Neuroinflammation Profiling of Brain Cytokines Following Repeated Blast Exposure
Abstract
1. Introduction
2. Results
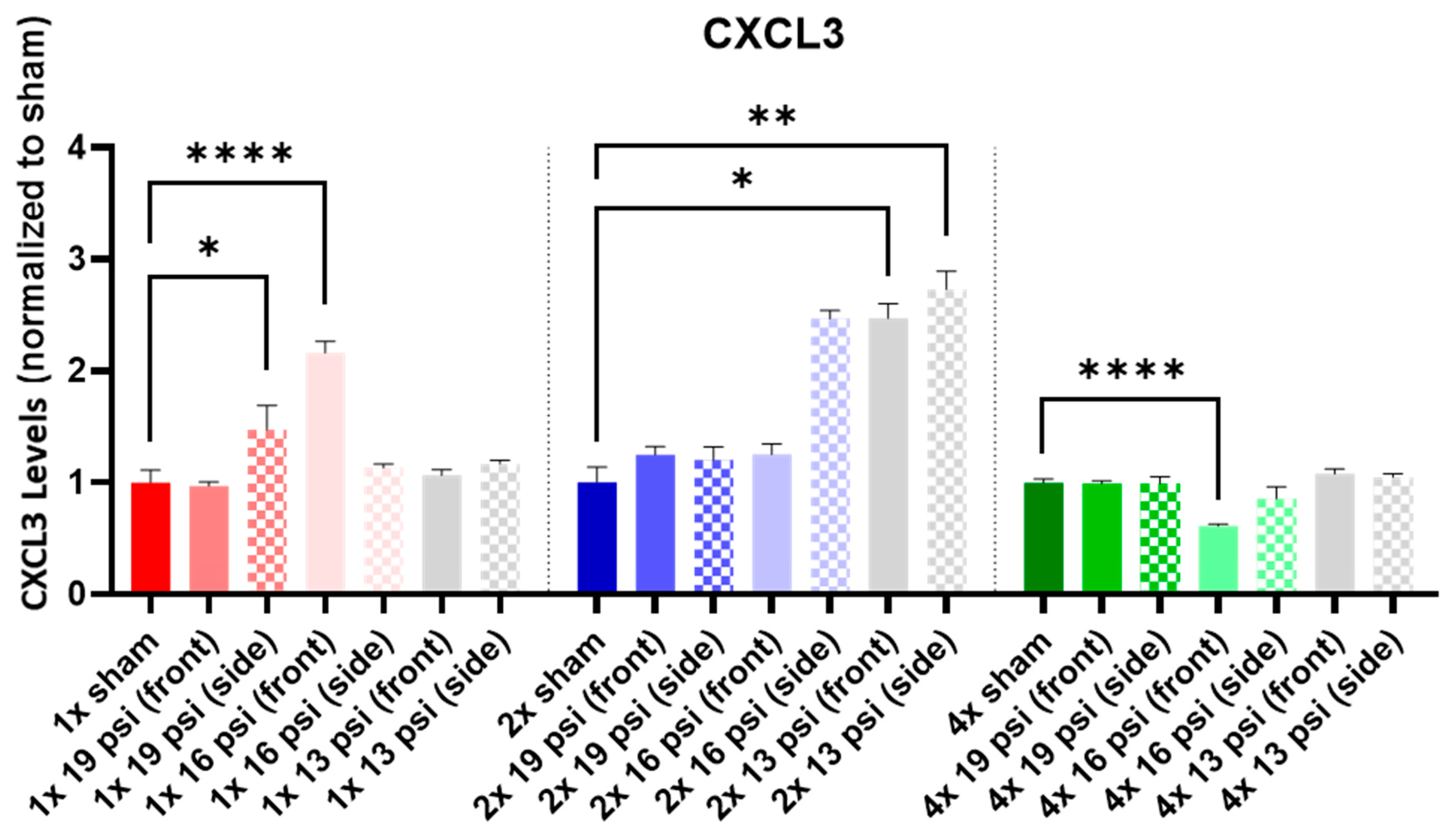
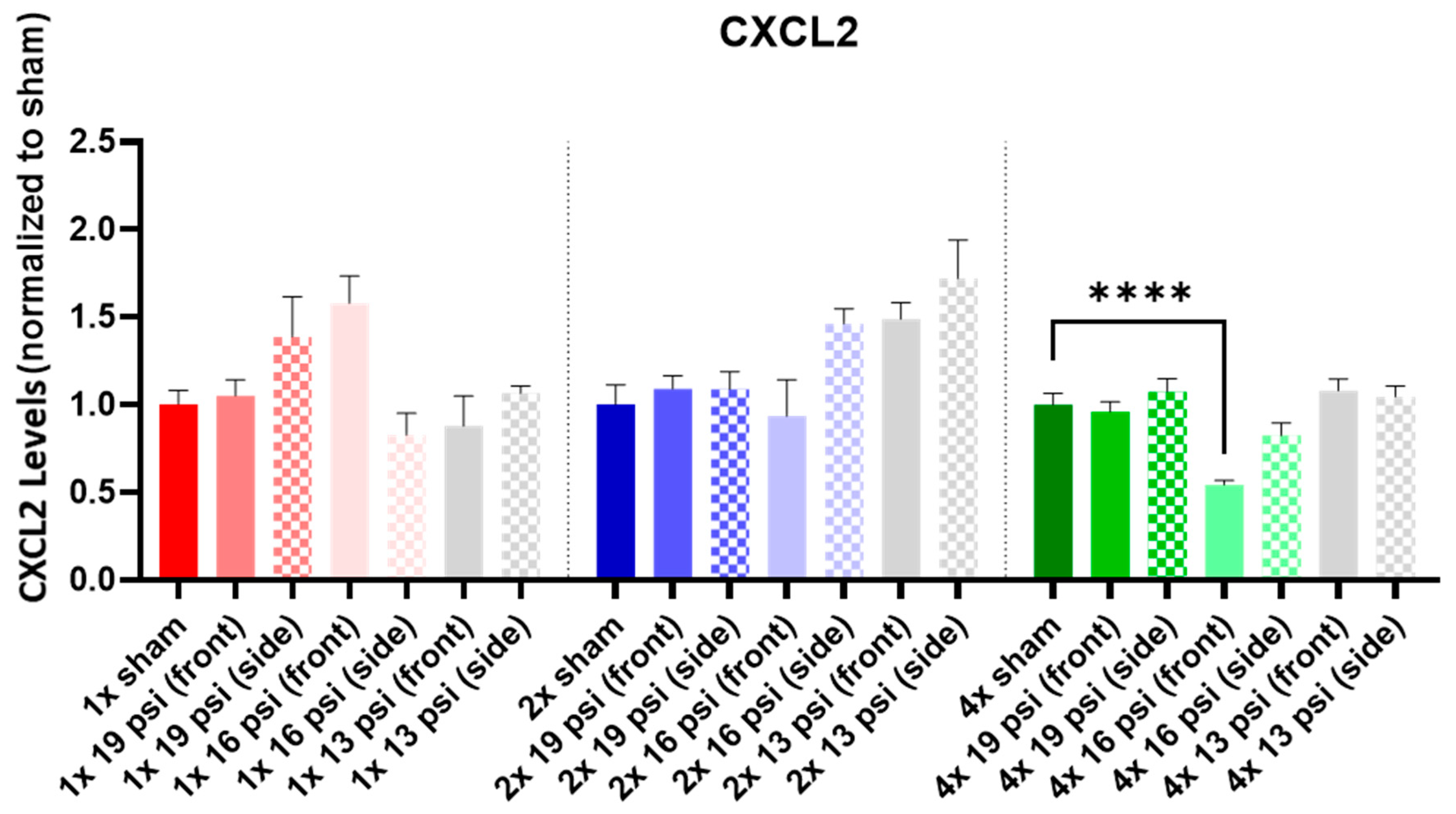
2.1. Chemokine (C-X-C) Motif Ligands
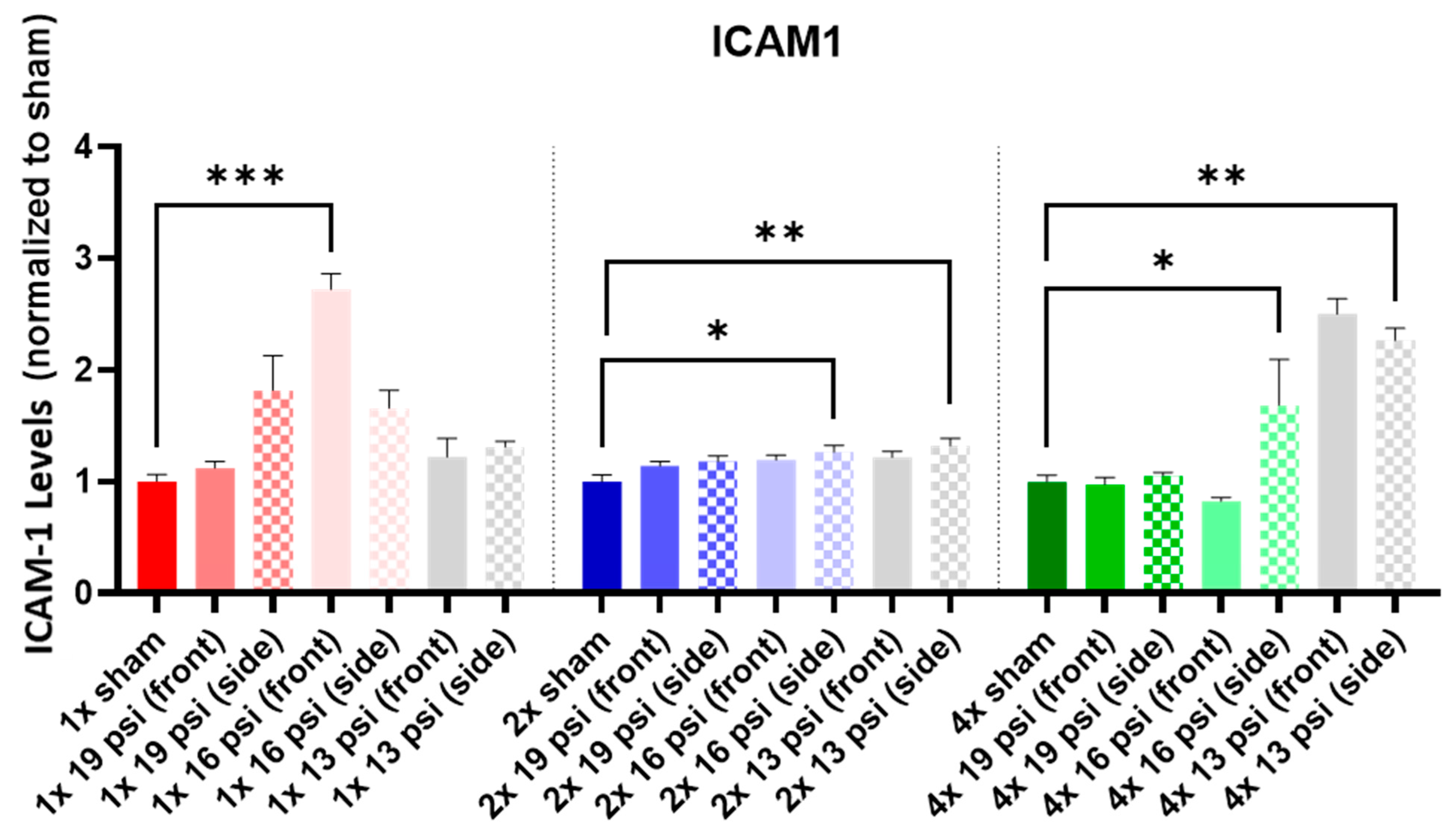
2.2. Intercellular Cell Adhesion Molecule 1 (ICAM-1)
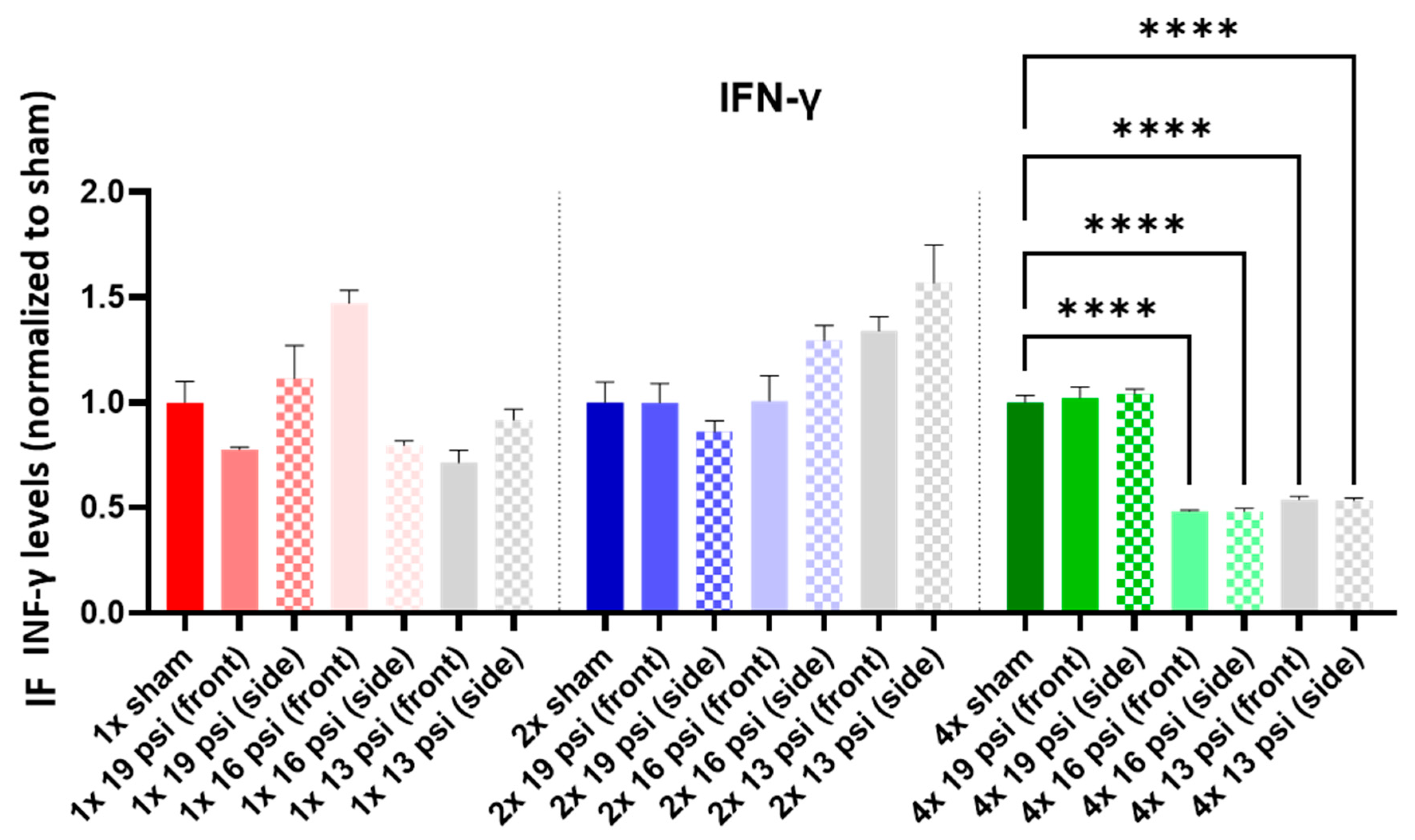
2.3. Interferon-Gamma (IFN-γ)
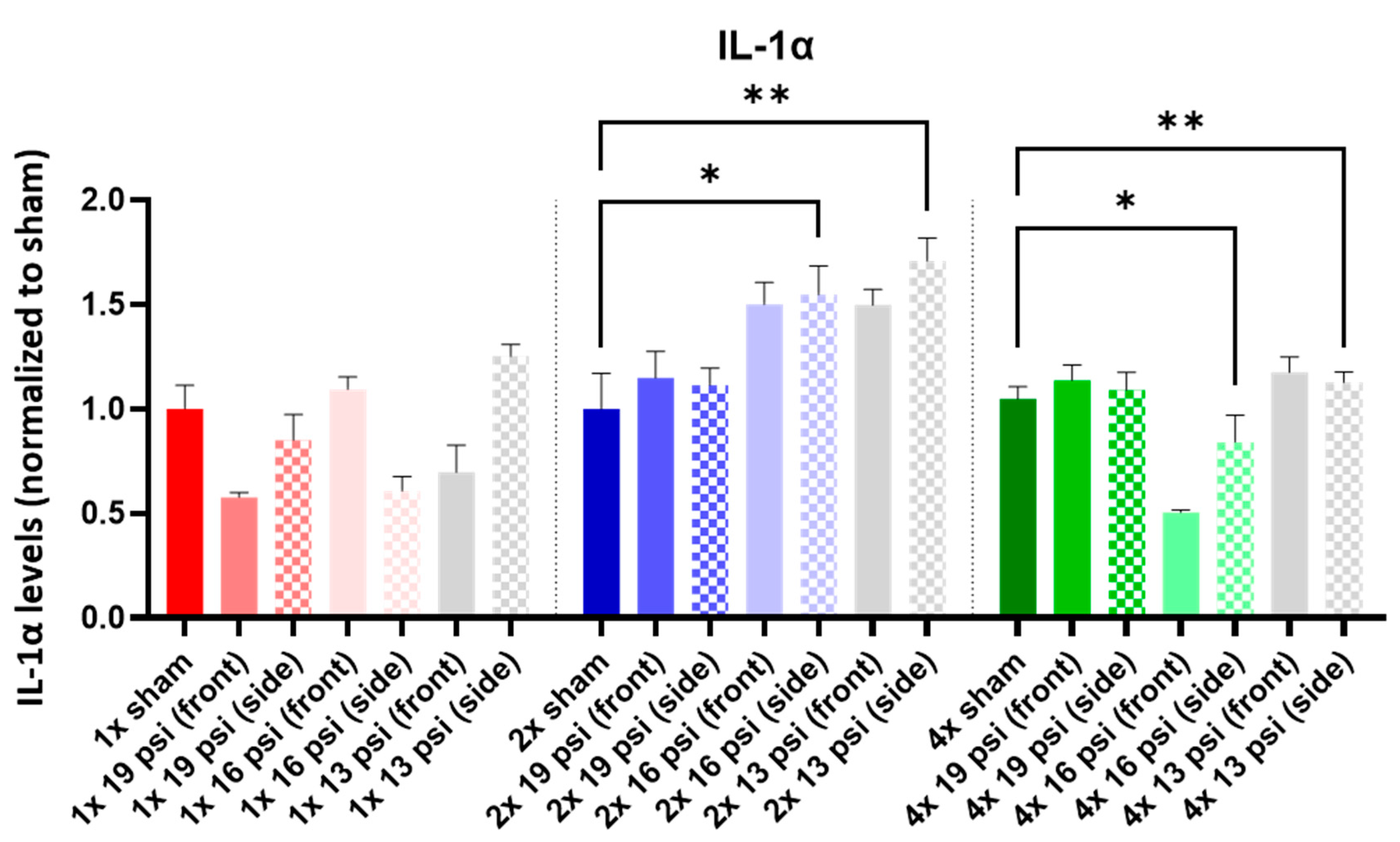
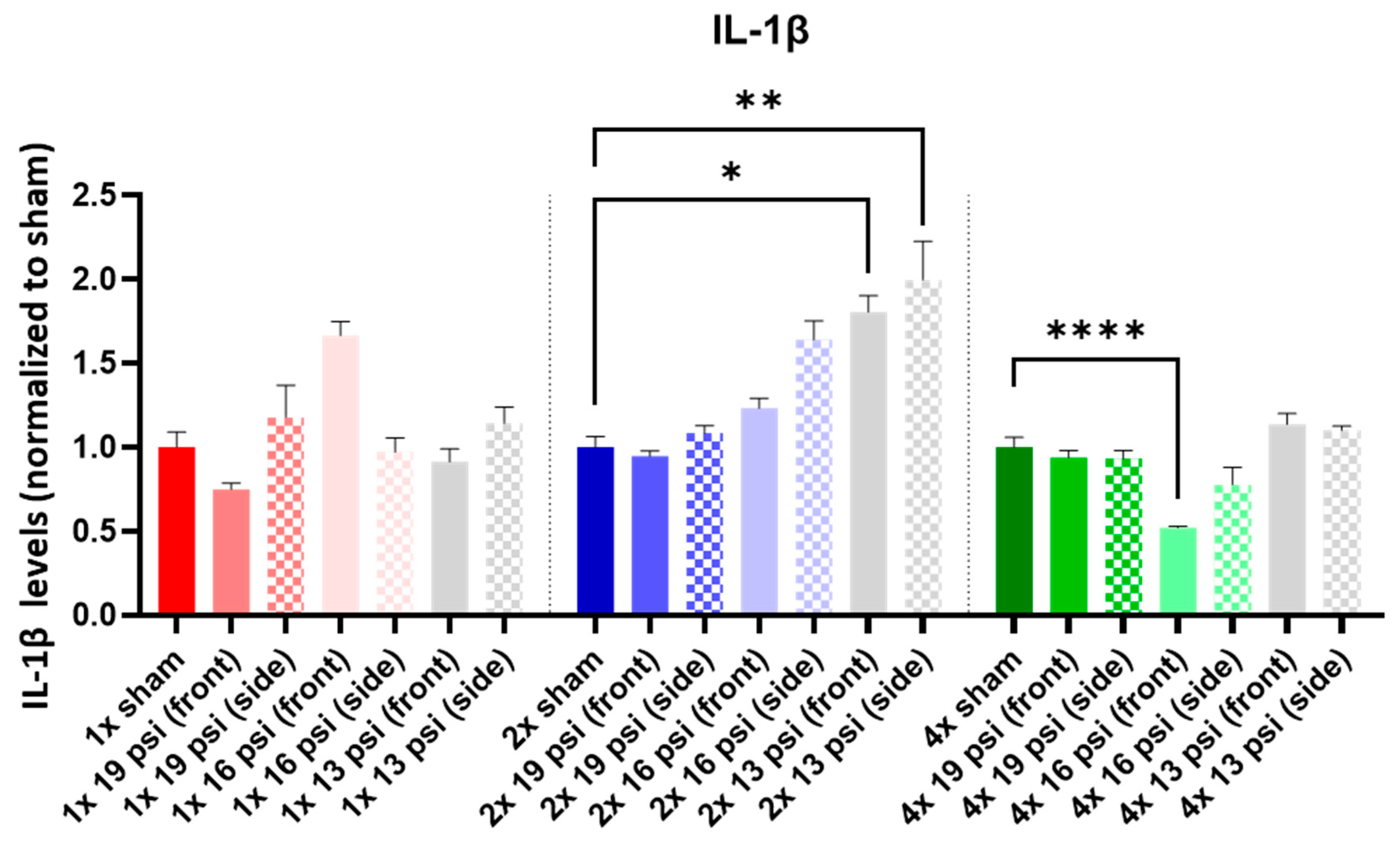
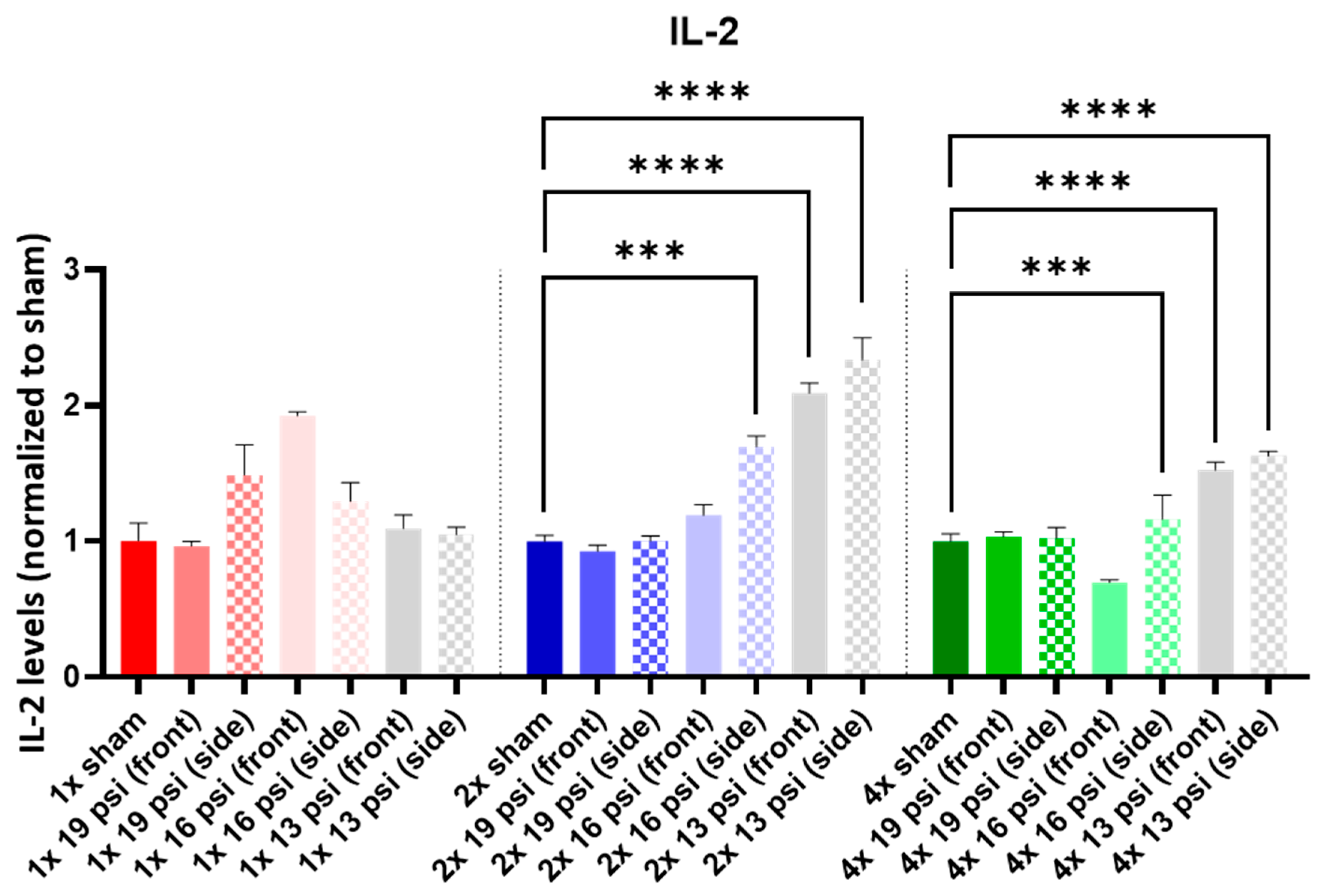
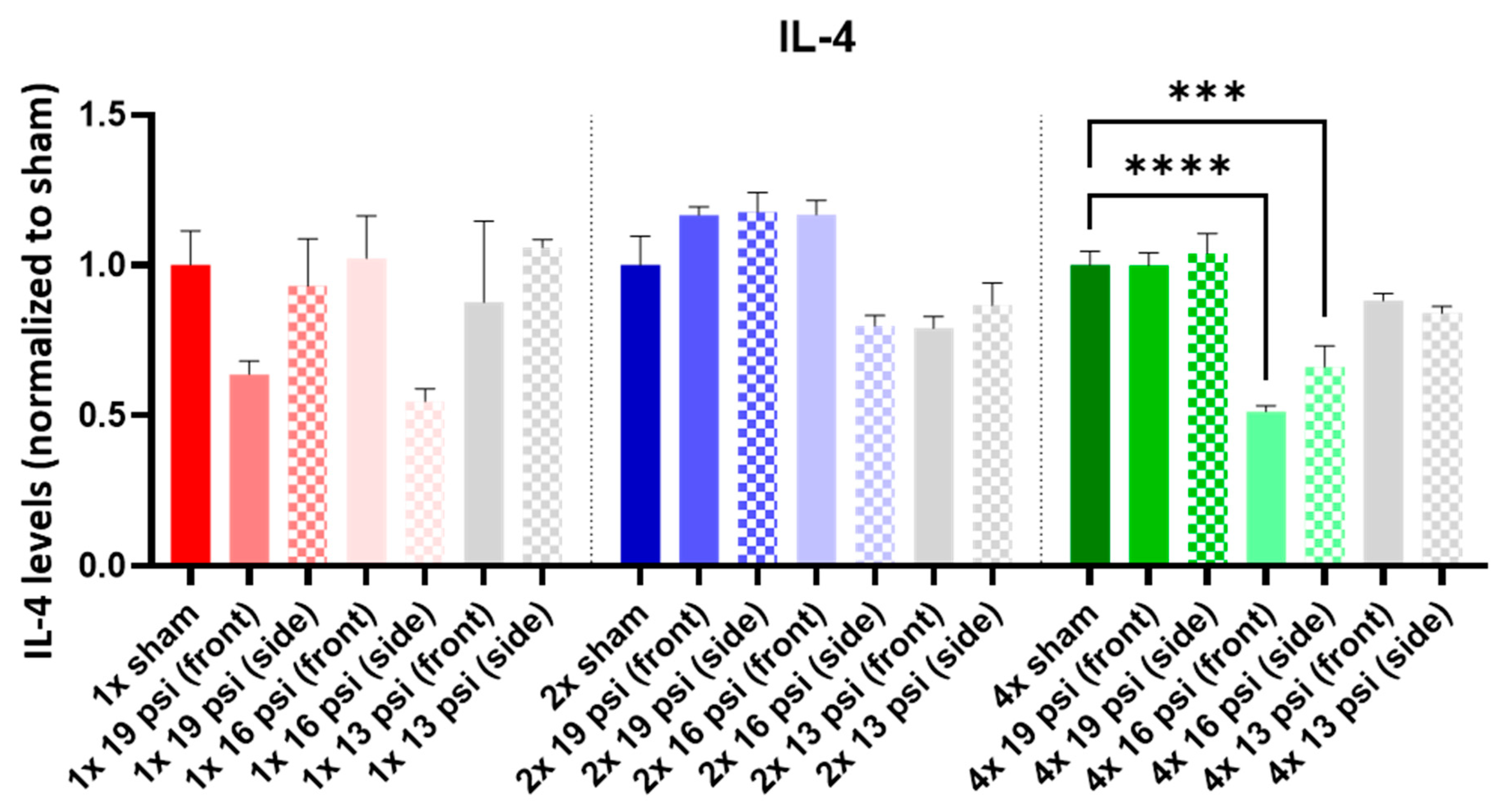
2.4. Interleukins (ILs)
2.5. Tissue Inhibitor of Metalloproteinases (TIMP) Metallopeptidase Inhibitor 1 (TIMP1)
2.6. Tumor Necrosis Factor-Alpha (TNF-α)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. BW Exposure
4.3. Protein Extraction
4.4. Multiplex ELISA
4.5. Statistical Analysis
5. Conclusions
6. Disclaimer
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Traumatic Brain Injury Center of Excellence (TBICoR). DoD Numbers for Traumatic Brain Injury: Worldwide Totals. 2018. Available online: https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers (accessed on 4 August 2023).
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild Traumatic Brain Injury in U.S. Soldiers Returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef]
- Walz, R.; Schwarzbold, M.; Diaz, A.; Martins, E.T.; Rufino, A.; Amante, L.N.; Thais, M.E.; Quevedo, J.; Hohl, A.; Linhares, M.N. Psychiatric disorders and traumatic brain injury. Neuropsychiatr. Dis. Treat. 2008, 4, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Morissette, S.B.; Woodward, M.; Kimbrel, N.A.; Meyer, E.C.; Kruse, M.I.; Dolan, S.; Gulliver, S.B. Deployment-Related TBI, Persistent Postconcussive Symptoms, PTSD, and Depression in OEF/OIF Veterans. Rehabil. Psychol. 2011, 56, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Laughter, S.; Khan, M.; Banaag, A.; Madsen, C.; Koehlmoos, T.P. Prevalence of Polytrauma Clinical Triad Among Active Duty Service Members. Mil. Med. 2021, 187, e856–e861. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Motamedi, V.; Osier, N.; Dell, K.; Arcurio, L.; Carr, W.; Walker, P.; Ahlers, S.; LoPresti, M.; Yarnell, A. Moderate blast exposure results in increased IL-6 and TNFα in peripheral blood. Brain Behav. Immun. 2017, 65, 90–94. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Liu, B.; Valdez, C.; Chavko, M.; Cancio, L.C. Low-Level Primary Blast Induces Neuroinflammation and Neurodegeneration in Rats. Mil. Med. 2019, 184, 265–272. [Google Scholar] [CrossRef]
- Sajja, V.S.S.S.; Tenn, C.; McLaws, L.J.; VandeVord, P.J. A temporal evaluation of cytokines in rats after blast exposure. Biomed. Sci. Instrum. 2012, 48, 374–379. [Google Scholar]
- Toklu, H.Z.; Yang, Z.; Oktay, S.; Sakarya, Y.; Kirichenko, N.; Matheny, M.K.; Muller-Delp, J.; Strang, K.; Scarpace, P.J.; Wang, K.K.; et al. Overpressure blast injury-induced oxidative stress and neuroinflammation response in rat frontal cortex and cerebellum. Behav. Brain Res. 2018, 340, 14–22. [Google Scholar] [CrossRef]
- Sosa, M.A.G.; De Gasperi, R.; Pryor, D.; Garcia, G.S.P.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Hogg, S.; Ache, B.; Janssen, W.G.; et al. Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol. Commun. 2021, 9, 167. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Bhaumik, R.; Zhang, H. Chemokines gene expression in the prefrontal cortex of depressed suicide victims and normal control subjects. Brain Behav. Immun. 2021, 94, 266–273. [Google Scholar] [CrossRef]
- Müller, N. The Role of Intercellular Adhesion Molecule-1 in the Pathogenesis of Psychiatric Disorders. Front. Pharmacol. 2019, 10, 1251. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Smid, G.E.; van Zuiden, M.; Geuze, E.; Kavelaars, A.; Heijnen, C.J.; Vermetten, E. Cytokine production as a putative biological mechanism underlying stress sensitization in high combat exposed soldiers. Psychoneuroendocrinology 2015, 51, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef]
- Mehta, D.; Voisey, J.; Bruenig, D.; Harvey, W.; Morris, C.P.; Lawford, B.; Young, R.M. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav. Immun. 2018, 74, 133–142. [Google Scholar] [CrossRef]
- Kanefsky, R.; Motamedi, V.; Mithani, S.; Mysliwiec, V.; Gill, J.M.; Pattinson, C.L. Mild traumatic brain injuries with loss of consciousness are associated with increased inflammation and pain in military personnel. Psychiatry Res. 2019, 279, 34–39. [Google Scholar] [CrossRef]
- Heyburn, L.; Abutarboush, R.; Goodrich, S.; Urioste, R.; Batuure, A.; Wheel, J.; Wilder, D.M.; Arun, P.; Ahlers, S.T.; Long, J.B.; et al. Repeated Low-Level Blast Acutely Alters Brain Cytokines, Neurovascular Proteins, Mechanotransduction, and Neurodegenerative Markers in a Rat Model. Front. Cell. Neurosci. 2021, 15, 636707. [Google Scholar] [CrossRef]
- Martindale, S.L.; Ord, A.S.; Rule, L.G.; Rowland, J.A. Effects of blast exposure on psychiatric and health symptoms in combat veterans. J. Psychiatr. Res. 2021, 143, 189–195. [Google Scholar] [CrossRef]
- Jones, N.; Thandi, G.; Fear, N.T.; Wessely, S.; Greenberg, N. The psychological effects of improvised explosive devices (IEDs) on UK military personnel in Afghanistan. Occup. Environ. Med. 2014, 71, 466–471. [Google Scholar] [CrossRef][Green Version]
- Arun, P.; Wilder, D.M.; Eken, O.; Urioste, R.; Batuure, A.; Sajja, S.; Van Albert, S.; Wang, Y.; Gist, I.D.; Long, J.B. Long-Term Effects of Blast Exposure: A Functional Study in Rats Using an Advanced Blast Simulator. J. Neurotrauma 2020, 37, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Heyburn, L.; Abutarboush, R.; Goodrich, S.; Urioste, R.; Batuure, A.; Statz, J.; Wilder, D.; Ahlers, S.T.; Long, J.B.; Sajja, V.S. Repeated Low-Level BW Leads to Endovascular Disruption and Alterations in TDP-43 and Piezo2 in a Rat Model of Blast TBI. Front. Neurol. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Szmydynger-Chodobska, J.; Strazielle, N.; Zink, B.J.; Ghersi-Egea, J.-F.; Chodobski, A. The Role of the Choroid Plexus in Neutrophil Invasion after Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2009, 29, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Mednova, I.A.; Boiko, A.S.; Buneva, V.N. Chemokine Dysregulation and Neuroinflammation in Schizophrenia: A Systematic Review. Int. J. Mol. Sci. 2022, 24, 2215. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.K.; Sharkey, J.; Andrews, P.J.; Loane, D.J.; Kumar, A.; Su, E.; Bell, M.; Taka, E.; Mazzio, E.A.; Goodman, C.B.; et al. The Temporal Expression, Cellular Localization, and Inhibition of the Chemokines MIP-2 and MCP-1 after Traumatic Brain Injury in the Rat. J. Neurotrauma 2009, 26, 507–525. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hung, T.-H.; Wang, Y.-H.; Lin, C.-W.; Wang, P.-Y.; Lee, C.-Y.; Chen, S.-F. Wogonin Improves Histological and Functional Outcomes, and Reduces Activation of TLR4/NF-κB Signaling after Experimental Traumatic Brain Injury. PLoS ONE 2012, 7, e30294. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Yang, X.; Liu, Y.; Liu, G.; Fan, K.; Ma, J. Cathepsin C aggravates neuroinflammation via promoting production of CCL2 and CXCL2 in glial cells and neurons in a cryogenic brain lesion. Neurochem. Int. 2021, 148, 105107. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.-Z.; Li, X.; Chen, Z.; Benedek, D.M.; Fullerton, C.S.; Wynn, G.; Naifeh, J.A.; Wu, H.; Benfer, N.; et al. Potential chemokine biomarkers associated with PTSD onset, risk and resilience as well as stress responses in US military service members. Transl. Psychiatry 2020, 10, 31. [Google Scholar] [CrossRef]
- Rancan, M.; Otto, V.I.; Hans, V.H.; Gerlach, I.; Jork, R.; Trentz, O.; Kossmann, T.; Morganti-Kossmann, M.C. Upregulation of ICAM-1 and MCP-1 but not of MIP-2 and sensorimotor deficit in response to traumatic axonal injury in rats. J. Neurosci. Res. 2001, 63, 438–446. [Google Scholar] [CrossRef]
- Otto, V.I.; Heinzel-Pleines, U.E.; Gloor, S.M.; Trentz, O.; Kossmann, T.; Morganti-Kossmann, M.C. sICAM-1 and TNF-α induce MIP-2 with distinct kinetics in astrocytes and brain microvascular endothelial cells. J. Neurosci. Res. 2000, 60, 733–742. [Google Scholar] [CrossRef]
- Yang, X.; Evans, R.W.; George, C.J.; Matthews, K.A.; Kovacs, M. Adiposity and Smoking Mediate the Relationship Between Depression History and Inflammation Among Young Adults. Int. J. Behav. Med. 2022, 29, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, J.; Jiang, Y.; Cao, Z.; Wu, M.; Sun, R.; Chen, Z.; Yu, P.; Ma, J.; Chen, Y.; et al. IL-6 and IL-8 are likely associated with psychological status in treatment naïve general population. J. Affect. Disord. 2022, 298, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ojo, J.O.; Greenberg, M.B.; Leary, P.; Mouzon, B.; Bachmeier, C.; Mullan, M.; Diamond, D.M.; Crawford, F. Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front. Behav. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Burke, J.D.; Young, H.A. IFN-γ: A cytokine at the right time, is in the right place. Semin. Immunol. 2020, 43, 101280. [Google Scholar] [CrossRef]
- Ryan, E.; Kelly, L.; Stacey, C.; Huggard, D.; Duff, E.; McCollum, D.; Leonard, A.; Boran, G.; Doherty, D.R.; Bolger, T.; et al. Mild-to-severe traumatic brain injury in children: Altered cytokines reflect severity. J. Neuroinflammation 2022, 19, 36. [Google Scholar] [CrossRef]
- Cho, H.; Sajja, V.; VandeVord, P.; Lee, Y. Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats RSS Download PDF. Neuroscience 2013, 253, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Y.; Yuan, Y. The Combination of Serum BDNF, Cortisol and IFN-Gamma Can Assist the Diagnosis of Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2021, 17, 2819–2829. [Google Scholar] [CrossRef]
- Yang, J.-J.; Jiang, W. Immune biomarkers alterations in post-traumatic stress disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 268, 39–46. [Google Scholar] [CrossRef]
- Tang, Z.; Ye, G.; Chen, X.; Pan, M.; Fu, J.; Fu, T.; Liu, Q.; Gao, Z.; Baldwin, D.S.; Hou, R. Peripheral proinflammatory cytokines in Chinese patients with generalised anxiety disorder. J. Affect. Disord. 2017, 225, 593–598. [Google Scholar] [CrossRef]
- Vaillant, A.A.J.; Qurie, A. Interleukin; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Thome, J.G.; Reeder, E.L.; Collins, S.M.; Gopalan, P.; Robson, M.J. Contributions of Interleukin-1 Receptor Signaling in Traumatic Brain Injury. Front. Behav. Neurosci. 2020, 13, 287. [Google Scholar] [CrossRef]
- Abboud, A.; Mi, Q.; Puccio, A.; Okonkwo, D.; Buliga, M.; Constantine, G.; Vodovotz, Y. Inflammation Following Traumatic Brain Injury in Humans: Insights from Data-Driven and Mechanistic Models into Survival and Death. Front. Pharmacol. 2016, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Morrison, F.G.; Miller, M.W.; Wolf, E.J.; Logue, M.W.; Maniates, H.; Kwasnik, D.; Cherry, J.D.; Svirsky, S.; Restaino, A.; Hildebrandt, A.; et al. Reduced interleukin 1A gene expression in the dorsolateral prefrontal cortex of individuals with PTSD and depression. Neurosci. Lett. 2019, 692, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Cheng, J.; Xiao, W.; Fan, K.; Gu, J.; Yu, B.; Yin, G.; Wu, J.; Ren, J.; et al. Impacts of Blast-Induced Traumatic Brain Injury on Expressions of Hepatic Cytochrome P450 1A2, 2B1, 2D1, and 3A2 in Rats. Cell. Mol. Neurobiol. 2017, 37, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ogłodek, E. Changes in the Serum Levels of Cytokines: IL-1β, IL-4, IL-8 and IL-10 in Depression with and without Posttraumatic Stress Disorder. Brain Sci. 2022, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, A.; Brennan, J.; Levin, H.S.; McCarthy, J.J.; Dash, P.K.; Redell, J.B.; Yamal, J.-M.; Robertson, C.S. Early versus Late Profiles of Inflammatory Cytokines after Mild Traumatic Brain Injury and Their Association with Neuropsychological Outcomes. J. Neurotrauma 2021, 38, 53–62. [Google Scholar] [CrossRef]
- Jiang, Q.; Wei, D.; He, X.; Gan, C.; Long, X.; Zhang, H. Phillyrin Prevents Neuroinflammation-Induced Blood–Brain Barrier Damage Following Traumatic Brain Injury via Altering Microglial Polarization. Front. Pharmacol. 2021, 12, 719823. [Google Scholar] [CrossRef]
- Pu, H.; Zheng, X.; Jiang, X.; Mu, H.; Xu, F.; Zhu, W.; Ye, Q.; Jizhang, Y.; Hitchens, T.K.; Shi, Y.; et al. Interleukin-4 improves white matter integrity and functional recovery after murine traumatic brain injury via oligodendroglial PPARγ. J. Cereb. Blood Flow Metab. 2021, 41, 511–529. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Buspavanich, P.; Adli, M.; Himmerich, H.; Berger, M.; Busche, M.; Schlattmann, P.; Bopp, S.; Bschor, T.; Richter, C.; Steinacher, B.; et al. Faster speed of onset of the depressive episode is associated with lower cytokine serum levels (IL-2, -4, -6, -10, TNF-α and IFN-γ) in patients with major depression. J. Psychiatr. Res. 2021, 141, 287–292. [Google Scholar] [CrossRef]
- Kiraly, D.D.; Horn, S.R.; Van Dam, N.T.; Costi, S.; Schwartz, J.; Kim-Schulze, S.; Patel, M.; Hodes, G.E.; Russo, S.J.; Merad, M.; et al. Altered peripheral immune profiles in treatment-resistant depression: Response to ketamine and prediction of treatment outcome. Transl. Psychiatry 2017, 7, e1065. [Google Scholar] [CrossRef]
- Freeman, L.C.; Ting, J.P.-Y. The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem. 2015, 136, 29–38. [Google Scholar] [CrossRef]
- Kerr, N.; Lee, S.W.; Perez-Barcena, J.; Crespi, C.; Ibañez, J.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W.; Vaccari, J.P.D.R. Inflammasome proteins as biomarkers of traumatic brain injury. PLoS ONE 2018, 13, e0210128. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, A.; Della Vedova, C.; Salani, F.; Viganotti, M.; D’Ippolito, M.; Caltagirone, C.; Formisano, R.; Sabatini, U.; Bossù, P. Increased Levels of Serum IL-18 Are Associated with the Long-Term Outcome of Severe Traumatic Brain Injury. Neuroimmunomodulation 2014, 21, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Song, A.-Q.; Gao, B.; Fan, J.-J.; Zhu, Y.-J.; Zhou, J.; Wang, Y.-L.; Xu, L.-Z.; Wu, W.-N. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J. Neuroinflammation 2020, 17, 178. [Google Scholar] [CrossRef]
- Ogłodek, E.A. The role of PON-1, GR, IL-18, and OxLDL in depression with and without posttraumatic stress disorder. Pharmacol. Rep. 2017, 69, 837–845. [Google Scholar] [CrossRef]
- Russell, K.L.; Berman, N.E.; Levant, B. Low brain DHA content worsens sensorimotor outcomes after TBI and decreases TBI-induced Timp1 expression in juvenile rats. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 97–105. [Google Scholar] [CrossRef]
- Foerstner, P.; Rehman, R.; Anastasiadou, S.; Haffner-Luntzer, M.; Sinske, D.; Ignatius, A.; Roselli, F.; Knoell, B. Neuroinflammation after Traumatic Brain Injury Is Enhanced in Activating Transcription Factor 3 Mutant Mice. J. Neurotrauma 2018, 35, 2317–2329. [Google Scholar] [CrossRef]
- Lorente, L. New Prognostic Biomarkers in Patients With Traumatic Brain Injury. Arch. Trauma Res. 2015, 4, e30165. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, K.; Zheng, P.; Fan, W.; Li, C.; Liu, H.; Shan, X. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of patients with moderate and severe traumatic brain injury. Neurol. India 2013, 61, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kang, Y.; Huang, L.; Wu, L.; Peng, Y. TIMP1 preserves the blood–brain barrier through interacting with CD63/integrin β1 complex and regulating downstream FAK/RhoA signaling. Acta Pharm. Sin. B 2020, 10, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Qi, K.; Yu, Y.; Lu, W.; Xu, W.; Yang, C.; Lin, Y. Analysis of Differentially Expressed Genes in the Dentate Gyrus and Anterior Cingulate Cortex in a Mouse Model of Depression. BioMed Res. Int. 2021, 2021, 5013565. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Sajja, V.S.; Statz, J.K.; Walker, L.P.B.; Gist, I.D.; Wilder, D.M.; Ahlers, S.T.; Long, J.B. Pulmonary injury risk curves and behavioral changes from blast overpressure exposures of varying frequency and intensity in rats. Sci Rep. 2020, 10, 16644. [Google Scholar] [CrossRef] [PubMed]
- Bergmann-Leitner, E.S.; Bobrov, A.G.; Bolton, J.S.; Rouse, M.D.; Heyburn, L.; Pavlovic, R.; Garry, B.I.; Alamneh, Y.; Long, J.; Swierczewski, B.; et al. Blast Waves Cause Immune System Dysfunction and Transient Bone Marrow Failure in a Mouse Model. Front. Bioeng. Biotechnol. 2022, 10, 821169. [Google Scholar] [CrossRef] [PubMed]
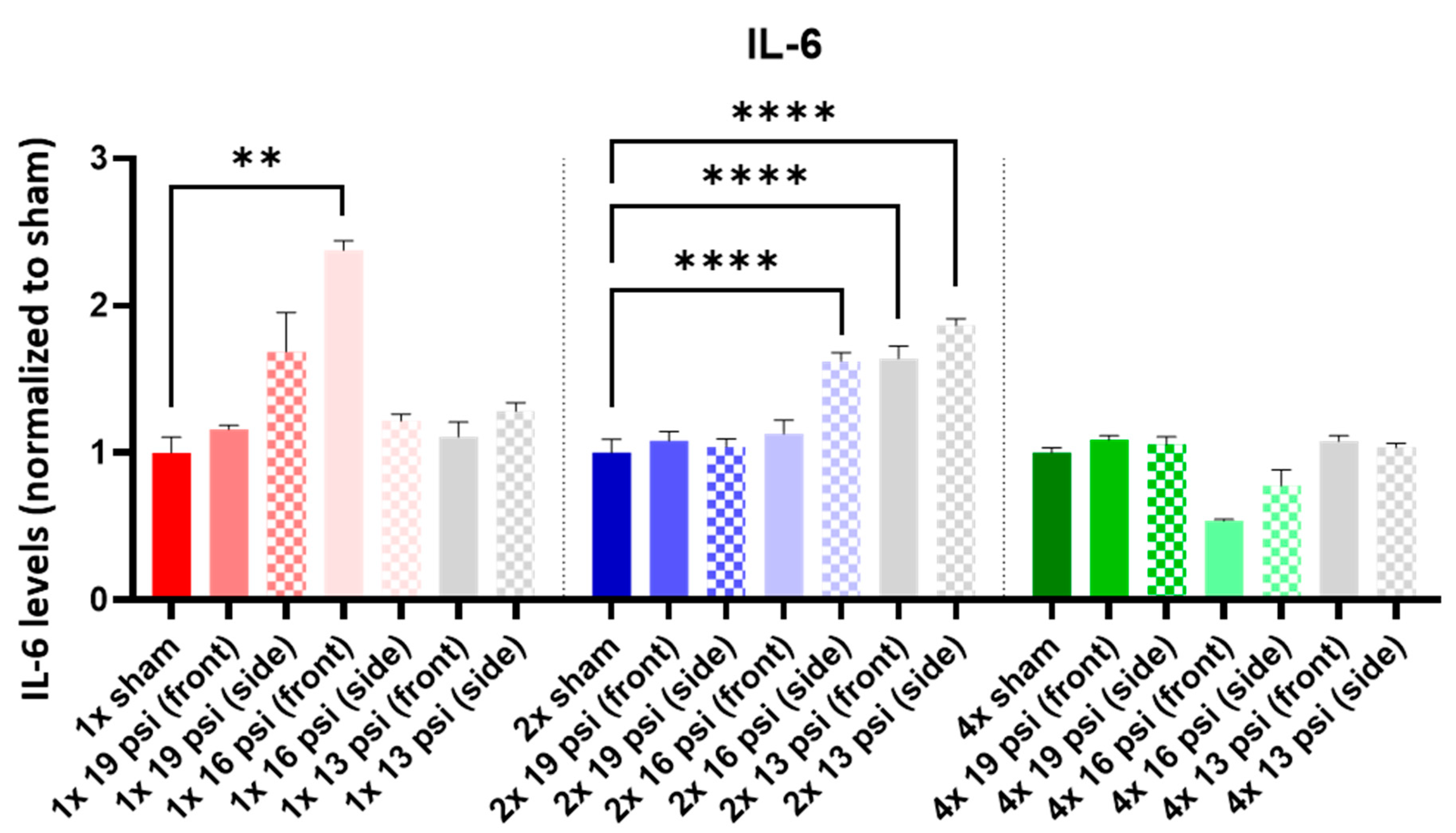
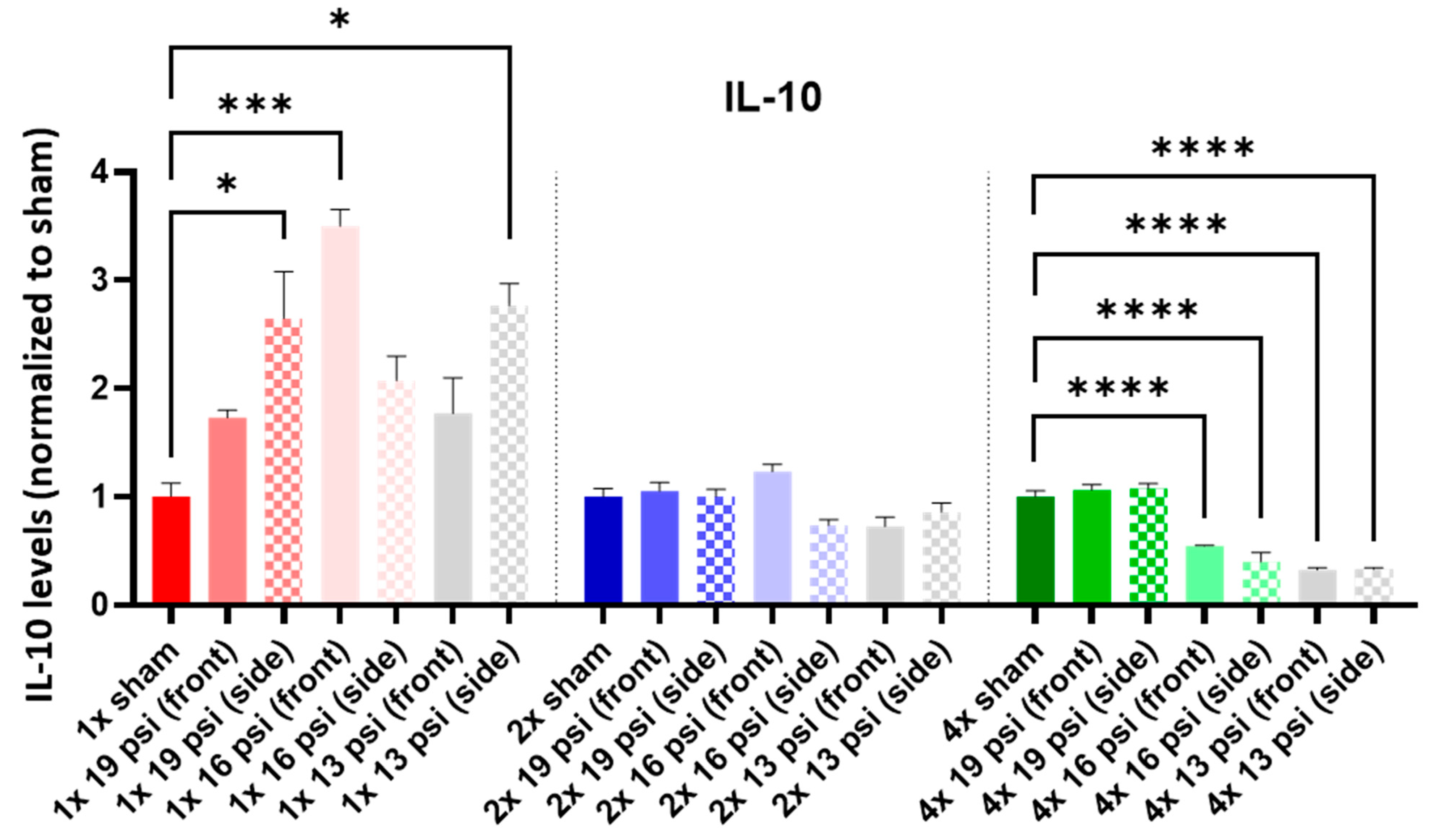
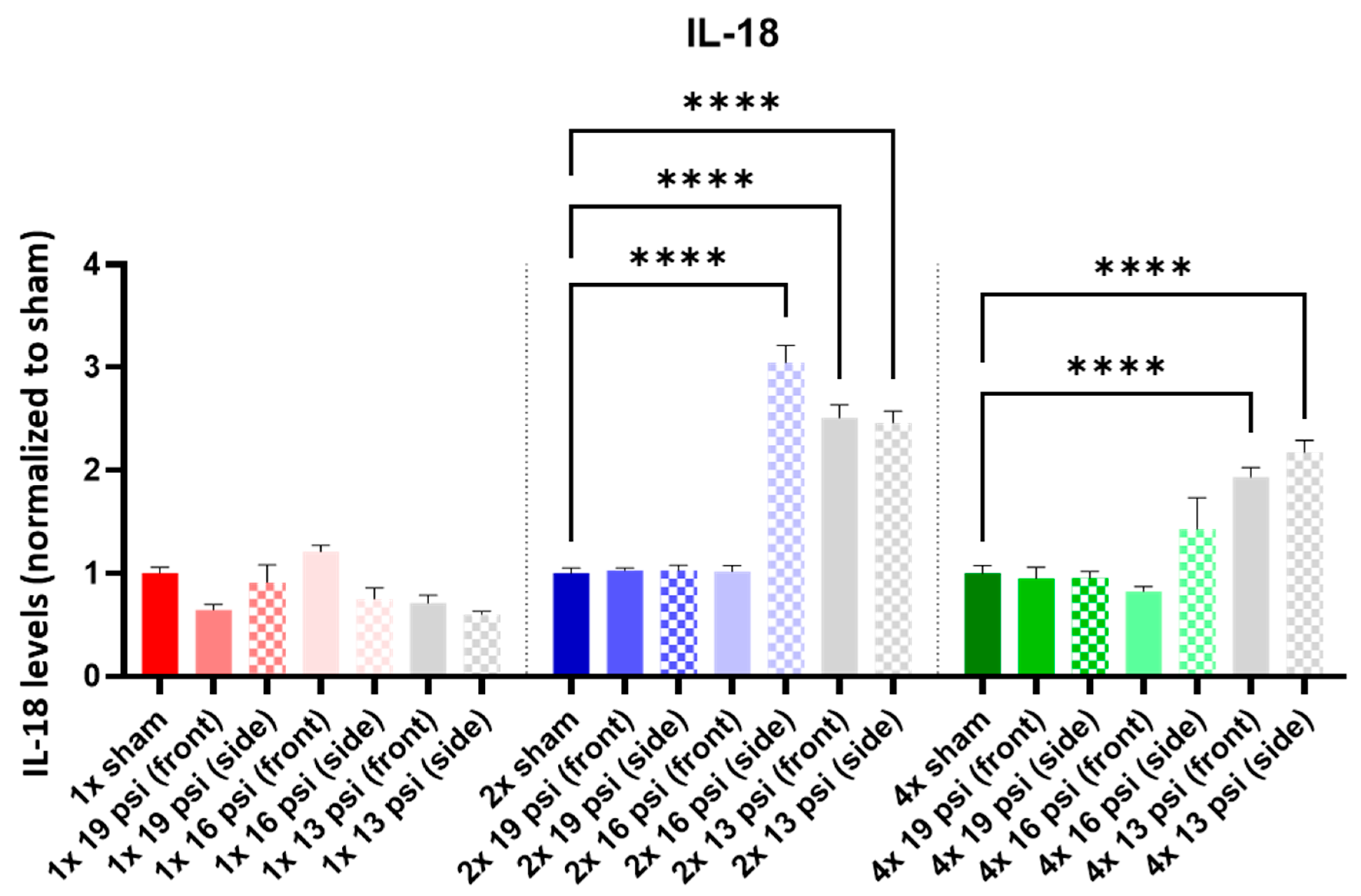

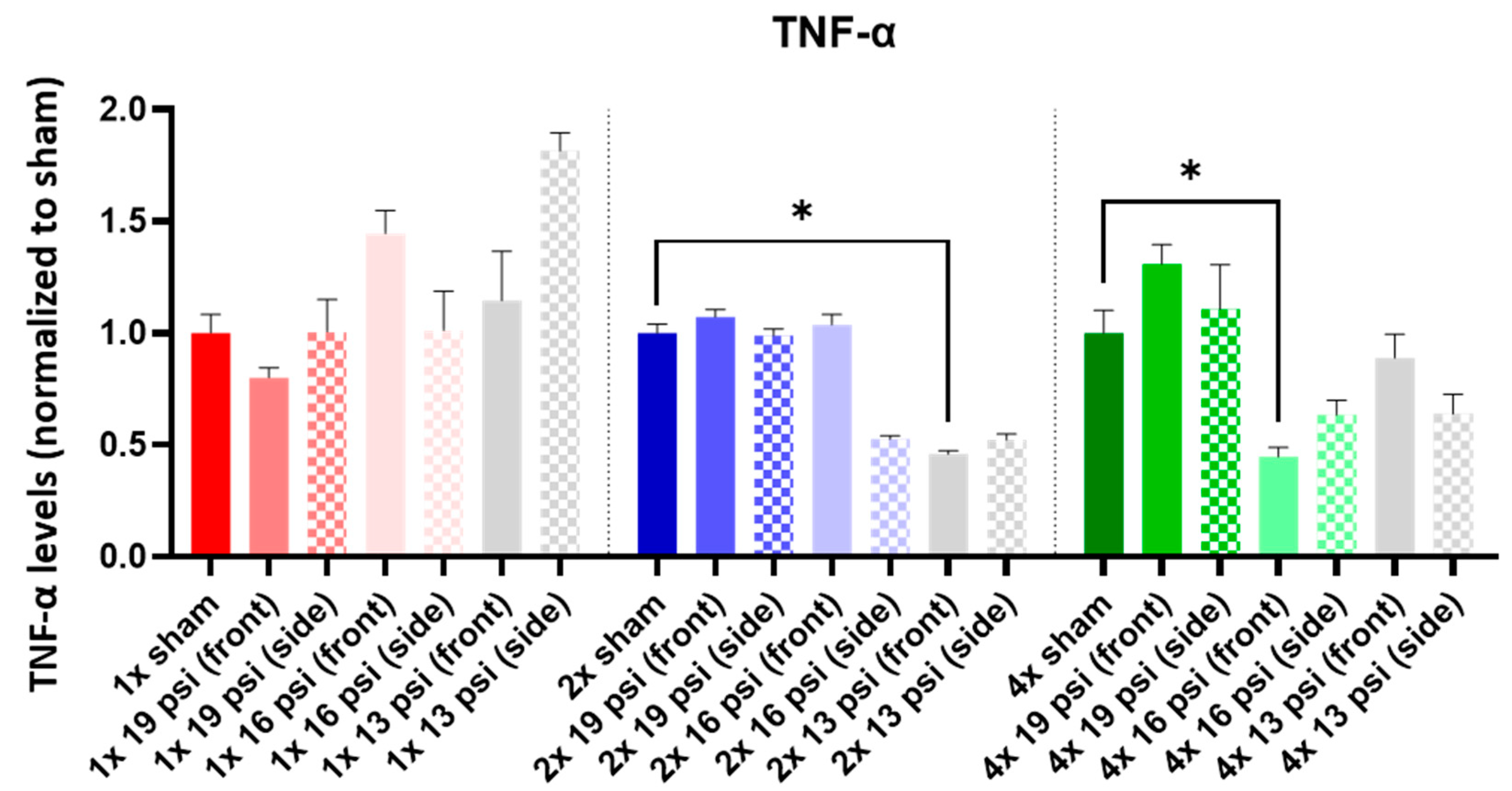
| 19 psi (Front) | 19 psi (Side) | 16 psi (Front) | 16 psi (Side) | 13 psi (Front) | 13 psi (Side) | |
|---|---|---|---|---|---|---|
| CXCL3 |  |  |  |  | ||
| CXCL2 |  | |||||
| ICAM-1 |  |  |  | |||
| IFN-γ |  |  |  |  | ||
| IL-1α | 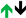 | 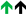 | ||||
| IL-1β | 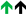 | 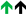 | ||||
| IL-2 |  |  |  |  | ||
| IL-4 |  |  | ||||
| IL-6 |  |  |  |  | ||
| IL-10 |  |  |  |  | 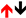 | |
| IL-18 | 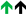 | 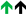 | 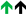 | |||
| TIMP1 | 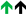 |  | 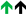 | |||
| TNF-α |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyburn, L.; Batuure, A.; Wilder, D.; Long, J.; Sajja, V.S. Neuroinflammation Profiling of Brain Cytokines Following Repeated Blast Exposure. Int. J. Mol. Sci. 2023, 24, 12564. https://doi.org/10.3390/ijms241612564
Heyburn L, Batuure A, Wilder D, Long J, Sajja VS. Neuroinflammation Profiling of Brain Cytokines Following Repeated Blast Exposure. International Journal of Molecular Sciences. 2023; 24(16):12564. https://doi.org/10.3390/ijms241612564
Chicago/Turabian StyleHeyburn, Lanier, Andrew Batuure, Donna Wilder, Joseph Long, and Venkatasivasai Sujith Sajja. 2023. "Neuroinflammation Profiling of Brain Cytokines Following Repeated Blast Exposure" International Journal of Molecular Sciences 24, no. 16: 12564. https://doi.org/10.3390/ijms241612564
APA StyleHeyburn, L., Batuure, A., Wilder, D., Long, J., & Sajja, V. S. (2023). Neuroinflammation Profiling of Brain Cytokines Following Repeated Blast Exposure. International Journal of Molecular Sciences, 24(16), 12564. https://doi.org/10.3390/ijms241612564






