediate DHRS
6. Conclusions and Unmet Needs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johansson, S.G.; Bieber, T.; Dahl, R.; Friedmann, P.S.; Lanier, B.Q.; Lockey, R.F.; Motala, C.; Ortega Martell, J.A.; Platts-Mills, T.A.; Ring, J.; et al. Revised Nomenclature for Allergy for Global Use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004, 113, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.S.; Pichler, W.; et al. International Consensus on Drug Allergy. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.A. Clinical Features and Severity Grading of Anaphylaxis. J. Allergy Clin. Immunol. 2004, 114, 371–376. [Google Scholar] [CrossRef]
- Jönsson, F.; De Chaisemartin, L.; Granger, V.; Gouel-Chéron, A.; Gillis, C.M.; Zhu, Q.; Dib, F.; Nicaise-Roland, P.; Ganneau, C.; Hurtado-Nedelec, M.; et al. An IgG-Induced Neutrophil Activation Pathway Contributes to Human Drug-Induced Anaphylaxis. Sci. Transl. Med. 2019, 11, eaat1479. [Google Scholar] [CrossRef] [PubMed]
- Bavbek, S.; Pagani, M.; Alvarez-Cuesta, E.; Castells, M.; Dursun, A.B.; Hamadi, S.; Madrigal-Burgaleta, R.; Sanchez-Sanchez, S.; Vultaggio, A. Hypersensitivity Reactions to Biologicals: An EAACI Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 39–54. [Google Scholar] [CrossRef]
- Mockenhaupt, M. Epidemiology and Causes of Severe Cutaneous Adverse Reactions to Drugs. In Drug Hypersensitivity; Karger Publishers: Basel, Karger, 2007. [Google Scholar]
- Paulmann, M.; Mockenhaupt, M. Severe Drug-Induced Skin Reactions: Clinical Features, Diagnosis, Etiology, and Therapy. J. Dtsch. Dermatol. Ges. 2015, 13, 625–645. [Google Scholar] [CrossRef]
- Sekula, P.; Dunant, A.; Mockenhaupt, M.; Naldi, L.; Bouwes Bavinck, J.N.; Halevy, S.; Kardaun, S.; Sidoroff, A.; Liss, Y.; Schumacher, M.; et al. Comprehensive Survival Analysis of a Cohort of Patients with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2013, 133, 1197–1204. [Google Scholar] [CrossRef]
- Guvenir, H.; Arikoglu, T.; Vezir, E.; Misirlioglu, E.D. Clinical Phenotypes of Severe Cutaneous Drug Hypersensitivity Reactions. Curr. Pharm. Des. 2019, 25, 3840–3854. [Google Scholar] [CrossRef]
- Mockenhaupt, M. Bullous Drug Reactions. Acta Derm. Venereol. 2020, 100, 122–134. [Google Scholar] [CrossRef]
- Lipowicz, S.; Sekula, P.; Ingen-Housz-Oro, S.; Liss, Y.; Sassolas, B.; Dunant, A.; Roujeau, J.-C.; Mockenhaupt, M. Prognosis of Generalized Bullous Fixed Drug Eruption: Comparison with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Br. J. Dermatol. 2013, 168, 726–732. [Google Scholar] [CrossRef]
- Aster, R.H. Blood Dyscrasias Caused by Hypersensitivity to Drugs. In Drug Hypersensitivity; Karger Publishers: Berlin, Germany, 2007; pp. 306–320. [Google Scholar] [CrossRef]
- Rawlins, M.D.; Thompson, J.W. Pathogenesis of Adverse Drug Reactions; Oxford University Press: New York, NY, USA, 1977; ISBN 0-19-263206-X. [Google Scholar]
- Phillips, E.J. Classifying ADRs--Does Dose Matter? Br. J. Clin. Pharmacol. 2016, 81, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Coombs, R.R.A.; Gell, P. Classification of Allergic Reactions Responsible for Clinical Hypersensitivity and Disease. In Clinical Aspects of Immunology; Coombs, R.R.A., Gells, P.G.H., Eds.; Oxford University Press: New York, NY, USA, 1976; p. 575. [Google Scholar]
- Pichler, W.J. Immune Pathomechanism and Classification of Drug Hypersensitivity. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1457–1471. [Google Scholar] [CrossRef]
- Pichler, W.J. Drug Allergy: Pathogenesis-UpToDate. Available online: https://www.uptodate.com/contents/drug-allergy-pathogenesis?search=drug allergy &source=search_result&selectedTitle=4~150&usage_type=default&display_rank=4 (accessed on 26 May 2023).
- Montañez, M.I.; Mayorga, C.; Bogas, G.; Barrionuevo, E.; Fernandez-Santamaria, R.; Martin-Serrano, A.; Laguna, J.J.; Torres, M.J.; Fernandez, T.D.; Doña, I. Epidemiology, Mechanisms, and Diagnosis of Drug-Induced Anaphylaxis. Front. Immunol. 2017, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Kuchen, S.; Robbins, R.; Sims, G.P.; Sheng, C.; Phillips, T.M.; Lipsky, P.E.; Ettinger, R. Essential Role of IL-21 in B Cell Activation, Expansion, and Plasma Cell Generation during CD4+ T Cell-B Cell Collaboration. J. Immunol. 2007, 179, 5886–5896. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iijima, K.; Dent, A.L.; Kita, H. Follicular Helper T Cells Mediate IgE Antibody Response to Airborne Allergens. J. Allergy Clin. Immunol. 2017, 139, 300–313.e7. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Peavy, R.D.; Gilfillan, A.M. Mechanisms of Mast Cell Signaling in Anaphylaxis. J. Allergy Clin. Immunol. 2009, 124, 639. [Google Scholar] [CrossRef]
- Torres, M.J.; Blanca, M. The Complex Clinical Picture of Beta-Lactam Hypersensitivity: Penicillins, Cephalosporins, Monobactams, Carbapenems, and Clavams. Med. Clin. N. Am. 2010, 94, 805–820. [Google Scholar] [CrossRef]
- Janeway, C.A.; Travers, P.; Walport, M.S.M. Allergy and Hypersensitivity. In Immunology: The Immune System in Health and Disease; Garland Publisher: New York, NY, USA, 2001. [Google Scholar]
- Pichler, W.J. Delayed Drug Hypersensitivity Reactions. Ann. Intern. Med. 2003, 139, 683–693. [Google Scholar] [CrossRef]
- Pichler, W.J. Drug Hypersensitivity Reactions: Classification and Relationship to T-Cell Activation. In Drug Hypersensitivity; Pichler, W.J., Ed.; Karger: Basel, Switzerland, 2007; pp. 168–189. [Google Scholar]
- Phillips, E.J.; Bigliardi, P.; Bircher, A.J.; Broyles, A.; Chang, Y.-S.; Chung, W.-H.; Lehloenya, R.; Mockenhaupt, M.; Peter, J.; Pirmohamed, M.; et al. Controversies in Drug Allergy: Testing for Delayed Reactions. J. Allergy Clin. Immunol. 2019, 143, 66–73. [Google Scholar] [CrossRef]
- Nassif, A.; Moslehi, H.; Le Gouvello, S.; Bagot, M.; Lyonnet, L.; Michel, L.; Boumsell, L.; Bensussan, A.; Roujeau, J.-C. Evaluation of the Potential Role of Cytokines in Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2004, 123, 850–855. [Google Scholar] [CrossRef]
- Neukomm, C.B.; Yawalkar, N.; Helbling, A.P.W. T-Cell Reactions to Drugs in Distinct Clinical Manifestations of Drug Allergy. Le. J. Investig. Allergol. Clin. Immunol. 2001, 11, 275–284. [Google Scholar]
- Venturi, V.; Price, D.A.; Douek, D.C.; Davenport, M.P. The Molecular Basis for Public T-Cell Responses? Nat. Rev. Immunol. 2008, 8, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chen, C.B.; Lin, M.Y.; Zhang, Z.Y.; Jia, X.Y.; Huang, M.; Zou, Y.F.; Chung, W.H. Genetics of Severe Cutaneous Adverse Reactions. Front. Med. 2021, 8, 652091. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Adam, J.; Watkins, S.; Wuillemin, N.; Yun, J.; Yerly, D. Drug Hypersensitivity: How Drugs Stimulate T Cells via Pharmacological Interaction with Immune Receptors. Int. Arch. Allergy Immunol. 2015, 168, 13–24. [Google Scholar] [CrossRef]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune Self-Reactivity Triggered by Drug-Modified HLA-Peptide Repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef]
- Norcross, M.A.; Luo, S.; Lu, L.; Boyne, M.T.; Gomarteli, M.; Rennels, A.D.; Woodcock, J.; Margulies, D.H.; McMurtrey, C.; Vernon, S.; et al. Abacavir Induces Loading of Novel Self-Peptides into HLA-B*57: 01: An Autoimmune Model for HLA-Associated Drug Hypersensitivity. AIDS 2012, 26, F21–F29. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncic, J.; de Oliveira, C.A.F.; et al. Drug Hypersensitivity Caused by Alteration of the MHC-Presented Self-Peptide Repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef]
- Illing, P.T.; Mifsud, N.A.; Purcell, A.W. Allotype Specific Interactions of Drugs and HLA Molecules in Hypersensitivity Reactions. Curr. Opin. Immunol. 2016, 42, 31–40. [Google Scholar] [CrossRef]
- Lucas, A.; Lucas, M.; Strhyn, A.; Keane, N.M.; McKinnon, E.; Pavlos, R.; Moran, E.M.; Meyer-Pannwitt, V.; Gaudieri, S.; D’Orsogna, L.; et al. Abacavir-Reactive Memory T Cells Are Present in Drug Naïve Individuals. PLoS ONE 2015, 10, e0117160. [Google Scholar] [CrossRef]
- Agrawal, B. Heterologous Immunity: Role in Natural and Vaccine-Induced Resistance to Infections. Front. Immunol. 2019, 10, 2631. [Google Scholar] [CrossRef]
- Gibson, A.; Deshpande, P.; Campbell, C.N.; Krantz, M.S.; Mukherjee, E.; Mockenhaupt, M.; Pirmohamed, M.; Palubinsky, A.M.; Phillips, E.J. Updates on the Immunopathology and Genomics of Severe Cutaneous Adverse Drug Reactions. J. Allergy Clin. Immunol. 2023, 151, 289–300.e4. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Kropf, R.; Bircher, A.; Pichler, W.J. Drug Hypersensitivity: Questionnaire. EAACI Interest Group on Drug Hypersensitivity. Allergy 1999, 54, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, B. Approach to the Patient with Drug Allergy. Med. Clin. N. Am. 2010, 94, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, R.; Ramírez, E.; Sendagorta, E.; Alamar, R.; Barranco, R.; Blanca-López, N.; Doña, I.; Fernández, J.; Garcia-Nunez, I.; García-Samaniego, J.; et al. Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of Dress Syndrome. J. Investig. Allergol. Clin. Immunol. 2020, 30, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, R.; Ramírez, E.; Bellón, T. Identifying the Culprit Drug in Severe Cutaneous Adverse Reactions (SCARs). Curr. Treat. Options Allergy 2021, 8, 194–209. [Google Scholar] [CrossRef]
- Cardona, V.; Luengo, O.; Garriga, T.; Labrador-Horrillo, M.; Sala-Cunill, A.; Izquierdo, A.; Soto, L.; Guilarte, M. Co-Factor-Enhanced Food Allergy. Allergy 2012, 67, 1316–1318. [Google Scholar] [CrossRef]
- Saff, R.R. Skin Testing as a Biomarker in Drug Allergy. Ann. Allergy Asthma Immunol. 2023, 130, 161–168. [Google Scholar] [CrossRef]
- Arimone, Y.; Bégaud, B.; Miremont-Salamé, G.; Fourrier-Réglat, A.; Molimard, M.; Moore, N.; Haramburu, F. A New Method for Assessing Drug Causation Provided Agreement with Experts’ Judgment. J. Clin. Epidemiol. 2006, 59, 308–314. [Google Scholar] [CrossRef]
- Pradhan, P.; Lavallée, M.; Akinola, S.; Escobar Gimenes, F.R.; Bérard, A.; Méthot, J.; Piché, M.-E.; Gonella, J.M.; Cloutier, L.; Leclerc, J. Causality Assessment of Adverse Drug Reaction: A Narrative Review to Find the Most Exhaustive and Easy-to-Use Tool in Post-Authorization Settings. J. Appl. Biomed. 2023, 21, 59–66. [Google Scholar] [CrossRef]
- Macedo, A.F.; Marques, F.B.; Ribeiro, C.F. Can Decisional Algorithms Replace Global Introspection in the Individual Causality Assessment of Spontaneously Reported ADRs? Drug Saf. 2006, 29, 697–702. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janacek, E.; Domecq, C.; Greenblatt, D.J. A Method for Estimating the Probability of Adverse Drug Reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Moore, N.; Paux, G.; Begaud, B.; Biour, M.; Loupi, E.; Boismare, F.; Royer, R.J. Adverse Drug Reaction Monitoring: Doing It the French Way. Lancet 1985, 2, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.; García, M. Causality Assessment in Reports on Adverse Drug Reactions. Algorithm of Spanish Pharmacovigilance System. Med. Clin. 2016, 147, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Karch, F.E.; Lasagna, L. Toward the Operational Identification of Adverse Drug Reactions. Clin. Pharmacol. Ther. 1977, 21, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, R.; Calderón, O.; Ramírez, E.; Fiandor, A.; Caballero, T.; Heredia, R.; Herranz, P.; Madero, R.; Quirce, S.; Bellón, T. Sensitivity and Specificity of the Lymphocyte Transformation Test in Drug Reaction with Eosinophilia and Systemic Symptoms Causality Assessment. Clin. Exp. Allergy 2018, 48, 325–333. [Google Scholar] [CrossRef]
- Sassolas, B.; Haddad, C.; Mockenhaupt, M.; Dunant, A.; Liss, Y.; Bork, K.; Haustein, U.F.; Vieluf, D.; Roujeau, J.C.; Le Louet, H. ALDEN, an Algorithm for Assessment of Drug Causality in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Comparison with Case-Control Analysis. Clin. Pharmacol. Ther. 2010, 88, 60–68. [Google Scholar] [CrossRef]
- Schwartz, R.A.; McDonough, P.H.; Lee, B.W. Toxic Epidermal Necrolysis: Part, I. Introduction, History, Classification, Clinical Features, Systemic Manifestations, Etiology, and Immunopathogenesis. J. Am. Acad. Dermatol. 2013, 69, 173.e1–173.e13. [Google Scholar] [CrossRef]
- Bellón, T.; Rodríguez-Martín, S.; Cabañas, R.; Ramírez, E.; Lerma, V.; González-Herrada, C.; González, O.; Sendagorta, E.; Fiandor, A.; de Abajo, F.J. Assessment of Drug Causality in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: Concordance between Lymphocyte Transformation Test and ALDEN. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Creamer, D.; Walsh, S.A.; Dziewulski, P.; Exton, L.S.; Lee, H.Y.; Dart, J.K.G.; Setterfield, J.; Bunker, C.B.; Ardern-Jones, M.R.; Watson, K.M.T.; et al. U.K. Guidelines for the Management of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in Adults 2016. Br. J. Dermatol. 2016, 174, 1194–1227. [Google Scholar] [CrossRef]
- Barbaud, A.; Castagna, J.; Soria, A. Skin Tests in the Work-up of Cutaneous Adverse Drug Reactions: A Review and Update. Contact Dermat. 2022, 86, 344–356. [Google Scholar] [CrossRef]
- Barbaud, A.; Romano, A. Skin Testing Approaches for Immediate and Delayed Hypersensitivity Reactions. Immunol. Allergy Clin. N. Am. 2022, 42, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Garvey, L.H.; Aberer, W.; Atanaskovic-Markovic, M.; Barbaud, A.; Bilo, M.B.; Bircher, A.; Blanca, M.; Bonadonna, B.; Campi, P.; et al. Skin Test Concentrations for Systemically Administered Drugs-An ENDA/EAACI Drug Allergy Interest Group Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Barbaud, A.; Weinborn, M.; Garvey, L.H.; Testi, S.; Kvedariene, V.; Bavbek, S.; Mosbech, H.; Gomes, E.; Aberer, W.; Elberink, H.N.G.O.; et al. Intradermal Tests with Drugs: An Approach to Standardization. Front. Med. 2020, 7, 156. [Google Scholar] [CrossRef]
- Romano, A.; Atanaskovic-Markovic, M.; Barbaud, A.; Bircher, A.J.; Brockow, K.; Caubet, J.C.; Celik, G.; Cernadas, J.; Chiriac, A.M.; Demoly, P.; et al. Towards a More Precise Diagnosis of Hypersensitivity to Beta-Lactams—An EAACI Position Paper. Allergy 2020, 75, 1300–1315. [Google Scholar] [CrossRef]
- Pagani, M.; Bavbek, S.; Alvarez-Cuesta, E.; Berna Dursun, A.; Bonadonna, P.; Castells, M.; Cernadas, J.; Chiriac, A.; Sahar, H.; Madrigal-Burgaleta, R.; et al. Hypersensitivity Reactions to Chemotherapy: An EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, R.; Breynaert, C.; Ahmedali, Y.; Bourrain, J.L.; Demoly, P.; Chiriac, A.M. Skin Testing for Suspected Iodinated Contrast Media Hypersensitivity. J. Allergy Clin. Immunol. Pract. 2018, 6, 1246–1254. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Koh, Y., II; Kim, J.H.; Ban, G.Y.; Lee, Y.K.; Hong, G.N.; Jin, U.R.; Choi, B.J.; Shin, Y.S.; Park, H.S.; et al. The Potential Utility of Iodinated Contrast Media (ICM) Skin Testing in Patients with ICM Hypersensitivity. J. Korean Med. Sci. 2015, 30, 245–251. [Google Scholar] [CrossRef]
- Brockow, K.; Romano, A.; Blanca, M.; Ring, J.; Pichler, W.; Demoly, P. General Considerations for Skin Test Procedures in the Diagnosis of Drug Hypersensitivity. Allergy Eur. J. Allergy Clin. Immunol. 2002, 57, 45–51. [Google Scholar] [CrossRef]
- Barbaud, A.; Gonçalo, M.; Bruynzeel, D.; Bircher, A. Guidelines for Performing Skin Tests with Drugs in the Investigation of Cutaneous Adverse Drug Reactions. Contact Dermat. 2001, 45, 321–328. [Google Scholar] [CrossRef]
- Barbaud, A.; Collet, E.; Milpied, B.; Assier, H.; Staumont, D.; Avenel-Audran, M.; Grange, A.; Amarger, S.; Girardin, P.; Guinnepain, M.T.; et al. A Multicentre Study to Determine the Value and Safety of Drug Patch Tests for the Three Main Classes of Severe Cutaneous Adverse Drug Reactions. Br. J. Dermatol. 2013, 168, 555–562. [Google Scholar] [CrossRef]
- Garvey, L.H.; Ebo, D.G.; Mertes, P.M.; Dewachter, P.; Garcez, T.; Kopac, P.; Laguna, J.J.; Chiriac, A.M.; Terreehorst, I.; Voltolini, S.; et al. An EAACI Position Paper on the Investigation of Perioperative Immediate Hypersensitivity Reactions. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Trautmann, A.; Böhm, I.; Scherer, K.; Barbaud, A.; Bavbek, S.; Bonadonna, P.; Cernadas, J.R.; Chiriac, A.M.; Gaeta, F.; et al. Practice Parameters for Diagnosing and Managing Iodinated Contrast Media Hypersensitivity. Allergy 2021, 76, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Romano, A.; Aberer, W.; Bircher, A.J.; Barbaud, A.; Bonadonna, P.; Faria, E.; Kanny, G.; Lerch, M.; Pichler, W.J.; et al. Skin Testing in Patients with Hypersensitivity Reactions to Iodinated Contrast Media—A European Multicenter Study. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Bavbek, S.; Dursun, A.B.; Bonadonna, P.; Caralli, M.; Cernadas, J.; Cortellini, G.; Costantino, M.T.; Gelincik, A.; Lucchini, G.; et al. Role of Skin Tests in the Diagnosis of Immediate Hypersensitivity Reactions to Taxanes: Results of a Multicenter Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Blanca, M.; Torres, M.J.; Garcia, J.J.; Romano, A.; Mayorga, C.; De Ramon, E.; Vega, J.M.; Miranda, A.; Juarez, C. Natural Evolution of Skin Test Sensitivity in Patients Allergic to β-Lactam Antibiotics. J. Allergy Clin. Immunol. 1999, 103, 918–924. [Google Scholar] [CrossRef]
- Castells, M.; Khan, D.A.; Phillips, E.J. Penicillin Allergy. N. Engl. J. Med. 2019, 381, 2338–2351. [Google Scholar] [CrossRef]
- Pinho, A.; Coutinho, I.; Gameiro, A.; Gouveia, M.; Gonçalo, M. Patch Testing-a Valuable Tool for Investigating Non-Immediate Cutaneous Adverse Drug Reactions to Antibiotics. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 280–287. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Douglas, A.P.; Goh, M.; Slavin, M.A.; Phillips, E.J. The Safety of Antibiotic Skin Testing in Severe T-Cell–mediated Hypersensitivity of Immunocompetent and Immunocompromised Hosts. J. Allergy Clin. Immunol. Pract. 2019, 7, 1341–1343.e1. [Google Scholar] [CrossRef]
- Bonadonna, P.; Lombardo, C.; Bortolami, O.; Bircher, A.; Scherer, K.; Barbaud, A.; Passalacqua, G.; Pagani, M. Hypersensitivity to Proton Pump Inhibitors: Diagnostic Accuracy of Skin Tests Compared to Oral Provocation Test. J. Allergy Clin. Immunol. 2012, 130, 547–549. [Google Scholar] [CrossRef]
- Scherer, K.; Tsakiris, D.; Bircher, A. Hypersensitivity Reactions to Anticoagulant Drugs. Curr. Pharm. Des. 2008, 14, 2863–2873. [Google Scholar] [CrossRef]
- Barbaud, A.; Garvey, L.H.; Arcolaci, A.; Brockow, K.; Mori, F.; Mayorga, C.; Bonadonna, P.; Atanaskovic-Markovic, M.; Moral, L.; Zanoni, G.; et al. Allergies and COVID-19 Vaccines: An ENDA/EAACI Position Paper. Allergy 2022, 77, 2292–2312. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bahna, S.L. Immediate Hypersensitivity Reactions to Corticosteroids. Ann. Allergy Asthma Immunol. 2015, 115, 178–182.e3. [Google Scholar] [CrossRef] [PubMed]
- Barbaud, A.; Waton, J. Systemic Allergy to Corticosteroids: Clinical Features and Cross Reactivity. Curr. Pharm. Des. 2016, 22, 6825–6831. [Google Scholar] [CrossRef]
- Laguna, J.J.; Archilla, J.; Doña, I.; Corominas, M.; Gastaminza, G.; Mayorga, C.; Berjes-Gimeno, P.; Tornero, P.; Martin, S.; Planas, A.; et al. Practical Guidelines for Perioperative Hypersensitivity Reactions. J. Investig. Allergol. Clin. Immunol. 2018, 28, 216–232. [Google Scholar] [CrossRef]
- Seitz, C.S.; Bröcker, E.B.; Trautmann, A. Allergy Diagnostic Testing in Clindamycin-Induced Skin Reactions. Int. Arch. Allergy Immunol. 2009, 149, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Barni, S.; Pucci, N.; Rossi, E.; Azzari, C.; de Martino, M.; Novembre, E. Sensitivity and Specificity of Skin Tests in the Diagnosis of Clarithromycin Allergy. Ann. Allergy. Asthma Immunol. 2010, 104, 417–419. [Google Scholar] [CrossRef]
- Arruti Oyarzabal, N.; Villarreal, O.; Bernedo, N.; Audicana, M.T.; Velasco, M.; Uriel, O.; Martinez, A.; Bellón, T. Positive Allergy Study (Intradermal, Patch, and Lymphocyte Transformation Tests) in a Case of Isoniazid-Induced Dress. J. Investig. Allergol. Clin. Immunol. 2016, 26, 119–120. [Google Scholar] [CrossRef]
- Empedrad, R.; Darter, A.L.; Earl, H.S.; Gruchalla, R.S. Nonirritating Intradermal Skin Test Concentrations for Commonly Prescribed Antibiotics. J. Allergy Clin. Immunol. 2003, 112, 629–630. [Google Scholar] [CrossRef]
- McGee, E.U.; Samuel, E.; Boronea, B.; Dillard, N.; Milby, M.N.; Lewis, S.J. Quinolone Allergy. Pharmacy 2019, 7, 97. [Google Scholar] [CrossRef]
- Doña, I.; Moreno, E.; Pérez-Sánchez, N.; Andreu, I.; Hernández Fernandez de Rojas, D.; Torres, M.J. Update on Quinolone Allergy. Curr. Allergy Asthma Rep. 2017, 17, 56. [Google Scholar] [CrossRef]
- Barbaud, A. Place of Excipients in Systemic Drug Allergy. Immunol. Allergy Clin. N. Am. 2014, 34, 671–679. [Google Scholar] [CrossRef]
- Caballero, M.L.; Quirce, S. Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review. J. Investig. Allergol. Clin. Immunol. 2020, 30, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Nin-Valencia, A.; Fiandor, A.; Lluch, M.; Quirce, S.; Caballero, Y.; Heredia-Revuelto, R.; Gónzález-Muñoz, M.; Caballero, M.L.; Cabañas, R. Safe Administration of SARS-CoV-2 Vaccine After Desensitization to a Biologic Containing Polysorbate 80 in a Patient with Polyethylene Glycol-Induced Severe Anaphylaxis and Sensitization to Polysorbate 80. J. Investig. Allergol. Clin. Immunol. 2023, 33, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Ewan, P. Grand Rounds Review Polyethylene GlycoleInduced Systemic Allergic Reactions (Anaphylaxis). J. Allergy Clin. Immunol. Pract. 2021, 9, 670–675. [Google Scholar] [CrossRef]
- Grueber, H.P.; Helbling, A.; Joerg, L. Skin Test Results and Cross-Reactivity Patterns in IgE- and T-Cell-Mediated Allergy to Gadolinium-Based Contrast Agents. Allergy. Asthma Immunol. Res. 2021, 13, 933–938. [Google Scholar] [CrossRef]
- Sola-Gazagnes, A.; Pecquet, C.; Berré, S.; Achenbach, P.; Pierson, L.A.; Virmoux-Buisson, I.; M’Bemba, J.; Elgrably, F.; Moguelet, P.; Boitard, C.; et al. Insulin Allergy: A Diagnostic and Therapeutic Strategy Based on a Retrospective Cohort and a Case-Control Study. Diabetologia 2022, 65, 1278–1290. [Google Scholar] [CrossRef]
- Opstrup, M.S.; Malling, H.J.; Krøigaard, M.; Mosbech, H.; Skov, P.S.; Poulsen, L.K.; Garvey, L.H. Standardized Testing with Chlorhexidine in Perioperative Allergy--a Large Single-Centre Evaluation. Allergy 2014, 69, 1390–1396. [Google Scholar] [CrossRef]
- Loli-Ausejo, D.; González de Abreu, J.M.; Fiandor, A.; Cabañas, R.; Domínguez-Ortega, F.; Caballero, M.L.; Lluch-Bernal, M.; Rodrigo García-Panto, C.; Núñez, M.C.; Quirce, S. Allergic Reactions After Administration of Pfizer-BioNTech COVID-19 Vaccine to Health Care Workers at a Tertiary Hospital. J. Investig. Allergol. Clin. Immunol. 2021, 31, 507–508. [Google Scholar] [CrossRef]
- Isabwe, G.A.C.; Garcia Neuer, M.; de las Vecillas Sanchez, L.; Lynch, D.M.; Marquis, K.; Castells, M. Hypersensitivity Reactions to Therapeutic Monoclonal Antibodies: Phenotypes and Endotypes. J. Allergy Clin. Immunol. 2018, 142, 159–170.e2. [Google Scholar] [CrossRef]
- Novelli, S.; Soto, L.; Caballero, A.; Moreno, M.E.; Lara, M.J.; Bayo, D.; Quintas, A.; Jimeno, P.; Zamora, M.I.; Bigorra, T.; et al. Assessment of Confirmed Clinical Hypersensitivity to Rituximab in Patients Affected with B-Cell Neoplasia. Adv. Hematol. 2020, 2020, 4231561. [Google Scholar] [CrossRef]
- Görgülü, B.; Seval, G.C.; Kendirlinan, R.; Toprak, S.K.; Özcan, M.; Bavbek, S. Rapid Drug Desensitization With Rituximab in 24 Cases: A Single-Center Experience. J. Investig. Allergol. Clin. Immunol. 2019, 29, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E.; Vultaggio, A.; Matucci, A. Acute Infusion Reactions Induced by Monoclonal Antibody Therapy. Expert Rev. Clin. Immunol. 2011, 7, 55–63. [Google Scholar] [CrossRef]
- Fréling, E.; Peyrin-Biroulet, L.; Poreaux, C.; Morali, A.; Waton, J.; Schmutz, J.L.; Guéant, J.L.; Barbaud, A. IgE Antibodies and Skin Tests in Immediate Hypersensitivity Reactions to Infliximab in Inflammatory Bowel Disease: Impact on Infliximab Retreatment. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1200–1208. [Google Scholar] [CrossRef]
- Madrigal-Burgaleta, R.; Alvarez-Cuesta, E.; Broyles, A.D.; Cuesta-Herranz, J.; Guzman-Melendez, M.A.; Maciag, M.C.; Phillips, E.J.; Trubiano, J.A.; Wong, J.T.; Ansotegui, I.; et al. Standards for Practical Intravenous Rapid Drug Desensitization & Delabeling: A WAO Committee Statement. World Allergy Organ. J. 2022, 15, 100640. [Google Scholar] [CrossRef]
- Rocchi, V.; Puxeddu, I.; Cataldo, G.; Del Corso, I.; Tavoni, A.; Bazzichi, L.; Bombardieri, S.; Migliorini, P. Hypersensitivity Reactions to Tocilizumab: Role of Skin Tests in Diagnosis. Rheumatology 2014, 53, 1527–1529. [Google Scholar] [CrossRef]
- Solensky, R.; Jacobs, J.; Lester, M.; Lieberman, P.; Mccafferty, F.; Nilsson, T.; Park, M.; Schwarz, G.; Soong, W.; Wagelie-Steffen, A.; et al. Penicillin Allergy Evaluation: A Prospective, Multicenter, Open-Label Evaluation of a Comprehensive Penicillin Skin Test Kit. J. Allergy Clin. Immunol. Pract. 2019, 7, 1876–1885.e3. [Google Scholar] [CrossRef]
- Benamara-Levy, M.; Haccard, F.; Jonville Bera, A.P.; Machet, L. Acute Generalized Exanthematous Pustulosis Due to Acetazolamide: Negative on Patch Testing and Confirmed by Delayed-Reading Intradermal Testing. Clin. Exp. Dermatol. 2014, 39, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Amsler, E.; Bernier, C.; Milpied, B.; Tétart, F.; Morice, C.; Dezoteux, F.; Ferrier-Le Bouedec, M.C.; Barbaud, A.; Staumont-Sallé, D.; et al. DRESS and AGEP Reactions to Iodinated Contrast Media: A French Case Series. J. Allergy Clin. Immunol. Pract. 2021, 9, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Di fonso, M.; Pocobelli, D.; Giannarini, L.; Venuti, A.; Garcovich, A. Two Cases of TEN Caused by Delayed Hypersensitivity to Betalactam. J. Invest. Allergol. Clin. Immnunol. 1993, 3, 53–55. [Google Scholar]
- Tagami, H. Delayed Hypersensitivity in Ampicillin-Induced Toxic Epidermal Necrolysis. Arch. Dermatol. 1983, 119, 910–913. [Google Scholar] [CrossRef]
- Roux, C.; Ben Said, B.; Milpied, B.; Bernier, C.; Staumont-Sallé, D.; Dezoteux, F.; Soria, A.; Barbaud, A.; Valeyrie-Allanore, L.; Tétart, F.; et al. Skin Testing and Drug Provocation Tests in Epidermal Necrolysis: A French Experience. J. Allergy Clin. Immunol. Pract. 2022, 10, 3252–3261.e2. [Google Scholar] [CrossRef]
- Cabañas, R.; Calderón, O.; Ramírez, E.; Fiandor, A.; Prior, N.; Caballero, T.; Herránz, P.; Bobolea, I.; López-Serrano, M.C.; Quirce, S.; et al. Piperacillin-Induced DRESS: Distinguishing Features Observed in a Clinical and Allergy Study of 8 Patients. J. Investig. Allergol. Clin. Immunol. 2014, 24, 425–430. [Google Scholar] [PubMed]
- Lehloenya, R.J.; Muloiwa, R.; Dlamini, S.; Gantsho, N.; Todd, G.; Dheda, K. Lack of Cross-Toxicity between Isoniazid and Ethionamide in Severe Cutaneous Adverse Drug Reactions: A Series of 25 Consecutive Confirmed Cases. J. Antimicrob. Chemother. 2015, 70, 2648–2651. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Hamelin, A.; de Risi Pugliese, T.; Amsler, E.; Barbaud, A. Are Drug Intradermal Tests Dangerous to Explore Cross-Reactivity and Co-Sensitization in DRESS? Br. J. Dermatol. 2019, 181, 611–612. [Google Scholar] [CrossRef]
- Liccioli, G.; Mori, F.; Parronchi, P.; Capone, M.; Fili, L.; Barni, S.; Sarti, L.; Giovannini, M.; Resti, M.; Novembre, E.M. Aetiopathogenesis of Severe Cutaneous Adverse Reactions (SCARs) in Children: A 9-year Experience in a Tertiary Care Paediatric Hospital Setting. Clin. Exp. Allergy 2020, 50, 61–73. [Google Scholar] [CrossRef]
- Teo, Y.X.; Friedmann, P.S.; Polak, M.E.; Ardern-Jones, M.R. Utility and Safety of Skin Tests in Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A Systematic Review. J. Allergy Clin. Immunol. Pract. 2023, 11, 481–491.e5. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.C.; Makowska, J.; et al. In Vitro Tests for Drug Hypersensitivity Reactions: An ENDA/EAACI Drug Allergy Interest Group Position Paper. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 1103–1134. [Google Scholar] [CrossRef]
- Romano, A.; Blanca, M.; Torres, M.J.; Bircher, A.; Aberer, W.; Brockow, K.; Pichler, W.J.; Demoly, P. ENDA EAACI Diagnosis of Nonimmediate Reactions to Beta-Lactam Antibiotics. Allergy 2004, 59, 1153–1160. [Google Scholar] [CrossRef]
- de Groot, A.C. Patch Testing in Drug Eruptions: Practical Aspects and Literature Review of Eruptions and Culprit Drugs. Dermat. Contact Atopic Occup. Drug 2022, 33, 16–30. [Google Scholar] [CrossRef]
- Woodruff, C.M.; Botto, N. The Role of Patch Testing in Evaluating Delayed Hypersensitivity Reactions to Medications. Clin. Rev. Allergy Immunol. 2022, 62, 548–561. [Google Scholar] [CrossRef]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavõ, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis Guideline for Diagnostic Patch Testing-Recommendations on Best Practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Hannuksela, M. Sensitivity of Various Skin Sites in the Repeated Open Application Test. Am. J. Contact Dermat. 1991, 2, 102–104. [Google Scholar] [CrossRef]
- Marques-Mejías, M.A.; Cabañas, R.; Ramírez, E.; Domínguez-Ortega, J.; Fiandor, A.; Trigo, E.; Quirce, S.; Bellón, T. Lymphocyte Transformation Test (LTT) in Allergy to Benznidazole: A Promising Approach. Front. Pharmacol. 2019, 10, 469. [Google Scholar] [CrossRef]
- Zinn, Z.; Gayam, S.; Chelliah, M.P.; Honari, G.; Teng, J. Patch Testing for Nonimmediate Cutaneous Adverse Drug Reactions. J. Am. Acad. Dermatol. 2018, 78, 421–423. [Google Scholar] [CrossRef]
- Andrade, P.; Brinca, A.; Gonçalo, M. Patch Testing in Fixed Drug Eruptions--a 20-Year Review. Contact Dermat. 2011, 65, 195–201. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.C. Patch Testing in Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A Literature Review. Contact Dermat. 2022, 86, 443–479. [Google Scholar] [CrossRef]
- Wolkenstein, P.; Chosidow, O.; Fléchet, M.L.; Robbiola, O.; Paul, M.; Dumé, L.; Revuz, J.; Roujeau, J.C. Patch Testing in Severe Cutaneous Adverse Drug Reactions, Including Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Contact Dermat. 1996, 35, 234–236. [Google Scholar] [CrossRef]
- Martí-Garrido, J.; Vázquez-Revuelta, P.; Lleonart-Bellfill, R.; Molina-Mata, K.; Muñoz-Sánchez, C.; Madrigal-Burgaleta, R. Pilot Experience Using Drug Provocation Testing for the Study of Hypersensitivity to Chemotherapy and Biological Agents. J. Investig. Allergol. Clin. Immunol. 2021, 31, 166–168. [Google Scholar] [CrossRef]
- Doña, I.; Labella, M.; Bogas, G.; Sáenz de Santa María, R.; Salas, M.; Ariza, A.; Torres, M.J. Antibiotic Allergy De-Labeling: A Pathway against Antibiotic Resistance. Antibiotics 2022, 11, 1055. [Google Scholar] [CrossRef]
- Prieto, A.; Muñoz, C.; Bogas, G.; Fernández-Santamaría, R.; Palomares, F.; Mayorga, C.; Salas, M.; Doña, I.; Torres, M.J. Single-Dose Prolonged Drug Provocation Test, without Previous Skin Testing, Is Safe for Diagnosing Children with Mild Non-Immediate Reactions to Beta-Lactams. Allergy 2021, 76, 2544–2554. [Google Scholar] [CrossRef]
- Moral, L.; Marco, N.; Toral, T.; Garde, J.; Fuentes, M.J.; García-Avilés, B.; Montahud, C.; Perona, J.; Fornies, M.J. No Need for Skin and in Vitro Tests in Most Children with Suspected Allergy to Beta-Lactam Antibiotics. Pediatr. Allergy Immunol. 2014, 25, 716–717. [Google Scholar] [CrossRef]
- Atakul, G. Oral Challenge without Penicillin Skin Tests in Children with Suspected Beta-Lactam Hypersensitivity. J. Child Sci. 2022, 12, e133–e137. [Google Scholar] [CrossRef]
- Iammatteo, M.; Alvarez Arango, S.; Ferastraoaru, D.; Akbar, N.; Lee, A.Y.; Cohen, H.W.; Jerschow, E. Safety and Outcomes of Oral Graded Challenges to Amoxicillin without Prior Skin Testing. J. Allergy Clin. Immunol. Pract. 2019, 7, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.; Shih, J.; Patel, K.; Scanlon, N. Direct Oral Amoxicillin Challenge without Preliminary Skin Testing in Adult Patients with Allergy and at Low Risk with Reported Penicillin Allergy. Allergy Asthma Proc. 2019, 40, 57–61. [Google Scholar] [CrossRef]
- Copaescu, A.M.; James, F.; Vogrin, S.; Rose, M.; Chua, K.; Holmes, N.E.; Turner, N.A.; Stone, C.; Phillips, E.; Trubiano, J. Use of a Penicillin Allergy Clinical Decision Rule to Enable Direct Oral Penicillin Provocation: An International Multicentre Randomised Control Trial in an Adult Population (PALACE): Study Protocol. BMJ Open 2022, 12, e063784. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Sanz, M.L.; Gamboa, P.; Garcia-Aviles, M.C.; Fernandez, J.; Torres, M.J. In Vitro Methods for Diagnosing Nonimmediate Hypersensitivity Reactions to Drugs. J. Investig. Allergol. Clin. Immunol. 2013, 23, 213–225. [Google Scholar]
- Brockow, K. Detection of Drug-Specific Immunoglobulin E (IgE) and Acute Mediator Release for the Diagnosis of Immediate Drug Hypersensitivity Reactions. J. Immunol. Methods 2021, 496, 113101. [Google Scholar] [CrossRef]
- Hamilton, R.G. Clinical Laboratory Assessment of Immediate-Type Hypersensitivity. J. Allergy Clin. Immunol. 2010, 125, S284–S296. [Google Scholar] [CrossRef]
- Lochmatter, P.; Zawodniak, A.; Pichler, W.J. In Vitro Tests in Drug Hypersensitivity Diagnosis. Immunol. Allergy Clin. N. Am. 2009, 29, 537–554. [Google Scholar]
- Bergmann, M.M.; Caubet, J.-C. Role of in Vivo and in Vitro Tests in the Diagnosis of Severe Cutaneous Adverse Reactions (SCAR) to Drug. Curr. Pharm. Des. 2019, 25, 3872–3880. [Google Scholar] [CrossRef]
- van der Poorten, M.L.M.; Van Gasse, A.L.; Hagendorens, M.M.; Faber, M.A.; De Puysseleyr, L.; Elst, J.; Mertens, C.M.; Sabato, V.; Ebo, D.G. Serum Specific IgE Antibodies in Immediate Drug Hypersensitivity. Clin. Chim. Acta 2020, 504, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Venemalm, L.; Bridts, C.H.; Degerbeck, F.; Hagberg, H.; De Clerck, L.S.; Stevens, W.J. Immunoglobulin E Antibodies to Rocuronium: A New Diagnostic Tool. Anesthesiology 2007, 107, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.G.O.; Adédoyin, J.; Van Hage, M.; Grönneberg, R.; Nopp, A. False-Positive Penicillin Immunoassay: An Unnoticed Common Problem. J. Allergy Clin. Immunol. 2013, 132, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Hjortlund, J.; Mortz, C.G.; Skov, P.S.; Bindslev-Jensen, C. Diagnosis of Penicillin Allergy Revisited: The Value of Case History, Skin Testing, Specific IgE and Prolonged Challenge. Allergy 2013, 68, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Klingebiel, C.; Vitte, J. Tryptase in Type I Hypersensitivity. Ann. Allergy. Asthma Immunol. 2023, 130, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mateja, A.; Wang, Q.; Chovanec, J.; Kim, J.; Wilson, K.J.; Schwartz, L.B.; Glover, S.C.; Carter, M.C.; Metcalfe, D.D.; Brittain, E.; et al. Defining Baseline Variability of Serum Tryptase Levels Improves Accuracy in Identifying Anaphylaxis. J. Allergy Clin. Immunol. 2022, 149, 1010–1017.e10. [Google Scholar] [CrossRef]
- Kalangara, J.; Vanijcharoenkarn, K.; Lynde, G.C.; McIntosh, N.; Kuruvilla, M. Approach to Perioperative Anaphylaxis in 2020: Updates in Diagnosis and Management. Curr. Allergy Asthma Rep. 2021, 21, 4. [Google Scholar] [CrossRef]
- Lieberman, P. The Basics of Histamine Biology. Ann. Allergy. Asthma Immunol. 2011, 106, S2–S5. [Google Scholar] [CrossRef]
- Ogawa, Y.; Grant, J.A. Mediators of Anaphylaxis. Immunol. Allergy Clin. N. Am. 2007, 27, 249–260. [Google Scholar] [CrossRef]
- Ebo, D.G.; Bridts, C.H.; Mertens, C.H.; Hagendorens, M.M.; Stevens, W.J.; De Clerck, L.S. Analyzing Histamine Release by Flow Cytometry (HistaFlow): A Novel Instrument to Study the Degranulation Patterns of Basophils. J. Immunol. Methods 2012, 375, 30–38. [Google Scholar] [CrossRef]
- Berroa, F.; Lafuente, A.; Javaloyes, G.; Ferrer, M.; Moncada, R.; Goikoetxea, M.J.; Urbain, C.M.; Sanz, M.L.; Gastaminza, G. The Usefulness of Plasma Histamine and Different Tryptase Cut-off Points in the Diagnosis of Peranaesthetic Hypersensitivity Reactions. Clin. Exp. Allergy 2014, 44, 270–277. [Google Scholar] [CrossRef]
- Takazawa, T.; Sabato, V.; Ebo, D.G. In Vitro Diagnostic Tests for Perioperative Hypersensitivity, a Narrative Review: Potential, Limitations, and Perspectives. Br. J. Anaesth. 2019, 123, e117–e125. [Google Scholar] [CrossRef]
- Torres, M.J.; Romano, A.; Blanca-Lopez, N.; Doña, I.; Canto, G.; Ariza, A.; Aranda, A.; Montañez, M.I.; Mayorga, C.; Blanca, M. Immunoglobulin E-Mediated Hypersensitivity to Amoxicillin: In Vivo and in Vitro Comparative Studies between an Injectable Therapeutic Compound and a New Commercial Compound. Clin. Exp. Allergy 2011, 41, 1595–1601. [Google Scholar] [CrossRef]
- Knol, E.F.; Mul, F.P.J.; Kuijpers, T.W.; Verhoeven, A.J.; Roos, D. Intracellular Events in Anti-IgE Nonreleasing Human Basophils. J. Allergy Clin. Immunol. 1992, 90, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Vigón, L.; Galán, M.; Torres, M.; Martín-Galiano, A.J.; Rodríguez-Mora, S.; Mateos, E.; Corona, M.; Malo, R.; Navarro, C.; Murciano-Antón, M.A.; et al. Association between HLA-C Alleles and COVID-19 Severity in a Pilot Study with a Spanish Mediterranean Caucasian Cohort. PLoS ONE 2022, 17, e0272867. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil Activation Test: Mechanisms and Considerations for Use in Clinical Trials and Clinical Practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Campos, L.; Galvão, V.R.; Kalil, J.; Castells, M.; Giavina-Bianchi, P. BAT in the Diagnosis of Drug Allergy: A Novel Tool in Clinical Daily Practice? Curr. Allergy Asthma Rep. 2019, 19, 20. [Google Scholar] [CrossRef]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The Clinical Utility of Basophil Activation Testing in Diagnosis and Monitoring of Allergic Disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.; Galvão, V.R.; Picard, M.; Caiado, J.; Castells, M.C. Basophil Activation Test Is a Relevant Biomarker of the Outcome of Rapid Desensitization in Platinum Compounds-Allergy. J. Allergy Clin. Immunol. Pract. 2017, 5, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Srinoulprasert, Y.; Rerkpattanapipat, T.; Sompornrattanaphan, M.; Wongsa, C.; Kanistanon, D. Clinical Value of in Vitro Tests for the Management of Severe Drug Hypersensitivity Reactions. Asia Pac. Allergy 2020, 10, e44. [Google Scholar] [CrossRef]
- Eberlein, B.; Suárez, I.L.; Darsow, U.; Ruëff, F.; Behrendt, H.; Ring, J. A New Basophil Activation Test Using CD63 and CCR3 in Allergy to Antibiotics. Clin. Exp. Allergy 2010, 40, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Leysen, J.; Bridts, C.H.; De Clerck, L.S.; Ebo, D.G. Rocuronium-Induced Anaphylaxis Is Probably Not Mitigated by Sugammadex: Evidence from an in Vitro Experiment. Anaesthesia 2011, 66, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, T.D.; Ariza, A.; Palomares, F.; Montanez, M.I.; Salas, M.; Martin-Serrano, A.; Fernandez, R.; Ruiz, A.; Blanca, M.; Mayorga, C.; et al. Hypersensitivity to Fluoroquinolones: The Expression of Basophil Activation Markers Depends on the Clinical Entity and the Culprit Fluoroquinolone. Medicine 2016, 95, e3679. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.J.; Kranzelbinder, B.; Sturm, E.M.; Heinemann, A.; Groselj-Strele, A.; Aberer, W. The Basophil Activation Test in the Diagnosis of Allergy: Technical Issues and Critical Factors. Allergy 2009, 64, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Tilch, J. The Lymphocyte Transformation Test in the Diagnosis of Drug Hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
- Tsuge, I.; Okumura, A.; Kondo, Y.; Itomi, S.; Kakami, M.; Kawamura, M.; Nakajima, Y.; Komatsubara, R.; Urisu, A. Allergen-Specific T-Cell Response in Patients with Phenytoin Hypersensitivity; Simultaneous Analysis of Proliferation and Cytokine Production by Carboxyfluorescein Succinimidyl Ester (CFSE) Dilution Assay. Allergol. Int. 2007, 56, 149–155. [Google Scholar] [CrossRef]
- Bellón, T.; Lerma, V.; Guijarro, J.; Ramírez, E.; Martínez, C.; Escudero, C.; Fiandor, A.M.; Barranco, R.; de Barrio, M.; de Abajo, F.; et al. LTT and HLA Testing as Diagnostic Tools in Spanish Vancomycin-Induced DRESS Cases: A Case-Control Study. Front. Pharmacol. 2022, 13, 959321. [Google Scholar] [CrossRef]
- Nyfeler, B.; Pichler, W.J. The Lymphocyte Transformation Test for the Diagnosis of Drug Allergy: Sensitivity and Specificity. Clin. Exp. Allergy 1997, 27, 175–181. [Google Scholar] [CrossRef]
- Luque, I.; Leyva, L.; José Torres, M.; Rosal, M.; Mayorga, C.; Segura, J.M.; Blanca, M.; Juárez, C. In Vitro T-Cell Responses to Beta-Lactam Drugs in Immediate and Nonimmediate Allergic Reactions. Allergy 2001, 56, 611–618. [Google Scholar] [CrossRef]
- Polak, M.E.; Belgi, G.; McGuire, C.; Pickard, C.; Healy, E.; Friedmann, P.S.; Ardern-Jones, M.R. In Vitro Diagnostic Assays Are Effective during the Acute Phase of Delayed-Type Drug Hypersensitivity Reactions. Br. J. Dermatol. 2013, 168, 539–549. [Google Scholar] [CrossRef]
- Mayorga, C.; Ebo, D.G.; Lang, D.M.; Pichler, W.J.; Sabato, V.; Park, M.A.; Makowska, J.; Atanaskovic-Markovic, M.; Bonadonna, P.; Jares, E. Controversies in Drug Allergy: In Vitro Testing. J. Allergy Clin. Immunol. 2019, 143, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hari, Y.; Frutig-Schnyder, K.; Hurni, M.; Yawalkar, N.; Zanni, M.P.; Schnyder, B.; Kappeler, A.; von Greyerz, S.; Braathen, L.R.; Pichler, W.J. T Cell Involvement in Cutaneous Drug Eruptions. Clin. Exp. Allergy 2001, 31, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, B.; Pichler, W.J. Skin and Laboratory Tests in Amoxicillin- and Penicillin-Induced Morbilliform Skin Eruption. Clin. Exp. Allergy 2000, 30, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Srinoulprasert, Y. Lymphocyte Transformation Test and Cytokine Detection Assays: Determination of Read out Parameters for Delayed-Type Drug Hypersensitivity Reactions. J. Immunol. Methods 2021, 496, 113098. [Google Scholar] [CrossRef]
- Beeler, A.; Engler, O.; Gerber, B.O.; Pichler, W.J. Long-Lasting Reactivity and High Frequency of Drug-Specific T Cells after Severe Systemic Drug Hypersensitivity Reactions. J. Allergy Clin. Immunol. 2006, 117, 455–462. [Google Scholar] [CrossRef]
- Beeler, A.; Zaccaria, L.; Kawabata, T.; Gerber, B.O.; Pichler, W.J. CD69 Upregulation on T Cells as an in Vitro Marker for Delayed-Type Drug Hypersensitivity. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 181–188. [Google Scholar] [CrossRef]
- Gex-Collet, C.; Helbling, A.; Pichler, W.J. Multiple Drug Hypersensitivity-Proof of Multiple Drug Hypersensitivity by Patch and Lymphocyte Transformation Tests. J. Investig. Allergol. Clin. Immunol. 2005, 15, 293–296. [Google Scholar]
- Jurado-Palomo, J.; Cabañas, R.; Prior, N.; Bobolea, I.D.; Fiandor-Román, A.M.; López-Serrano, M.C.; Quirce, S.; Bellón, T. Use of the Lymphocyte Transformation Test in the Diagnosis of DRESS Syndrome Induced by Ceftriaxone and Iperacillintazobactam: Two Case Reports. J. Investig. Allergol. Clin. Immunol. 2010, 20, 433. [Google Scholar]
- Kano, Y.; Hirahara, K.; Mitsuyama, Y.; Takahashi, R.; Shiohara, T. Utility of the Lymphocyte Transformation Test in the Diagnosis of Drug Sensitivity: Dependence on Its Timing and the Type of Drug Eruption. Allergy 2007, 62, 1439–1444. [Google Scholar] [CrossRef]
- Karami, Z.; Mesdaghi, M.; Karimzadeh, P.; Mansouri, M.; Taghdiri, M.M.; Kayhanidoost, Z.; Jebelli, B.; Shekarriz Foumani, R.; Babaie, D.; Chavoshzadeh, Z. Evaluation of Lymphocyte Transformation Test Results in Patients with Delayed Hypersensitivity Reactions Following the Use of Anticonvulsant Drugs. Int. Arch. Allergy Immunol. 2016, 170, 158–162. [Google Scholar] [CrossRef]
- Lochmatter, P.; Beeler, A.; Kawabata, T.T.; Gerber, B.O.; Pichler, W.J. Drug-Specific in Vitro Release of IL-2, IL-5, IL-13 and IFN-γ in Patients with Delayed-Type Drug Hypersensitivity. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mattsson, J.; Schnyder, K.; Fontana, S.; Largiadèr, C.R.; Pichler, W.J.; Yerly, D. Allopurinol Hypersensitivity Is Primarily Mediated by Dose-Dependent Oxypurinol-Specific T Cell Response. Clin. Exp. Allergy 2013, 43, 1246–1255. [Google Scholar] [CrossRef]
- Elzagallaai, A.A.; Knowles, S.R.; Rieder, M.J.; Bend, J.R.; Shear, N.H.; Koren, G. In Vitro Testing for the Diagnosis of Anticonvulsant Hypersensitivity Syndrome: A Systematic Review. Mol. Diagn. Ther. 2009, 13, 313–330. [Google Scholar] [CrossRef]
- Ye, Y.M.; Hur, G.Y.; Kim, S.H.; Ban, G.Y.; Jee, Y.K.; Naisbitt, D.J.; Park, H.S.; Kim, S.H. Drug-Specific CD4+ T-Cell Immune Responses Are Responsible for Antituberculosis Drug-Induced Maculopapular Exanthema and Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome. Br. J. Dermatol. 2017, 176, 378–386. [Google Scholar] [CrossRef]
- Chung, W.-H.; Pan, R.-Y.; Chu, M.-T.; Chin, S.-W.; Huang, Y.-L.; Wang, W.-C.; Chang, J.-Y.; Hung, S.-I. Oxypurinol-Specific T Cells Possess Preferential TCR Clonotypes and Express Granulysin in Allopurinol-Induced Severe Cutaneous Adverse Reactions. J. Investig. Dermatol. 2015, 135, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Porebski, G. In Vitro Assays in Severe Cutaneous Adverse Drug Reactions: Are They Still Research Tools or Diagnostic Tests Already? Int. J. Mol. Sci. 2017, 18, 1737. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.C.; Albengres, E.; Moritz, S.; Piacentino, A.; Cuny, M.; Revuz, J.; Touraine, R. Lymphocyte Transformation Test in Drug-Induced Toxic Epidermal Necrolysis. Int. Arch. Allergy Immunol. 1985, 78, 22–24. [Google Scholar] [CrossRef]
- Porebski, G.; Pecaric-Petkovic, T.; Groux-Keller, M.; Bosak, M.; Kawabata, T.T.; Pichler, W.J. In Vitro Drug Causality Assessment in Stevens-Johnson Syndrome-Alternatives for Lymphocyte Transformation Test. Clin. Exp. Allergy 2013, 43, 1027–1037. [Google Scholar] [CrossRef]
- Britschgi, M.; Steiner, U.C.; Schmid, S.; Depta, J.P.; Senti, G.; Bircher, A.; Burkhart, C.; Yawalkar, N.; Pichler, W.J. T-Cell Involvement in Drug-Induced Acute Generalized Exanthematous Pustulosis. J. Clin. Investig. 2001, 107, 1433–1441. [Google Scholar] [CrossRef]
- Girardi, M.; Duncan, K.O.; Tigelaar, R.E.; Imaeda, S.; Watsky, K.L.; McNiff, J.M. Cross-Comparison of Patch Test and Lymphocyte Proliferation Responses in Patients with a History of Acute Generalized Exanthematous Pustulosis. Am. J. Dermatopathol. 2005, 27, 343–346. [Google Scholar] [CrossRef]
- Kabashima, R.; Sugita, K.; Sawada, Y.; Hino, R.; Nakamura, M.; Tokura, Y. Increased Circulating Th17 Frequencies and Serum IL-22 Levels in Patients with Acute Generalized Exanthematous Pustulosis. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 485–488. [Google Scholar] [CrossRef]
- Schmid, D.A.; Depta, J.P.H.; Pichler, W.J. T Cell-Mediated Hypersensitivity to Quinolones: Mechanisms and Cross-Reactivity. Clin. Exp. Allergy 2006, 36, 59–69. [Google Scholar] [CrossRef]
- Anliker, M.D.; Wüthrich, B. Acute Generalized Exanthematous Pustulosis Due to Sulfamethoxazol with Positive Lymphocyte Transformation Test (LTT). J. Investig. Allergol. Clin. Immunol. 2003, 13, 66–68. [Google Scholar] [PubMed]
- Beltraminelli, H.S.; Lerch, M.; Arnold, A.; Bircher, A.J.; Haeusermann, P. Acute Generalized Exanthematous Pustulosis Induced by the Antifungal Terbinafine: Case Report and Review of the Literature. Br. J. Dermatol. 2005, 152, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Buettiker, U.; Keller, M.; Pichler, W.J.; Braathen, L.R.; Yawalkar, N. Oral Prednisolone Induced Acute Generalized Exanthematous Pustulosis Due to Corticosteroids of Group A Confirmed by Epicutaneous Testing and Lymphocyte Transformation Tests. Dermatology 2006, 213, 40–43. [Google Scholar] [CrossRef]
- Goeschke, B.; Braathen, L.R. Acute Generalized Exanthematic Pustulosis: A Case and an Overview of Side Effects Affecting the Skin Caused by Celecoxib and Other COX-2 Inhibitors Reported so Far. Dermatology 2004, 209, 53–56. [Google Scholar] [CrossRef]
- Kardaun, S.H.; de Monchy, J.G. Acute Generalized Exanthematous Pustulosis Caused by Morphine, Confirmed by Positive Patch Test and Lymphocyte Transformation Test. J. Am. Acad. Dermatol. 2006, 55, S21–S23. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Takayasu, S. Drug Induced Acute Generalized Exanthematous Pustulosis. J. Dermatol. 1996, 23, 623–627. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Okubo, Y.; Yamamoto, T.; Ito, T.; Tsuboi, R. Case of Acute Generalized Exanthematous Pustulosis Caused by Ampicillin/Cloxacillin Sodium in a Pregnant Woman. J. Dermatol. 2008, 35, 362–364. [Google Scholar] [CrossRef]
- Nakamizo, S.; Kobayashi, S.; Usui, T.; Miyachi, Y.; Kabashima, K. Clopidogrel-Induced Acute Generalized Exanthematous Pustulosis with Elevated Th17 Cytokine Levels as Determined by a Drug Lymphocyte Stimulation Test. Br. J. Dermatol. 2010, 162, 1402–1403. [Google Scholar] [CrossRef]
- Thomas, E.; Bellón, T.; Barranco, P.; Padial, A.; Tapia, B.; Morel, E.; Alves-Ferreira, J.; Martín-Esteban, M. Acute Generalized Exanthematous Pustulosis Due to Tetrazepam. J. Investig. Allergol. Clin. Immunol. 2008, 18, 119. [Google Scholar] [PubMed]
- Trueb, R.M.; Burg, G. Acute Generalized Exanthematous Pustulosis Due to Doxycycline. Dermatology 1993, 186, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Meier-Schiesser, B.; Feldmeyer, L.; Jankovic, D.; Mellett, M.; Satoh, T.K.; Yerly, D.; Navarini, A.; Abe, R.; Yawalkar, N.; Chung, W.H.; et al. Culprit Drugs Induce Specific IL-36 Overexpression in Acute Generalized Exanthematous Pustulosis. J. Investig. Dermatol. 2019, 139, 848–858. [Google Scholar] [CrossRef]
- Rozieres, A.; Hennino, A.; Rodet, K.; Gutowski, M.C.; Gunera-Saad, N.; Berard, F.; Cozon, G.; Bienvenu, J.; Nicolas, J.F. Detection and Quantification of Drug-Specific T Cells in Penicillin Allergy. Allergy 2009, 64, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Trubiano, J.A.; Strautins, K.; Redwood, A.J.; Pavlos, R.; Konvinse, K.C.; Aung, A.K.; Slavin, M.A.; Thursky, K.A.; Grayson, M.L.; Phillips, E.J. The Combined Utility of Ex Vivo IFN-γ Release Enzyme-Linked ImmunoSpot Assay and In Vivo Skin Testing in Patients with Antibiotic-Associated Severe Cutaneous Adverse Reactions. J. Allergy Clin. Immunol. Pract. 2018, 6, 1287–1296.e1. [Google Scholar] [CrossRef] [PubMed]
- Konvinse, K.C.; Trubiano, J.A.; Pavlos, R.; James, I.; Shaffer, C.M.; Bejan, C.A.; Schutte, R.J.; Ostrov, D.A.; Pilkinton, M.A.; Rosenbach, M.; et al. HLA-A*32:01 Is Strongly Associated with Vancomycin-Induced Drug Reaction with Eosinophilia and Systemic Symptoms. J. Allergy Clin. Immunol. 2019, 144, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Copaescu, A.; Gibson, A.; Li, Y.; Trubiano, J.A.; Phillips, E.J. An Updated Review of the Diagnostic Methods in Delayed Drug Hypersensitivity. Front. Pharmacol. 2021, 11, 573573. [Google Scholar] [CrossRef] [PubMed]
- Suthumchai, N.; Srinoulprasert, Y.; Thantiworasit, P.; Rerknimitr, P.; Tuchinda, P.; Chularojanamontri, L.; Rerkpattanapipat, T.; Chanprapaph, K.; Disphanurat, W.; Chakkavittumrong, P.; et al. The Measurement of Drug-Induced Interferon γ-Releasing Cells and Lymphocyte Proliferation in Severe Cutaneous Adverse Reactions. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 992–998. [Google Scholar] [CrossRef]
- Klaewsongkram, J.; Thantiworasit, P.; Suthumchai, N.; Rerknimitr, P.; Sukasem, C.; Tuchinda, P.; Chularojanamontri, L.; Srinoulprasert, Y.; Rerkpattanapipat, T.; Chanprapaph, K.; et al. In Vitro Test to Confirm Diagnosis of Allopurinol-Induced Severe Cutaneous Adverse Reactions. Br. J. Dermatol. 2016, 175, 994–1002. [Google Scholar] [CrossRef]
- Klaewsongkram, J.; Sukasem, C.; Thantiworasit, P.; Suthumchai, N.; Rerknimitr, P.; Tuchinda, P.; Chularojanamontri, L.; Srinoulprasert, Y.; Rerkpattanapipat, T.; Chanprapaph, K.; et al. Analysis of HLA-B Allelic Variation and IFN-γ ELISpot Responses in Patients with Severe Cutaneous Adverse Reactions Associated with Drugs. J. Allergy Clin. Immunol. Pract. 2019, 7, 219–227.e4. [Google Scholar] [CrossRef]
- Copaescu, A.; Mouhtouris, E.; Vogrin, S.; James, F.; Chua, K.Y.L.; Holmes, N.E.; Douglas, A.; Slavin, M.A.; Cleland, H.; Zubrinich, C.; et al. The Role of In Vivo and Ex Vivo Diagnostic Tools in Severe Delayed Immune-Mediated Adverse Antibiotic Drug Reactions. J. Allergy Clin. Immunol. Pract. 2021, 9, 2010–2015.e4. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, B.; Rozieres, A.; Nosbaum, A.; Nicolas, J.F.; Berard, F. Amikacin-Induced Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: Delayed Skin Test and ELISPOT Assay Results Allow the Identification of the Culprit Drug. J. Allergy Clin. Immunol. 2012, 130, 1413–1414. [Google Scholar] [CrossRef]
- Ben-Said, B.; Arnaud-Butel, S.; Rozières, A.; Rodet, K.; Bérard, F.; Nicolas, J.F.; Nosbaum, A. Allergic Delayed Drug Hypersensitivity Is More Frequently Diagnosed in Drug Reaction, Eosinophilia and Systemic Symptoms (DRESS) Syndrome than in Exanthema Induced by Beta-Lactam Antibiotics. J. Dermatol. Sci. 2015, 80, 71–74. [Google Scholar] [CrossRef]
- Klaewsongkram, J.; Buranapraditkun, S.; Thantiworasit, P.; Rerknimitr, P.; Tuchinda, P.; Chularojanamontri, L.; Rerkpattanapipat, T.; Chanprapaph, K.; Disphanurat, W.; Chakkavittumrong, P.; et al. The Role of In Vitro Detection of Drug-Specific Mediator-Releasing Cells to Diagnose Different Phenotypes of Severe Cutaneous Adverse Reactions. Allergy. Asthma Immunol. Res. 2021, 13, 896–907. [Google Scholar] [CrossRef]
- Hernandez-Fuentes, M.P.; Warrens, A.N.; Lechler, R.I. Immunologic Monitoring. Immunol. Rev. 2003, 196, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Yerly, D.; (University of Bern, Bern, Switzerland). Personal communication, 2023.
- Li, Y.; Deshpande, P.; Hertzman, R.J.; Palubinsky, A.M.; Gibson, A.; Phillips, E.J. Genomic Risk Factors Driving Immune-Mediated Delayed Drug Hypersensitivity Reactions. Front. Genet. 2021, 12, 641905. [Google Scholar] [CrossRef] [PubMed]
- Mounzer, K.; Hsu, R.; Fusco, J.S.; Brunet, L.; Henegar, C.E.; Vannappagari, V.; Stainsby, C.M.; Shaefer, M.S.; Ragone, L.; Fusco, G.P. HLA-B*57:01 Screening and Hypersensitivity Reaction to Abacavir between 1999 and 2016 in the OPERA® Observational Database: A Cohort Study. AIDS Res. Ther. 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Balu, R.; Phillips, E.; Brachman, P.; Martorell, C.; Burman, W.; Stancil, B.; Mosteller, M.; Brothers, C.; Wannamaker, P.; et al. High Sensitivity of Human Leukocyte Antigen-B*5701 as a Marker for Immunologically Confirmed Abacavir Hypersensitivity in White and Black Patients. Clin. Infect. Dis. 2008, 46, 1111–1118. [Google Scholar] [CrossRef]
- Chung, W.-H.; Hung, S.-I.; Hong, H.-S.; Hsih, M.-S.; Yang, L.-C.; Ho, H.-C.; Wu, J.-Y.; Chen, Y.-T. A Marker for Stevens–Johnson Syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef]
- Fan, W.L.; Shiao, M.S.; Hui, R.C.Y.; Su, S.C.; Wang, C.W.; Chang, Y.C.; Chung, W.H. HLA Association with Drug-Induced Adverse Reactions. J. Immunol. Res. 2017, 2017, 3186328. [Google Scholar] [CrossRef]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperavičiūtė, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; de Bakker, P.I.W.; et al. HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef]
- Genin, E.; Chen, D.-P.; Hung, S.-I.; Sekula, P.; Schumacher, M.; Chang, P.-Y.; Tsai, S.-H.; Wu, T.-L.; Bellón, T.; Tamouza, R.; et al. HLA-A*31:01 and Different Types of Carbamazepine-Induced Severe Cutaneous Adverse Reactions: An International Study and Meta-Analysis. Pharmacogenomics J. 2014, 14, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Ripa, M.; Burastero, S.; Benanti, G.; Bagnasco, D.; Nannipieri, S.; Monardo, R.; Ponta, G.; Asperti, C.; Cilona, M.B.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses. Microorganisms 2023, 11, 346. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, Z.; Zhu, Q.; Zhang, J.; Yang, L.; Qin, S.; Shao, L.; Zhang, Y.; Xuan, J.; Li, Q.; et al. HLA-B*58:01 Allele Is Associated with Augmented Risk for Both Mild and Severe Cutaneous Adverse Reactions Induced by Allopurinol in Han Chinese. Pharmacogenomics 2012, 13, 1193–1201. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, C.B.; Wang, C.W.; Hung, S.I.; Chung, W.H. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Risk Factors, Causality Assessment and Potential Prevention Strategies. Expert Rev. Clin. Immunol. 2020, 16, 373–387. [Google Scholar] [CrossRef]
- Zhang, F.-R.; Liu, H.; Irwanto, A.; Fu, X.-A.; Li, Y.; Yu, G.-Q.; Yu, Y.-X.; Chen, M.-F.; Low, H.-Q.; Li, J.-H.; et al. HLA-B*13:01 and the Dapsone Hypersensitivity Syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Vidal, C.; Chu, H.C.; Do, N.T.Q.; Tran, T.T.L.; Le, H.T.M.; Fulton, R.B.; Li, J.; Fernando, S.L. Validation of a Novel Real-Time PCR Assay for Detection of HLA-B*15:02 Allele for Prevention of Carbamazepine-Induced Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in Individuals of Asian Ancestry. Hum. Immunol. 2016, 77, 1140–1146. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Vidal, C.; Chi, H.C.; Do, N.T.Q.; Fulton, R.; Li, J.; Fernando, S.L. A Novel Multiplex Polymerase Chain Reaction Assay for Detection of Both HLA-A*31:01/HLA-B*15:02 Alleles, Which Confer Susceptibility to Carbamazepine-Induced Severe Cutaneous Adverse Reactions. HLA 2017, 90, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Rwandamuriye, F.X.; Chopra, A.; Konvinse, K.C.; Choo, L.; Trubiano, J.A.; Shaffer, C.M.; Watson, M.; Mallal, S.A.; Phillips, E.J. A Rapid Allele-Specific Assay for HLA-A*32:01 to Identify Patients at Risk for Vancomycin-Induced Drug Reaction with Eosinophilia and Systemic Symptoms. J. Mol. Diagn. 2019, 21, 782–789. [Google Scholar] [CrossRef]
- White, K.D.; Abe, R.; Ardern-Jones, M.; Beachkofsky, T.; Bouchard, C.; Carleton, B.; Chodosh, J.; Cibotti, R.; Davis, R.; Denny, J.C.; et al. SJS/TEN 2017: Building Multidisciplinary Networks to Drive Science and Translation. J. Allergy Clin. Immunol. Pract. 2018, 6, 38–69. [Google Scholar] [CrossRef]
- Alfirevic, A.; Pirmohamed, M. Genomics of Adverse Drug Reactions. Trends Pharmacol. Sci. 2017, 38, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Manson, L.E.N.; Swen, J.J.; Guchelaar, H.J. Diagnostic Test Criteria for HLA Genotyping to Prevent Drug Hypersensitivity Reactions: A Systematic Review of Actionable HLA Recommendations in CPIC and DPWG Guidelines. Front. Pharmacol. 2020, 11, 567048. [Google Scholar] [CrossRef]
- Chen, P.; Lin, J.-J.; Lu, C.-S.; Ong, C.-T.; Hsieh, P.F.; Yang, C.-C.; Tai, C.-T.; Wu, S.-L.; Lu, C.-H.; Hsu, Y.-C.; et al. Carbamazepine-Induced Toxic Effects and HLA-B*1502 Screening in Taiwan. N. Engl. J. Med. 2011, 364, 1126–1133. [Google Scholar] [CrossRef]
- Mushiroda, T.; Takahashi, Y.; Onuma, T.; Yamamoto, Y.; Kamei, T.; Hoshida, T.; Takeuchi, K.; Otsuka, K.; Okazaki, M.; Watanabe, M.; et al. Association of HLA-A*31:01 Screening With the Incidence of Carbamazepine-Induced Cutaneous Adverse Reactions in a Japanese Population. JAMA Neurol. 2018, 75, 842–849. [Google Scholar] [CrossRef]
- Ko, T.M.; Tsai, C.Y.; Chen, S.Y.; Chen, K.S.; Yu, K.H.; Chu, C.S.; Huang, C.M.; Wang, C.R.; Weng, C.T.; Yu, C.L.; et al. Use of HLA-B*58:01 Genotyping to Prevent Allopurinol Induced Severe Cutaneous Adverse Reactions in Taiwan: National Prospective Cohort Study. BMJ 2015, 351. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Kim, D.K.; Kim, S.H.; Kim, S.; Chae, D.W.; Yang, M.S.; Oh, Y.K.; Lee, J.P.; Jung, J.W.; Shin, J.; et al. Efficacy of the HLA-B ∗ 58:01 Screening Test in Preventing Allopurinol-Induced Severe Cutaneous Adverse Reactions in Patients with Chronic Renal Insufficiency—A Prospective Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 1271–1276. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Bao, F.; Wang, C.; Sun, L.; Zhang, H.; Yu, G.; Mi, Z.; Li, J.; Li, L.; et al. Evaluation of Prospective HLA-B*13:01 Screening to Prevent Dapsone Hypersensitivity Syndrome in Patients with Leprosy. JAMA Dermatol. 2019, 155, 666–672. [Google Scholar] [CrossRef]
- Chang, C.J.; Chen, C.B.; Hung, S.I.; Ji, C.; Chung, W.H. Pharmacogenetic Testing for Prevention of Severe Cutaneous Adverse Drug Reactions. Front. Pharmacol. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Gerogianni, K.; Tsezou, A.; Dimas, K. Drug-Induced Skin Adverse Reactions: The Role of Pharmacogenomics in Their Prevention. Mol. Diagn. Ther. 2018, 22, 297–314. [Google Scholar] [CrossRef]

| Drug | Result | SI 1 |
|---|---|---|
| Beta-lactam antibiotics [162] | Negative | <3 |
| Positive | >3 | |
| Iodinated contrast media [162] | Negative | <4 |
| Positive | >4 | |
| Vancomycin [164] | Negative | <3 |
| Positive | >3 | |
| Other drugs [162] | Negative | <2 |
| Doubtful | 2–3 | |
| Positive | >3 |
| Drug | Clinical Entity | Risk HLA Allele | OR | PPV | NPV | NNT 1 | Ethnic Risk Groups |
|---|---|---|---|---|---|---|---|
| Abacavir | Hypersensitivity | B*57:01 | 960 | 55% | 100% | 14 | European, African American |
| Carbamazepine | SJS/TEN | B*15:02 | >1000 | 3% | 100% (Han Chinese) | 1000 | Han Chinese and Southeast Asian countries |
| Carbamazepine | DRESS/MPE | A*31:01 | 57.6 | 0.89% | 99.98% | 2857 | European |
| A*31:01 | 23.0 | 0.59% | 99.97% | 4000 | Han Chinese | ||
| Alopurinol | SSJ/NET, DRESS, MPE | B*58:01 | 580 | 2% | 100% (Han Chinese) | 500 | Han Chinese and Southeast Asian countries |
| Oxcarbazepine | SSJ/NET | B*15:02 | 0.73% | 99.97% | 1715 | Han Chinese | |
| Dapsone | DRESS | B*13:01 | 20 | 7.8% | 99.80% | 84 | Han Chinese |
| Vancomycin | DRESS | A*32:01 | 70 | 20% | 75 | European descent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pérez, R.; de las Vecillas, L.; Cabañas, R.; Bellón, T. Tools for Etiologic Diagnosis of Drug-Induced Allergic Conditions. Int. J. Mol. Sci. 2023, 24, 12577. https://doi.org/10.3390/ijms241612577
Rodríguez-Pérez R, de las Vecillas L, Cabañas R, Bellón T. Tools for Etiologic Diagnosis of Drug-Induced Allergic Conditions. International Journal of Molecular Sciences. 2023; 24(16):12577. https://doi.org/10.3390/ijms241612577
Chicago/Turabian StyleRodríguez-Pérez, Rosa, Leticia de las Vecillas, Rosario Cabañas, and Teresa Bellón. 2023. "Tools for Etiologic Diagnosis of Drug-Induced Allergic Conditions" International Journal of Molecular Sciences 24, no. 16: 12577. https://doi.org/10.3390/ijms241612577
APA StyleRodríguez-Pérez, R., de las Vecillas, L., Cabañas, R., & Bellón, T. (2023). Tools for Etiologic Diagnosis of Drug-Induced Allergic Conditions. International Journal of Molecular Sciences, 24(16), 12577. https://doi.org/10.3390/ijms241612577






