Tackling Sleeping Sickness: Current and Promising Therapeutics and Treatment Strategies
Abstract
1. Introduction
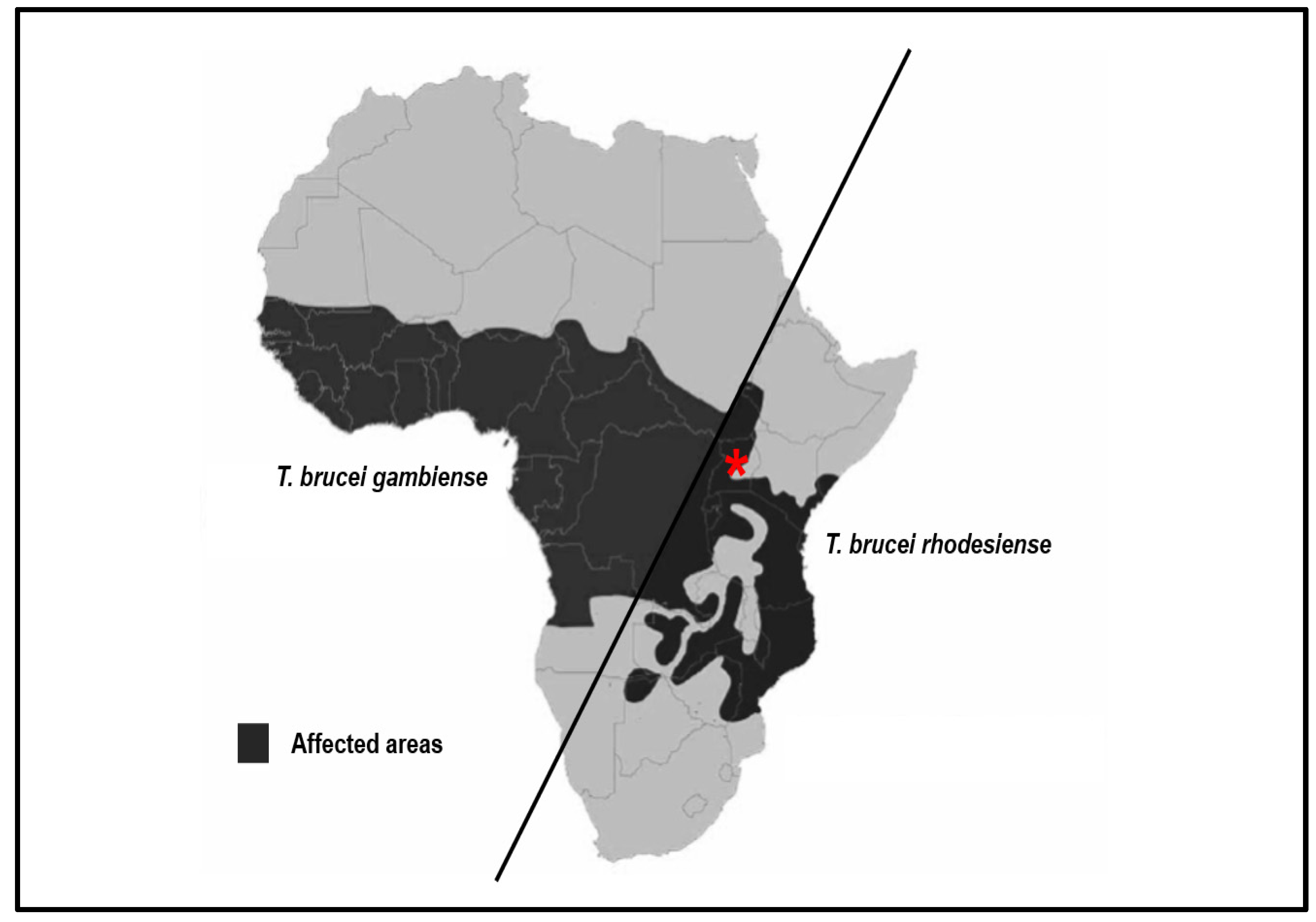
2. Disease Burden of Human African Trypanosomiasis
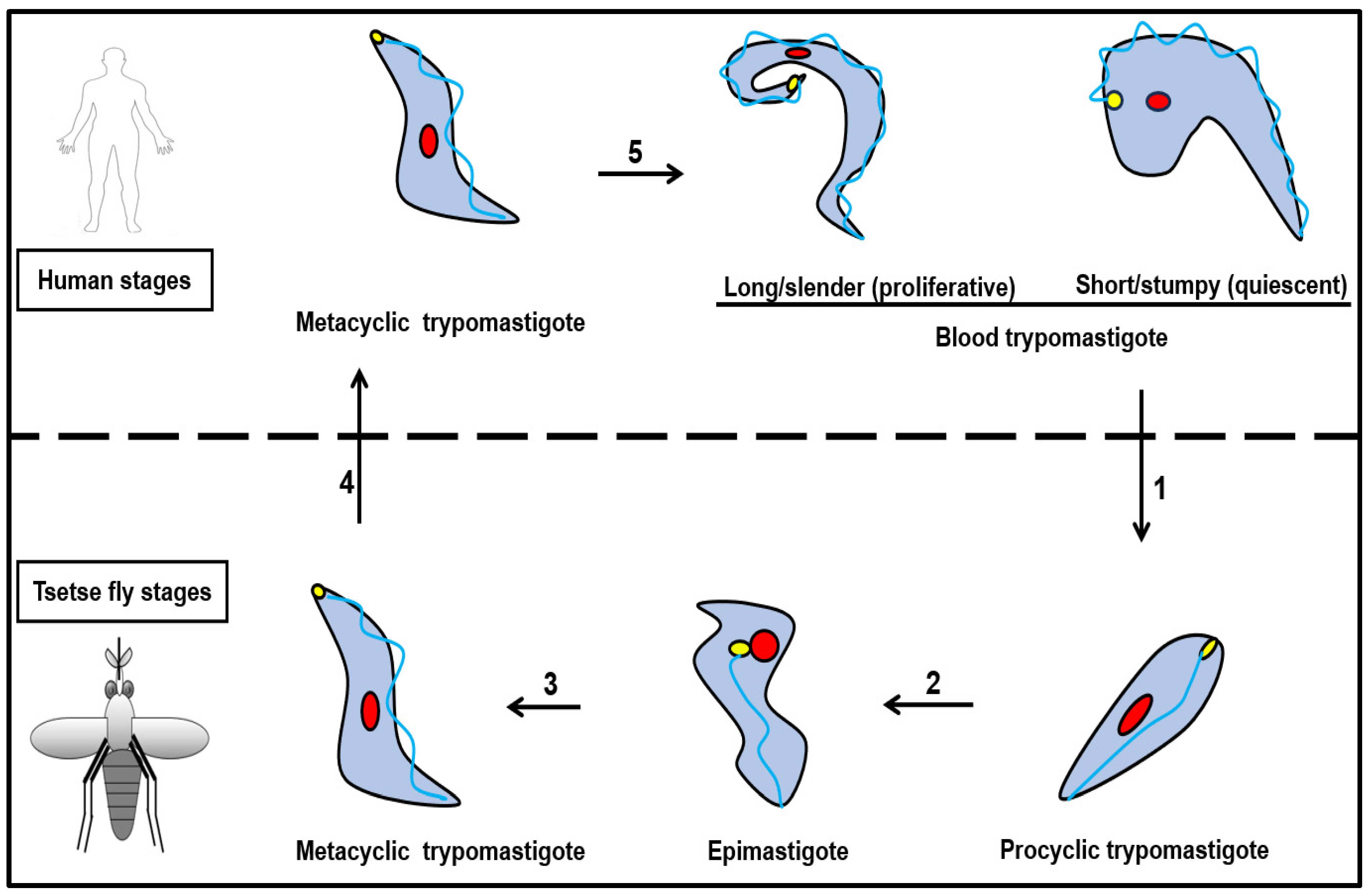
3. Lifecycle of Trypanosoma brucei
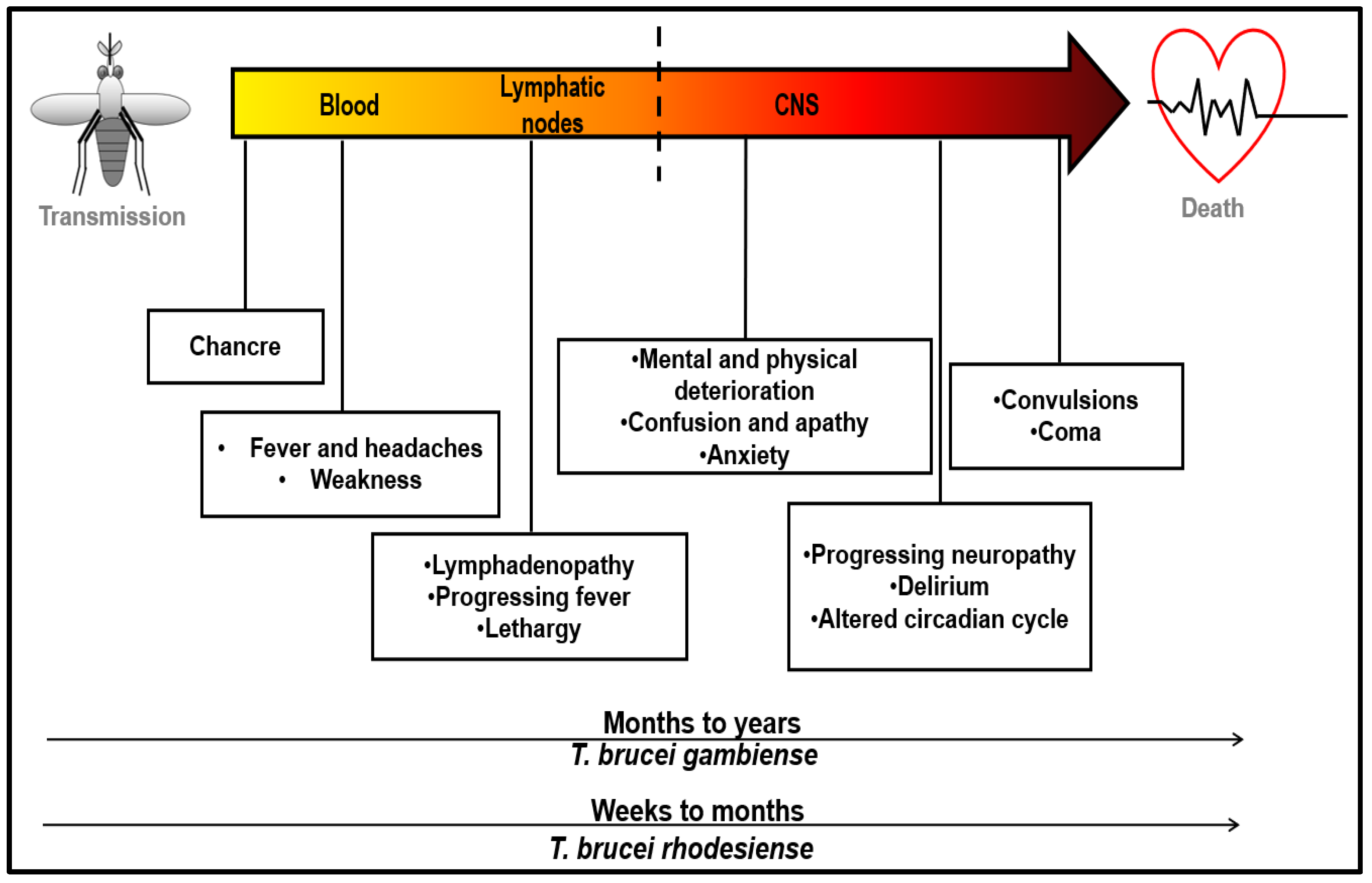
4. Symptoms and Disease Progression
5. Disease Diagnosis
6. Anti-Trypanosomal Drug Treatments and Resistance Mechanisms
6.1. Approved Drug Treatments
6.2. Drug Resistance Mechanisms
6.3. Physiological Challenges to Effective Drug Delivery
6.4. Recent Advances in Drug Development
6.5. Validated and Potential Drug Targets
6.5.1. N-Myristoyltransferase
6.5.2. Ubiquitination/Proteasome
6.5.3. Cyclic Adenosine Monophosphate-Specific Phosphodiesterases B1 and B2
6.5.4. Oxidative Stress/Polyamine Synthesis—Trypanothione System
6.5.5. RNA-Editing Ligase and Pteridine Ligase
6.5.6. Repurposing Anti-Cancer Drugs as Anti-Parasitic Treatments
6.5.7. Heat Shock Protein 70 and 90
Trypanosoma brucei Hsp70
Trypanosoma brucei Hsp90
6.5.8. Adenosine Analogues
7. Vaccine Development
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raper, J.; Portela, M.P.M.; Lugli, E.; Frevert, U.; Tomlinson, S. Trypanosome lytic factors: Novel mediators of human innate immunity. Curr. Opin. Microbiol. 2001, 4, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.H.; Souto-Padrón, T.; Dias, F.A.; Gomes, M.T.; Rodrigues, G.C.; Zimmermann, L.T.; e Silva, T.L.A.; Vermelho, A.B. Trypanosomatids: Odd Organisms, Devastating Diseases. Open Parasitol. J. 2010, 4, 30–59. [Google Scholar] [CrossRef]
- Alsan, M. The Effect of the TseTse Fly on African Development. Am. Econ. Rev. 2015, 105, 382–410. [Google Scholar] [CrossRef]
- Kennedy, P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013, 12, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar]
- Cayla, M.; Rojas, F.; Silvester, E.; Venter, F.; Matthews, K.R. African trypanosomes. Parasit. Vectors 2019, 12, 190. [Google Scholar]
- Holmes, P. Tsetse-transmitted trypanosomes—Their biology, disease impact and control. J. Invertebr. Pathol. 2013, 112, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Maudlin, I. African trypanosomiasis. Ann. Trop. Med. Parasitol. 2006, 100, 679–701. [Google Scholar] [CrossRef]
- Simarro, P.; Diarra, A.; Ruiz Postigo, J.A.; Franco, J.R.; Jannin, J.G. The Human African Trypanosomiasis Control and Surveillance Programme of the World Health Organization 2000–2009: The Way Forward. PLoS Negl. Trop. Dis. 2011, 5, e1007. [Google Scholar]
- Gruvel, J. Considérations générales sur la signification de la transmission mécanique des trypanosomoses chez le bétail. Int. J. Trop. Insect Sci. 1980, 1, 55–57. [Google Scholar] [CrossRef]
- Lindner, A.K.; Priotto, G. The Unknown Risk of Vertical Transmission in Sleeping Sickness—A Literature Review. PLoS Negl. Trop. Dis. 2010, 4, e783. [Google Scholar] [CrossRef] [PubMed]
- Simarro, P.; Franco, J.; Diarra, A.; Jannin, J. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014, 6, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E. Human African trypanosomiasis of the CNS: Current issues and challenges. J. Clin. Investig. 2004, 113, 496–504. [Google Scholar]
- Kennedy, P.G.E.; Rodgers, J. Clinical and Neuropathogenetic Aspects of Human African Trypanosomiasis. Front. Immunol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Torr, S.J.; Chamisa, A.; Mangwiro, T.N.C.; Vale, G.A. Where, When and Why Do Tsetse Contact Humans? Answers from Studies in a National Park of Zimbabwe. PLoS Negl. Trop. Dis. 2012, 6, e1791. [Google Scholar] [CrossRef]
- Simo, G.; Mbida, J.; Eyenga, V.; Asonganyi, T.; Njiokou, F.; Grébaut, P. Challenges towards the elimination of Human African Trypanosomiasis in the sleeping sickness focus of Campo in southern Cameroon. Parasit. Vectors 2014, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Berrang-Ford, L.; Odiit, M.; Maiso, F.; Waltner-Toews, D.; McDermott, J. Sleeping sickness in Uganda: Revisiting current and historical distributions. Afr. Health Sci. 2006, 6, 223–231. [Google Scholar]
- Berrang-Ford, L.; Wamboga, C.; Kakembo, A.S.L. Trypanososma brucei rhodesiense Sleeping Sickness, Uganda. Emerg. Infect. Dis. 2012, 18, 1686–1687. [Google Scholar] [CrossRef]
- Wamwiri, F.N.; Changasi, R.E. Tsetse Flies (Glossina) as Vectors of Human African Trypanosomiasis: A Review. BioMed Res. Int. 2016, 2016, 6201350. [Google Scholar] [CrossRef]
- Tirados, I.; Esterhuizen, J.; Kovacic, V.; Mangwiro, T.N.C.; Vale, G.A.; Hastings, I.; Solano, P.; Lehane, M.J.; Torr, S.J. Tsetse Control and Gambian Sleeping Sickness; Implications for Control Strategy. PLoS Negl. Trop. Dis. 2015, 9, e0003822. [Google Scholar] [CrossRef]
- Egeru, A.; Opio, J.; Siya, A.; Barasa, B.; Magaya, J.P.; Namaalwa, J.J. Tsetse Invasion as an Emerging Threat to Socioecological Resilience of Pastoral Communities in Karamoja, Uganda. Sustainability 2020, 12, 1599. [Google Scholar] [CrossRef]
- Louis, F.J.; Simarro, P.P. Rough start for the fight against sleeping sickness in French equatorial Africa. Med. Trop. Rev. Corps Sante Colon. 2005, 65, 251–257. [Google Scholar]
- Steverding, D. The history of African trypanosomiasis. Parasit. Vectors 2008, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Hide, G. History of Sleeping Sickness in East Africa. Clin. Microbiol. Rev. 1999, 12, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Pepin, J.; Stich, A.H.R. Human African trypanosomiasis: An emerging public health crisis. Br. Med. Bull. 1998, 54, 341–355. [Google Scholar] [CrossRef]
- Moore, A.; Richer, M. Re-emergence of epidemic sleeping sickness in southern Sudan. Trop. Med. Int. Health 2001, 6, 342–347. [Google Scholar] [CrossRef]
- Nieuwenhove, S.V.; Betu-Ku-Mesu, V.K.; Diabakana, P.M.; Declercq, J.; Bilenge, C.M.M. Sleeping sickness resurgence in the DRC: The past decade. Trop. Med. Int. Health 2001, 6, 335–341. [Google Scholar] [CrossRef]
- Stanghellini, A.; Josenando, T. The situation of sleeping sickness in Angola: A calamity. Trop. Med. Int. Health 2001, 6, 330–334. [Google Scholar] [CrossRef][Green Version]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Ruiz-Postigo, J.A.; Jannin, J.G. The journey towards elimination of gambiense human African trypanosomiasis: Not far, nor easy. Parasitology 2014, 141, 748–760. [Google Scholar] [CrossRef]
- WHO. Trypanosomiasis, Human African (Sleeping Sickness). 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 21 May 2022).
- Simarro, P.; Cecchi, G.; Franco, J.; Paone, M.; Diarra, A.; Ruiz-Postigo, J.; Fèvre, E.; Mattioli, R.; Jannin, M.; Ndung’u, J. Estimating and Mapping the Population at Risk of Sleeping Sickness. PLoS Negl. Trop. Dis. 2012, 6, e1859. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, F.; Lima, M.A.; Flevaud, L.; Ritmeijer, K. Human African Trypanosomiasis in Areas without Surveillance. Emerg. Infect. Dis. 2010, 16, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, D.; Massari, M.; Claudio, A.; Ermanno, G.; Claudio, F.; Brini, M.; Capatti, C.; Suter, F. African Sleeping Sickness in Tourists Returning from Tanzania: The First 2 Italian Cases from a Small Outbreak among European Travelers. Clin. Infect. Dis. 2002, 34, e18–e22. [Google Scholar] [PubMed]
- Meehl, G.; Stocker, T.; Collins, W.; Friedlingstein, P.; Gaye, T.; Gregory, J.; Kitoh, A.; Knutti, R.; Murphy, J.; Noda, A.; et al. IPCC, 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007; pp. 747–846. [Google Scholar]
- Urbaniak, M.D.; Guther, M.L.S.; Ferguson, M.A.J. Comparative SILAC proteomic analysis of Trypanosoma brucei bloodstream and procyclic lifecycle stages. PLoS ONE 2012, 7, e36619. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.E.G. History of sleeping sickness (African trypanosomiasis). Infect. Dis. Clin. N. Am. 2004, 18, 231–245. [Google Scholar] [CrossRef]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Chappuis, F.; Loutan, L.; Simarro, P.; Lejon, V.; Büscher, P. Options for Field Diagnosis of Human African Trypanosomiasis. Clin. Microbiol. Rev. 2005, 18, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Cappello, M.; Li, S.; Chen, X.; Li, C.-B.; Harrison, L.; Narashimhan, S.; Beard, C.B.; Aksoy, S. Tsetse thrombin inhibitor: Bloodmeal-induced expression of an anticoagulant in salivary glands and gut tissue of Glossina morsitans morsitans. Proc. Natl. Acad. Sci. USA 1998, 95, 14290–14295. [Google Scholar] [CrossRef]
- MacGregor, P.; Savill, N.J.; Hall, D.; Matthews, K.R. Transmission Stages Dominate Trypanosome Within-Host Dynamics during Chronic Infections. Cell Host Microbe 2011, 9, 310–318. [Google Scholar] [CrossRef]
- Reuner, B.; Vassella, E.; Yutzy, B.; Boshart, M. Cell density triggers slender to stumpy differentiation of Trypanosoma brucei bloodstream forms in culture. Mol. Biochem. Parasitol. 1997, 90, 269–280. [Google Scholar] [CrossRef]
- Seed, J.; Wenck, M. Role of the long slender to short stumpy transition in the life cycle of the african trypanosomes. Kinetoplastid Biol. Dis. 2003, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Mant, M.J.; Parker, K.R. Two Platelet Aggregation Inhibitors in Tsetse (Glossina) Saliva with Studies of Roles of Thrombin and Citrate in in Vitro Platelet Aggregation. Br. J. Haematol. 2008, 48, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Caljon, G.; De Ridder, K.; De Baetselier, P.; Coosemans, M.; Van Den Abbeele, J. Identification of a Tsetse Fly Salivary Protein with Dual Inhibitory Action on Human Platelet Aggregation. PLoS ONE 2010, 5, e9671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Silva, T.L.A.E.; Cronin, L.; Savage, A.F.; O’Neill, M.; Nerima, B.; Okedi, L.M.; Aksoy, S. Immunogenicity and Serological Cross-Reactivity of Saliva Proteins among Different Tsetse Species. PLoS Negl. Trop. Dis. 2015, 9, e0004038. [Google Scholar] [CrossRef]
- Trindade, S.; Rijo-Ferreira, F.; Carvalho, T.; Pinto-Neves, D.; Guegan, F.; Aresta-Branco, F.; Bento, F.; Young, S.A.; Pinto, A.; Van Den Abbeele, J.; et al. Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice. Cell Host Microbe 2016, 19, 837–848. [Google Scholar] [CrossRef]
- Hoare, C.A.; Wallace, F.G. Developmental Stages of Trypanosomatid Flagellates: A New Terminology. Nature 1966, 212, 1385–1386. [Google Scholar] [CrossRef]
- Wheeler, R.J.; Gluenz, E.; Gull, K. The Limits on Trypanosomatid Morphological Diversity. PLoS ONE 2013, 8, e79581. [Google Scholar] [CrossRef] [PubMed]
- Losos, G.J.; Ikede, B.O. Review of Pathology of Diseases in Domestic and Laboratory Animals Caused by Trypanosoma congolense, T. vivax, T. brucei, T. rhodesiense and T. gambiense. Vet. Pathol. 1972, 9, 1–79. [Google Scholar] [CrossRef]
- Caljon, G.; Van Reet, N.; De Trez, C.; Vermeersch, M.; Pérez-Morga, D.; Van Den Abbeele, J. The Dermis as a Delivery Site of Trypanosoma brucei for Tsetse Flies. PLoS Pathog. 2016, 12, e1005744. [Google Scholar] [CrossRef]
- Capewell, P.; Cren-Travaillé, C.; Marchesi, F.; Johnston, P.; Clucas, C.; Benson, R.A.; Gorman, T.-A.; Calvo-Alvarez, E.; Crouzols, A.; Jouvion, G.; et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. eLife 2016, 5, e17716. [Google Scholar] [CrossRef]
- Tanowitz, H.B.; Scherer, P.E.; Mota, M.M.; Figueiredo, L.M. Adipose Tissue: A Safe Haven for Parasites? Trends Parasitol. 2017, 33, 276–284. [Google Scholar] [PubMed]
- Poudyal, N.R.; Paul, K.S. Fatty acid uptake in Trypanosoma brucei: Host resources and possible mechanisms. Front. Cell. Infect. Microbiol. 2022, 12, 949409. [Google Scholar] [PubMed]
- Claes, F.; Vodnala, S.K.; van Reet, N.; Boucher, N.; Lunden-Miguel, H.; Baltz, T.; Goddeeris, B.M.; Büscher, P.; Rottenberg, M.E. Bioluminescent Imaging of Trypanosoma brucei Shows Preferential Testis Dissemination Which May Hamper Drug Efficacy in Sleeping Sickness. PLoS Negl. Trop. Dis. 2009, 3, e486. [Google Scholar] [CrossRef] [PubMed]
- Biteau, N.; Asencio, C.; Izotte, J.; Rousseau, B.; Fèvre, M.; Pillay, D.; Baltz, T. Trypanosoma brucei gambiense Infections in Mice Lead to Tropism to the Reproductive Organs, and Horizontal and Vertical Transmission. PLoS Negl. Trop. Dis. 2016, 10, e0004350. [Google Scholar] [CrossRef]
- Butter, F.; Bucerius, F.; Michel, M.; Cicova, Z.; Mann, M.; Janzen, C.J. Comparative proteomics of two life cycle stages of stable isotope-labeled Trypanosoma brucei reveals novel components of the parasite’s host adaptation machinery. Mol. Cell. Proteomics MCP 2013, 12, 172–179. [Google Scholar] [CrossRef]
- Szöör, B.; Haanstra, J.R.; Gualdrón-López, M.; Michels, P.A. Evolution, dynamics and specialized functions of glycosomes in metabolism and development of trypanosomatids. Curr. Opin. Microbiol. 2014, 22, 79–87. [Google Scholar] [CrossRef]
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related protozoan pathogens, different diseases. J. Clin. Investig. 2008, 118, 1301–1310. [Google Scholar] [CrossRef]
- MacLean, L.M.; Odiit, M.; Chisi, J.E.; Kennedy, P.G.E.; Sternberg, J.M. Focus–Specific Clinical Profiles in Human African Trypanosomiasis Caused by Trypanosoma brucei rhodesiense. PLoS Negl. Trop. Dis. 2010, 4, e906. [Google Scholar] [CrossRef]
- Kennedy, P.G.E. The continuing problem of human African trypanosomiasis (sleeping sickness). Ann. Neurol. 2008, 64, 116–126. [Google Scholar] [CrossRef]
- Bisser, S.; Lejon, V.; Preux, P.M.; Bouteille, B.; Stanghellini, A.; Jauberteau, M.O.; Büscher, P.; Dumas, M. Blood–cerebrospinal fluid barrier and intrathecal immunoglobulins compared to field diagnosis of central nervous system involvement in sleeping sickness. J. Neurol. Sci. 2002, 193, 127–135. [Google Scholar] [CrossRef]
- Buguet, A.; Bisser, S.; Josenando, T.; Chapotot, F.; Cespuglio, R. Sleep structure: A new diagnostic tool for stage determination in sleeping sickness. Acta Trop. 2005, 93, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.; Boudot, C.; Courtioux, B. Overview of the Diagnostic Methods Used in the Field for Human African Trypanosomiasis: What Could Change in the Next Years? BioMed Res. Int. 2015, 2015, 583262. [Google Scholar] [PubMed]
- Stich, A. Human African trypanosomiasis. BMJ 2002, 325, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A. An Overview of Trypanosoma brucei Infections: An Intense Host–Parasite Interaction. Front. Microbiol. 2016, 7, 2126. [Google Scholar] [PubMed]
- de Raadt, P.; Koten, J.W. Myocarditis in Rhodesiense trypanosomiasis. East Afr. Med. J. 1968, 45, 128–132. [Google Scholar]
- Petzke, F.; Heppner, C.; Mbulamberi, D.; Winkelmann, W.; Chrousos, G.P.; Allolio, B.; Reincke, M. Hypogonadism in Rhodesian sleeping sickness: Evidence for acute and chronic dysfunction of the hypothalamic-pituitary-gonadal axis. Fertil. Steril. 1996, 65, 68–75. [Google Scholar]
- Kuepfer, I.; Hhary, E.; Allan, M.; Edielu, A.; Burri, C.; Blum, J. Clinical Presentation of T.b. rhodesiense Sleeping Sickness in Second Stage Patients from Tanzania and Uganda. PLoS Negl. Trop. Dis. 2011, 5, e968. [Google Scholar] [CrossRef]
- Büscher, P.; Mumba Ngoyi, D.; Kaboré, J.; Lejon, V.; Robays, J.; Jamonneau, V.; Bebronne, N.; Van der Veken, W.; Biéler, S. Improved Models of Mini Anion Exchange Centrifugation Technique (mAECT) and Modified Single Centrifugation (MSC) for Sleeping Sickness Diagnosis and Staging. PLoS Negl. Trop. Dis. 2009, 3, e471. [Google Scholar] [CrossRef]
- Camara, M.; Camara, O.; Ilboudo, H.; Sakande, H.; Kaboré, J.; N’Dri, L.; Jamonneau, V.; Bucheton, B. Sleeping sickness diagnosis: Use of buffy coats improves the sensitivity of the mini anion exchange centrifugation test: mAECT-bc and sleeping sickness diagnostic. Trop. Med. Int. Health 2010, 15, 796–799. [Google Scholar]
- Truc, P.; Lejon, V.; Magnus, E.; Jamonneau, V.; Nangouma, A.; Verloo, D.; Penchenier, L.; Büscher, P. Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T b gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in West and Central Africa. Bull. World Health Organ. 2002, 80, 882–886. [Google Scholar]
- Bisser, S.; Lumbala, C.; Nguertoum, E.; Kande, V.; Flevaud, L.; Vatunga, G.; Boelaert, M.; Büscher, P.; Josenando, T.; Bessell, P.R.; et al. Sensitivity and Specificity of a Prototype Rapid Diagnostic Test for the Detection of Trypanosoma brucei gambiense Infection: A Multi-centric Prospective Study. PLoS Negl. Trop. Dis. 2016, 10, e0004608. [Google Scholar]
- Bisser, S.; N’Siesi, F.; Lejon, V.; Preux, P.; Van Nieuwenhove, S.; Miaka Mia Bilenge, C.; Büscher, P. Equivalence Trial of Melarsoprol and Nifurtimox Monotherapy and Combination Therapy for the Treatment of Second-Stage Trypanosoma brucei gambiense Sleeping Sickness. J. Infect. Dis. 2007, 195, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar]
- Kansiime, F.; Adibaku, S.; Wamboga, C.; Idi, F.; Kato, C.D.; Yamuah, L.; Vaillant, M.; Kioy, D.; Olliaro, P.; Matovu, E. A multicentre, randomised, non-inferiority clinical trial comparing a nifurtimox-eflornithine combination to standard eflornithine monotherapy for late stage Trypanosoma brucei gambiense human African trypanosomiasis in Uganda. Parasit. Vectors 2018, 11, 105. [Google Scholar]
- Munoz-Calderon, A.; Díaz-Bello, Z.; Ramírez, J.; Noya, O.; de Noya, B. Nifurtimox response of Trypanosoma cruzi isolates from an outbreak of Chagas disease in Caracas, Venezuela. J. Vector Borne Dis. 2019, 56, 237. [Google Scholar]
- Masocha, W.; Rottenberg, M.E.; Kristensson, K. Migration of African trypanosomes across the blood–brain barrier. Physiol. Behav. 2007, 92, 110–114. [Google Scholar] [CrossRef]
- Priotto, G.; Pinoges, L.; Fursa, I.B.; Burke, B.; Nicolay, N.; Grillet, G.; Hewison, C.; Balasegaram, M. Safety and effectiveness of first line eflornithine for Trypanosoma brucei gambiense sleeping sickness in Sudan: Cohort study. BMJ 2008, 336, 705–708. [Google Scholar] [PubMed]
- Sanderson, L.; Dogruel, M.; Rodgers, J.; De Koning, H.P.; Thomas, S.A. Pentamidine Movement across the Murine Blood-Brain and Blood-Cerebrospinal Fluid Barriers: Effect of Trypanosome Infection, Combination Therapy, P-Glycoprotein, and Multidrug Resistance-Associated Protein. J. Pharmacol. Exp. Ther. 2009, 329, 967–977. [Google Scholar] [PubMed]
- Singaro, J.R.S.; Yapo, F.B.; Doua, F.; Miezan, T.W.; Baltz, T. The Efficacy of Pentamidine in the Treatment of Early-Late Stage Trypanosoma brucei gambiense Trypanosomiasis *. Am. J. Trop. Med. Hyg. 1996, 55, 586–588. [Google Scholar]
- Sekhar, G.N.; Georgian, A.R.; Sanderson, L.; Vizcay-Barrena, G.; Brown, R.C.; Muresan, P.; Fleck, R.A.; Thomas, S.A. Organic cation transporter 1 (OCT1) is involved in pentamidine transport at the human and mouse blood-brain barrier (BBB). PLoS ONE 2017, 12, e0173474. [Google Scholar] [CrossRef]
- Gehrig, S.; Efferth, T. Development of drug resistance in Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. Treatment of human African trypanosomiasis with natural products (Review). Int. J. Mol. Med. 2008, 22, 411–419. [Google Scholar]
- Barrett, M.P.; Boykin, D.W.; Brun, R.; Tidwell, R.R. Human African trypanosomiasis: Pharmacological re-engagement with a neglected disease: Drugs for human African trypanosomiasis. Br. J. Pharmacol. 2007, 152, 1155–1171. [Google Scholar]
- Van Bogaert, I.; Haemers, A. Eflornithine: A new drug in the treatment of sleeping sickness. Pharm. Weekbl. 1989, 11, 69–75. [Google Scholar]
- Barrett, S.V.; Barrett, M.P. Anti-sleeping sickness drugs and cancer chemotherapy. Parasitol. Today Pers. Ed 2000, 16, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.S.; Comini, M.A.; Resende, B.V.; Santi, A.M.M.; Zoboli, A.P.; Moreira, D.S.; Murta, S.M.F. Ornithine decarboxylase or gamma-glutamylcysteine synthetase overexpression protects Leishmania (Vianna) guyanensis against antimony. Exp. Parasitol. 2017, 175, 36–43. [Google Scholar] [PubMed]
- Ariyanayagam, M.R.; Oza, S.L.; Guther, M.L.S.; Fairlamb, A.H. Phenotypic analysis of trypanothione synthetase knockdown in the African trypanosome. Biochem. J. 2005, 391, 425–432. [Google Scholar] [CrossRef]
- Meyskens, F.L.; Gerner, E.W. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 945–951. [Google Scholar]
- Priotto, G.; Kasparian, S.; Mutombo, W.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Baudin, E.; Buard, V.; Kazadi-Kyanza, S.; et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicentre, randomised, phase III, non-inferiority trial. Lancet 2009, 374, 56–64. [Google Scholar]
- Schmidt, R.S.; Macêdo, J.P.; Steinmann, M.E.; Salgado, A.G.; Bütikofer, P.; Sigel, E.; Rentsch, D.; Mäser, P. Transporters of Trypanosoma brucei —Phylogeny, physiology, pharmacology. FEBS J. 2018, 285, 1012–1023. [Google Scholar] [CrossRef]
- Hassan, H.F.; Coombs, G.H. Purine and pyrimidine metabolism in parasitic protozoa. FEMS Microbiol. Rev. 1988, 4, 47–83. [Google Scholar]
- Chaudhary, K.; Roos, D.S. Protozoan genomics for drug discovery. Nat. Biotechnol. 2005, 23, 1089–1091. [Google Scholar] [CrossRef]
- Alsford, S.; Field, M.C.; Horn, D. Receptor-mediated endocytosis for drug delivery in African trypanosomes: Fulfilling Paul Ehrlich’s vision of chemotherapy. Trends Parasitol. 2013, 29, 207–212. [Google Scholar]
- Bridges, D.J.; Gould, M.K.; Nerima, B.; Mäser, P.; Burchmore, R.J.S.; De Koning, H.P. Loss of the High-Affinity Pentamidine Transporter Is Responsible for High Levels of Cross-Resistance between Arsenical and Diamidine Drugs in African Trypanosomes. Mol. Pharmacol. 2007, 71, 1098–1108. [Google Scholar] [CrossRef]
- Baker, N.; Glover, L.; Munday, J.C.; Aguinaga Andrés, D.; Barrett, M.P.; de Koning, H.P.; Horn, D. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl. Acad. Sci. USA 2012, 109, 10996–11001. [Google Scholar] [CrossRef]
- Matovu, E.; Stewart, M.L.; Geiser, F.; Brun, R.; Mäser, P.; Wallace, L.J.M.; Burchmore, R.J.; Enyaru, J.C.K.; Barrett, M.P.; Kaminsky, R.; et al. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2003, 2, 1003–1008. [Google Scholar] [CrossRef]
- Munday, J.C.; Eze, A.A.; Baker, N.; Glover, L.; Clucas, C.; Aguinaga Andres, D.; Natto, M.J.; Teka, I.A.; McDonald, J.; Lee, R.S.; et al. Trypanosoma brucei aquaglyceroporin 2 is a high-affinity transporter for pentamidine and melaminophenyl arsenic drugs and the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P. Uptake of Pentamidine in Trypanosoma brucei brucei is Mediated by Three Distinct Transporters: Implications for Cross-Resistance with Arsenicals. Mol. Pharmacol. 2001, 59, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.C.; Settimo, L.; De Koning, H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front. Pharmacol. 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Bassarak, B.; Uzcátegui, N.L.; Schönfeld, C.; Duszenko, M. Functional Characterization of Three Aquaglyceroporins from Trypanosoma brucei in Osmoregulation and Glycerol Transport. Cell. Physiol. Biochem. 2011, 27, 411–420. [Google Scholar] [CrossRef]
- Pyana Pati, P.; Van Reet, N.; Mumba Ngoyi, D.; Ngay Lukusa, I.; Karhemere Bin Shamamba, S.; Büscher, P. Melarsoprol Sensitivity Profile of Trypanosoma brucei gambiense Isolates from Cured and Relapsed Sleeping Sickness Patients from the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2014, 8, e3212. [Google Scholar] [CrossRef]
- Graf, F.E.; Baker, N.; Munday, J.C.; De Koning, H.P.; Horn, D.; Mäser, P. Chimerization at the AQP2–AQP3 locus is the genetic basis of melarsoprol–pentamidine cross-resistance in clinical Trypanosoma brucei gambiense isolates. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 65–68. [Google Scholar] [CrossRef]
- Quintana, J.F.; Bueren-Calabuig, J.; Zuccotto, F.; De Koning, H.P.; Horn, D.; Field, M.C. Instability of aquaglyceroporin (AQP) 2 contributes to drug resistance in Trypanosoma brucei. PLoS Negl. Trop. Dis. 2020, 14, e0008458. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.H.; Munday, J.C.; Campagnaro, G.D.; Gurvic, D.; Svensson, F.; Okpara, C.E.; Kumar, A.; Quintana, J.; Martin Abril, M.E.; Milić, P.; et al. Positively selected modifications in the pore of TbAQP2 allow pentamidine to enter Trypanosoma brucei. eLife 2020, 9, e56416. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Baker, N.; Rothert, M.; Henke, B.; Jeacock, L.; Horn, D.; Beitz, E. Pentamidine Is Not a Permeant but a Nanomolar Inhibitor of the Trypanosoma brucei Aquaglyceroporin-2. PLoS Pathog. 2016, 12, e1005436. [Google Scholar] [CrossRef] [PubMed]
- Unciti-Broceta, J.D.; Arias, J.L.; Maceira, J.; Soriano, M.; Ortiz-González, M.; Hernández-Quero, J.; Muñóz-Torres, M.; De Koning, H.P.; Magez, S.; Garcia-Salcedo, J.A. Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis. PLoS Pathog. 2015, 11, e1004942. [Google Scholar] [CrossRef]
- Stijlemans, B.; Conrath, K.; Cortez-Retamozo, V.; Van Xong, H.; Wyns, L.; Senter, P.; Revets, H.; De Baetselier, P.; Muyldermans, S.; Magez, S. Efficient Targeting of Conserved Cryptic Epitopes of Infectious Agents by Single Domain Antibodies. J. Biol. Chem. 2004, 279, 1256–1261. [Google Scholar] [CrossRef]
- Jeacock, L.; Baker, N.; Wiedemar, N.; Mäser, P.; Horn, D. Aquaglyceroporin-null trypanosomes display glycerol transport defects and respiratory-inhibitor sensitivity. PLoS Pathog. 2017, 13, e1006307. [Google Scholar] [CrossRef]
- Zoltner, M.; Campagnaro, G.D.; Taleva, G.; Burrell, A.; Cerone, M.; Leung, K.-F.; Achcar, F.; Horn, D.; Vaughan, S.; Gadelha, C.; et al. Suramin exposure alters cellular metabolism and mitochondrial energy production in African trypanosomes. J. Biol. Chem. 2020, 295, 8331–8347. [Google Scholar] [CrossRef]
- Wiedemar, N.; Graf, F.E.; Zwyer, M.; Ndomba, E.; Kunz Renggli, C.; Cal, M.; Schmidt, R.S.; Wenzler, T.; Mäser, P. Beyond immune escape: A variant surface glycoprotein causes suramin resistance in Trypanosoma brucei: Suramin resistance in T. brucei. Mol. Microbiol. 2018, 107, 57–67. [Google Scholar] [CrossRef]
- Zeelen, J.; Van Straaten, M.; Verdi, J.; Hempelmann, A.; Hashemi, H.; Perez, K.; Jeffrey, P.D.; Hälg, S.; Wiedemar, N.; Mäser, P.; et al. Structure of trypanosome coat protein VSGsur and function in suramin resistance. Nat. Microbiol. 2021, 6, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Wiedemar, N.; Zwyer, M.; Zoltner, M.; Cal, M.; Field, M.C.; Mäser, P. Expression of a specific variant surface glycoprotein has a major impact on suramin sensitivity and endocytosis in Trypanosoma brucei. FASEB BioAdv. 2019, 1, 595–608. [Google Scholar] [CrossRef]
- Mutuku, C.N.; Bateta, R.; Rono, M.K.; Njunge, J.M.; Awuoche, E.O.; Ndung’u, K.; Mang’era, C.M.; Akoth, M.O.; Adung’a, V.O.; Ondigo, B.N.; et al. Physiological and proteomic profiles of Trypanosoma brucei rhodesiense parasite isolated from suramin responsive and non-responsive HAT patients in Busoga, Uganda. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 57–67. [Google Scholar] [CrossRef]
- Vincent, I.M.; Creek, D.; Watson, D.G.; Kamleh, M.A.; Woods, D.J.; Wong, P.E.; Burchmore, R.J.S.; Barrett, M.P. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog. 2010, 6, e1001204. [Google Scholar] [CrossRef]
- Crilly, N.P.; Mugnier, M.R. Thinking outside the blood: Perspectives on tissue-resident Trypanosoma brucei. PLoS Pathog. 2021, 17, e1009866. [Google Scholar] [CrossRef]
- De Rycker, M.; Wyllie, S.; Horn, D.; Read, K.D.; Gilbert, I.H. Anti-trypanosomatid drug discovery: Progress and challenges. Nat. Rev. Microbiol. 2023, 21, 35–50. [Google Scholar] [CrossRef]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial persister cell formation and dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Blanchard, N.; Besteiro, S. The Bradyzoite: A Key Developmental Stage for the Persistence and Pathogenesis of Toxoplasmosis. Pathogens 2020, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.F.; Jayawardhana, S.; Lewis, M.D.; Taylor, M.C.; Kelly, J.M. Biological factors that impinge on Chagas disease drug development. Parasitology 2017, 144, 1871–1880. [Google Scholar] [CrossRef]
- Sánchez-Valdéz, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. eLife 2018, 7, e34039. [Google Scholar] [CrossRef]
- Silva Pereira, S.; Trindade, S.; De Niz, M.; Figueiredo, L.M. Tissue tropism in parasitic diseases. Open Biol. 2019, 9, 190036. [Google Scholar] [CrossRef]
- Trindade, S.; De Niz, M.; Costa-Sequeira, M.; Bizarra-Rebelo, T.; Bento, F.; Dejung, M.; Narciso, M.V.; López-Escobar, L.; Ferreira, J.; Butter, F.; et al. Slow growing behavior in African trypanosomes during adipose tissue colonization. Nat. Commun. 2022, 13, 7548. [Google Scholar] [CrossRef]
- Machado, H.; Bizarra-Rebelo, T.; Costa-Sequeira, M.; Trindade, S.; Carvalho, T.; Rijo-Ferreira, F.; Rentroia-Pacheco, B.; Serre, K.; Figueiredo, L.M. Trypanosoma brucei triggers a broad immune response in the adipose tissue. PLoS Pathog. 2021, 17, e1009933. [Google Scholar] [CrossRef]
- Reuter, C.; Imdahl, F.; Hauf, L.; Vafadarnejad, E.; Fey, P.; Finger, T.; Walles, H.; Saliba, A.-E.; Groeber-Becker, F.; Engstler, M. Vector-borneTrypanosoma brucei parasites develop in artificial human skin and persist as skin tissue forms. bioRxiv 2021. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First Global Approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Ortiz, J.F.; Fabara, S.P.; Eissa-Garcés, A.; Reddy, D.; Collins, K.D.; Tirupathi, R. Efficacy and Toxicity of Fexinidazole and Nifurtimox Plus Eflornithine in the Treatment of African Trypanosomiasis: A Systematic Review. Cureus 2021, 13, e16881. [Google Scholar] [PubMed]
- Kande Betu Ku Mesu, V.; Mutombo Kalonji, W.; Bardonneau, C.; Valverde Mordt, O.; Ngolo Tete, D.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Mahenzi Mbembo, H.; et al. Oral fexinidazole for stage 1 or early stage 2 African Trypanosoma brucei gambiense trypanosomiasis: A prospective, multicentre, open-label, cohort study. Lancet Glob. Health 2021, 9, e999–e1008. [Google Scholar]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.Y.; Wyllie, S.; Patterson, S.; Oza, S.L.; Read, K.D.; Fairlamb, A.H. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 2893–2900. [Google Scholar] [CrossRef]
- Alsford, S.; Eckert, S.; Baker, N.; Glover, L.; Sanchez-Flores, A.; Leung, K.F.; Turner, D.J.; Field, M.C.; Berriman, M.; Horn, D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature 2012, 482, 232–236. [Google Scholar] [CrossRef]
- Thomas, J.A.; Baker, N.; Hutchinson, S.; Dominicus, C.; Trenaman, A.; Glover, L.; Alsford, S.; Horn, D. Insights into antitrypanosomal drug mode-of-action from cytology-based profiling. PLoS Negl. Trop. Dis. 2018, 12, e0006980. [Google Scholar] [CrossRef] [PubMed]
- Drugs for Neglected Diseases Initiative. Fexinidazole for T. brucei. DNDi—Best Science for the Most Neglected. 16 May 2023. Available online: https://dndi.org/research-development/portfolio/fexinidazole-tb-rhodesiense/ (accessed on 5 May 2023).
- Torrico, F.; Gascón, J.; Ortiz, L.; Pinto, J.; Rojas, G.; Palacios, A.; Barreira, F.; Blum, B.; Schijman, A.G.; Vaillant, M.; et al. A Phase 2, Randomized, Multicenter, Placebo-Controlled, Proof-of-Concept Trial of Oral Fexinidazole in Adults With Chronic Indeterminate Chagas Disease. Clin. Infect. Dis. 2023, 76, e1186–e1194. [Google Scholar] [CrossRef] [PubMed]
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Mäser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.S.; Barrett, M.P. New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story. Trop. Med. Infect. Dis. 2020, 5, 29. [Google Scholar] [PubMed]
- Waithaka, A.; Clayton, C. Clinically relevant benzoxaboroles inhibit mRNA processing in Trypanosoma brucei. BMC Res. Notes 2022, 15, 371. [Google Scholar] [CrossRef]
- Steketee, P.C.; Vincent, I.M.; Achcar, F.; Giordani, F.; Kim, D.-H.; Creek, D.J.; Freund, Y.; Jacobs, R.; Rattigan, K.; Horn, D.; et al. Benzoxaborole treatment perturbs S-adenosyl-L-methionine metabolism in Trypanosoma brucei. PLoS Negl. Trop. Dis. 2018, 12, e0006450. [Google Scholar] [CrossRef]
- Wall, R.J.; Rico, E.; Lukac, I.; Zuccotto, F.; Elg, S.; Gilbert, I.H.; Freund, Y.; Alley, M.R.K.; Field, M.C.; Wyllie, S.; et al. Clinical and veterinary trypanocidal benzoxaboroles target CPSF3. Proc. Natl. Acad. Sci. USA 2018, 115, 9616–9621. [Google Scholar] [CrossRef]
- Kumeso, V.K.; Kalonji, W.M.; Rembry, S.; Mordt, O.; Ngolo Tete, D.; Prêtre, A.; Delhomme, S.; Kyhi, M.; Camara, M.; Catusse, J.; et al. Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: A multicentre, open-label, single-arm, phase 2/3 trial. Lancet Infect. Dis. 2023, 23, 463–470. [Google Scholar]
- Jacobs, R.T.; Nare, B.; Wring, S.A.; Orr, M.D.; Chen, D.; Sligar, J.M.; Jenks, M.X.; Noe, R.A.; Bowling, T.S.; Mercer, L.T.; et al. SCYX-7158, an Orally-Active Benzoxaborole for the Treatment of Stage 2 Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2011, 5, e1151. [Google Scholar] [CrossRef]
- Dominski, Z.; Yang, X.; Marzluff, W.F. The Polyadenylation Factor CPSF-73 Is Involved in Histone-Pre-mRNA Processing. Cell 2005, 123, 37–48. [Google Scholar] [CrossRef]
- Mandel, C.R.; Kaneko, S.; Zhang, H.; Gebauer, D.; Vethantham, V.; Manley, J.L.; Tong, L. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature 2006, 444, 953–956. [Google Scholar] [CrossRef]
- Akama, T.; Zhang, Y.-K.; Freund, Y.R.; Berry, P.; Lee, J.; Easom, E.E.; Jacobs, R.T.; Plattner, J.J.; Witty, M.J.; Peter, R.; et al. Identification of a 4-fluorobenzyl l-valinate amide benzoxaborole (AN11736) as a potential development candidate for the treatment of Animal African Trypanosomiasis (AAT). Bioorg. Med. Chem. Lett. 2018, 28, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Yeramian, P.D.; Allen, J.L.; Merolle, A.; Serge, K.K.; Mpanya, A.; Lutumba, P.; Mesu, V.K.B.K.; Bilenge, C.M.M.; Lubaki, J.-P.F.; et al. Efficacy, Safety, and Dose of Pafuramidine, a New Oral Drug for Treatment of First Stage Sleeping Sickness, in a Phase 2a Clinical Study and Phase 2b Randomized Clinical Studies. PLoS Negl. Trop. Dis. 2016, 10, e0004362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pohlig, G.; Bernhard, S.C.; Blum, J.; Burri, C.; Mpanya, A.; Lubaki, J.-P.F.; Mpoto, A.M.; Munungu, B.F.; N’tombe, P.M.; Deo, G.K.M.; et al. Efficacy and Safety of Pafuramidine versus Pentamidine Maleate for Treatment of First Stage Sleeping Sickness in a Randomized, Comparator-Controlled, International Phase 3 Clinical Trial. PLoS Negl. Trop. Dis. 2016, 10, e0004363. [Google Scholar] [CrossRef]
- Mathis, A.M.; Holman, J.L.; Sturk, L.M.; Ismail, M.A.; Boykin, D.W.; Tidwell, R.R.; Hall, J.E. Accumulation and Intracellular Distribution of Antitrypanosomal Diamidine Compounds DB75 and DB820 in African Trypanosomes. Antimicrob. Agents Chemother. 2006, 50, 2185–2191. [Google Scholar] [CrossRef]
- Thuita, J.K.; Wang, M.Z.; Kagira, J.M.; Denton, C.L.; Paine, M.F.; Mdachi, R.E.; Murilla, G.A.; Ching, S.; Boykin, D.W.; Tidwell, R.R.; et al. Pharmacology of DB844, an Orally Active aza Analogue of Pafuramidine, in a Monkey Model of Second Stage Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2012, 6, e1734. [Google Scholar] [CrossRef] [PubMed]
- Thuita, J.K.; Wolf, K.K.; Murilla, G.A.; Bridges, A.S.; Boykin, D.W.; Mutuku, J.N.; Liu, Q.; Jones, S.K.; Gem, C.O.; Ching, S.; et al. Chemotherapy of Second Stage Human African Trypanosomiasis: Comparison between the Parenteral Diamidine DB829 and Its Oral Prodrug DB868 in Vervet Monkeys. PLoS Negl. Trop. Dis. 2015, 9, e0003409. [Google Scholar]
- De Koning, H.P. The Drugs of Sleeping Sickness: Their Mechanisms of Action and Resistance, and a Brief History. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef]
- Sturk, L.M.; Brock, J.L.; Bagnell, C.R.; Hall, J.E.; Tidwell, R.R. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75) and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 2004, 91, 131–143. [Google Scholar] [CrossRef]
- Rodgers, J.; Jones, A.; Gibaud, S.; Bradley, B.; McCabe, C.; Barrett, M.P.; Gettinby, G.; Kennedy, P.G.E. Melarsoprol Cyclodextrin Inclusion Complexes as Promising Oral Candidates for the Treatment of Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2011, 5, e1308. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Jackson, A.P.; Sanders, M.; Berry, A.; McQuillan, J.; Aslett, M.A.; Quail, M.A.; Chukualim, B.; Capewell, P.; MacLeod, A.; Melville, S.E.; et al. The Genome Sequence of Trypanosoma brucei gambiense, Causative Agent of Chronic Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2010, 4, e658. [Google Scholar] [CrossRef]
- Gibson, W. The origins of the trypanosome genome strains Trypanosoma brucei brucei TREU 927, T. b. gambiense DAL 972, T. vivax Y486 and T. congolense IL3000. Parasit. Vectors 2012, 5, 71. [Google Scholar] [CrossRef]
- Kamps, M.P.; Buss, J.E.; Sefton, B.M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc. Natl. Acad. Sci. USA 1985, 82, 4625–4628. [Google Scholar] [CrossRef] [PubMed]
- Buss, J.E.; Kamps, M.P.; Gould, K.; Sefton, B.M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J. Virol. 1986, 58, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Towler, D.A.; Adams, S.P.; Eubanks, S.R.; Towery, D.S.; Jackson-Machelski, E.; Glaser, L.; Gordon, J.I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc. Natl. Acad. Sci. USA 1987, 84, 2708–2712. [Google Scholar] [CrossRef]
- Zha, J.; Weiler, S.; Oh, K.J.; Wei, M.C.; Korsmeyer, S.J. Posttranslational N-Myristoylation of BID as a Molecular Switch for Targeting Mitochondria and Apoptosis. Science 2000, 290, 1761–1765. [Google Scholar] [CrossRef]
- Price, H.P.; Menon, M.R.; Panethymitaki, C.; Goulding, D.; McKean, P.G.; Smith, D.F. Myristoyl-CoA:Protein N-Myristoyltransferase, an Essential Enzyme and Potential Drug Target in Kinetoplastid Parasites. J. Biol. Chem. 2003, 278, 7206–7214. [Google Scholar] [CrossRef]
- Price, H.P.; Güther, M.L.S.; Ferguson, M.A.J.; Smith, D.F. Myristoyl-CoA:protein N-myristoyltransferase depletion in trypanosomes causes avirulence and endocytic defects. Mol. Biochem. Parasitol. 2010, 169, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Giang, D.K.; Cravatt, B.F. A Second Mammalian N-Myristoyltransferase. J. Biol. Chem. 1998, 273, 6595–6598. [Google Scholar] [CrossRef] [PubMed]
- Frearson, J.A.; Brand, S.; McElroy, S.P.; Cleghorn, L.A.T.; Smid, O.; Stojanovski, L.; Price, H.P.; Guther, M.L.S.; Torrie, L.S.; Robinson, D.A.; et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature 2010, 464, 728–732. [Google Scholar] [CrossRef]
- Hertz-Fowler, C.; Ersfeld, K.; Gull, K. CAP5.5, a life-cycle-regulated, cytoskeleton-associated protein is a member of a novel family of calpain-related proteins in Trypanosoma brucei. Mol. Biochem. Parasitol. 2001, 116, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Price, H.P.; Panethymitaki, C.; Goulding, D.; Smith, D.F. Functional analysis of TbARL1, an N-myristoylated Golgi protein essential for viability in bloodstream trypanosomes. J. Cell Sci. 2005, 118, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Price, H.P.; Stark, M.; Smith, D.F. Trypanosoma brucei ARF1 Plays a Central Role in Endocytosis and Golgi–Lysosome Trafficking. Mol. Biol. Cell 2007, 18, 864–873. [Google Scholar] [CrossRef][Green Version]
- Denny, P.W.; Gokool, S.; Russell, D.G.; Field, M.C.; Smith, D.F. Acylation-dependent Protein Export inLeishmania. J. Biol. Chem. 2000, 275, 11017–11025. [Google Scholar] [CrossRef]
- Wingard, J.N.; Ladner, J.; Vanarotti, M.; Fisher, A.J.; Robinson, H.; Buchanan, K.T.; Engman, D.M.; Ames, J.B. Structural insights into membrane targeting by the flagellar calcium-binding protein (FCaBP), a myristoylated and palmitoylated calcium sensor in Trypanosoma cruzi. J. Biol. Chem. 2008, 283, 23388–23396. [Google Scholar] [CrossRef]
- Mills, E.; Price, H.P.; Johner, A.; Emerson, J.E.; Smith, D.F. Kinetoplastid PPEF phosphatases: Dual acylated proteins expressed in the endomembrane system of Leishmania. Mol. Biochem. Parasitol. 2007, 152, 22–34. [Google Scholar] [CrossRef]
- Brand, S.; Cleghorn, L.A.T.; McElroy, S.P.; Robinson, D.A.; Smith, V.C.; Hallyburton, I.; Harrison, J.R.; Norcross, N.R.; Spinks, D.; Bayliss, T.; et al. Discovery of a Novel Class of Orally Active Trypanocidal N-Myristoyltransferase Inhibitors. J. Med. Chem. 2012, 55, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Norcross, N.R.; Thompson, S.; Harrison, J.R.; Smith, V.C.; Robinson, D.A.; Torrie, L.S.; McElroy, S.P.; Hallyburton, I.; Norval, S.; et al. Lead Optimization of a Pyrazole Sulfonamide Series of Trypanosoma brucei N-Myristoyltransferase Inhibitors: Identification and Evaluation of CNS Penetrant Compounds as Potential Treatments for Stage 2 Human African Trypanosomiasis. J. Med. Chem. 2014, 57, 9855–9869. [Google Scholar] [CrossRef]
- Bayliss, T.; Robinson, D.A.; Smith, V.C.; Brand, S.; McElroy, S.P.; Torrie, L.S.; Mpamhanga, C.; Norval, S.; Stojanovski, L.; Brenk, R.; et al. Design and Synthesis of Brain Penetrant Trypanocidal N-Myristoyltransferase Inhibitors. J. Med. Chem. 2017, 60, 9790–9806. [Google Scholar] [CrossRef]
- Boer, D.R.; Bijlmakers, M.-J. Differential Inhibition of Human and Trypanosome Ubiquitin E1S by TAK-243 Offers Possibilities for Parasite Selective Inhibitors. Sci. Rep. 2019, 9, 16195. [Google Scholar] [CrossRef]
- Bijlmakers, M.-J. Ubiquitination and the Proteasome as Drug Targets in Trypanosomatid Diseases. Front. Chem. 2021, 8, 630888. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Hicke, L. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2001, 2, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Schwartz, D.; Elias, J.E.; Thoreen, C.C.; Cheng, D.; Marsischky, G.; Roelofs, J.; Finley, D.; Gygi, S.P. A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 2003, 21, 921–926. [Google Scholar] [CrossRef]
- Pickart, C.M.; Fushman, D. Polyubiquitin chains: Polymeric protein signals. Curr. Opin. Chem. Biol. 2004, 8, 610–616. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef]
- Scheffner, M.; Nuber, U.; Huibregtse, J.M. Protein ubiquitination involving an E1–E2–E3 enzyme ubiquitin thioester cascade. Nature 1995, 373, 81–83. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Scotti, E.; Chen, Z.J.; Tontonoz, P. Both K63 and K48 ubiquitin linkages signal lysosomal degradation of the LDL receptor. J. Lipid Res. 2013, 54, 1410–1420. [Google Scholar] [CrossRef]
- Nguyen, L.K.; Dobrzyński, M.; Fey, D.; Kholodenko, B.N. Polyubiquitin chain assembly and organization determine the dynamics of protein activation and degradation. Front. Physiol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Chung, W.-L.; Leung, K.F.; Carrington, M.; Field, M.C. Ubiquitylation is Required for Degradation of Transmembrane Surface Proteins in Trypanosomes. Traffic 2008, 9, 1681–1697. [Google Scholar] [CrossRef]
- Alsford, S.; Turner, D.J.; Obado, S.O.; Sanchez-Flores, A.; Glover, L.; Berriman, M.; Hertz-Fowler, C.; Horn, D. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res. 2011, 21, 915–924. [Google Scholar] [CrossRef]
- Gupta, I.; Aggarwal, S.; Singh, K.; Yadav, A.; Khan, S. Ubiquitin Proteasome pathway proteins as potential drug targets in parasite Trypanosoma cruzi. Sci. Rep. 2018, 8, 8399. [Google Scholar] [CrossRef] [PubMed]
- Dewar, C.E.; Oeljeklaus, S.; Mani, J.; Mühlhäuser, W.W.D.; von Känel, C.; Zimmermann, J.; Ochsenreiter, T.; Warscheid, B.; Schneider, A. Mistargeting of aggregation prone mitochondrial proteins activates a nucleus-mediated posttranscriptional quality control pathway in trypanosomes. Nat. Commun. 2022, 13, 3084. [Google Scholar] [CrossRef] [PubMed]
- Barghout, S.H.; Schimmer, A.D. E1 Enzymes as Therapeutic Targets in Cancer. Pharmacol. Rev. 2021, 73, 1–56. [Google Scholar] [CrossRef]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.N.; Myburgh, E.; Gao, M.-Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef]
- Wyllie, S.; Brand, S.; Thomas, M.; De Rycker, M.; Chung, C.-W.; Pena, I.; Bingham, R.P.; Bueren-Calabuig, J.A.; Cantizani, J.; Cebrian, D.; et al. Preclinical candidate for the treatment of visceral leishmaniasis that acts through proteasome inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 9318–9323. [Google Scholar] [CrossRef]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B.; et al. Discovery and Characterization of Clinical Candidate LXE408 as a Kinetoplastid-Selective Proteasome Inhibitor for the Treatment of Leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 1. Anti-infective drugs for regulatory registration. Parasit. Vectors 2023, 16, 82. [Google Scholar]
- Oberholzer, M.; Marti, G.; Baresic, M.; Kunz, S.; Hemphill, A.; Seebeck, T. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEBl and TbrPDEB2: Flagellar enzymes that are essential for parasite virulence. FASEB J. 2007, 21, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, E.; Ryter, D.; Schranz-Zumkehr, J.; Oberholzer, M.; Kunz, S.; Seebeck, T. The N Terminus of Phosphodiesterase TbrPDEB1 of Trypanosoma brucei Contains the Signal for Integration into the Flagellar Skeleton. Eukaryot. Cell 2010, 9, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P.; Gould, M.K.; Sterk, G.J.; Tenor, H.; Kunz, S.; Luginbuehl, E.; Seebeck, T. Pharmacological Validation of Trypanosoma brucei Phosphodiesterases as Novel Drug Targets. J. Infect. Dis. 2012, 206, 229–237. [Google Scholar] [CrossRef]
- Seebeck, T.; Sterk, G.J.; Ke, H. Phosphodiesterase inhibitors as a new generation of antiprotozoan drugs: Exploiting the benefit of enzymes that are highly conserved between host and parasite. Future Med. Chem. 2011, 3, 1289–1306. [Google Scholar]
- Gould, M.K.; Bachmaier, S.; Ali, J.A.M.; Alsford, S.; Tagoe, D.N.A.; Munday, J.C.; Schnaufer, A.C.; Horn, D.; Boshart, M.; De Koning, H.P. Cyclic AMP Effectors in African Trypanosomes Revealed by Genome-Scale RNA Interference Library Screening for Resistance to the Phosphodiesterase Inhibitor CpdA. Antimicrob. Agents Chemother. 2013, 57, 4882–4893. [Google Scholar] [CrossRef]
- Bachmaier, S.; Gould, M.K.; Polatoglou, E.; Omelianczyk, R.; Brennand, A.E.; Aloraini, M.A.; Munday, J.C.; Horn, D.; Boshart, M.; De Koning, H.P. Novel kinetoplastid-specific cAMP binding proteins identified by RNAi screening for cAMP resistance in Trypanosoma brucei. Front. Cell. Infect. Microbiol. 2023, 13, 1204707. [Google Scholar] [CrossRef]
- Van Der Mey, M.; Hatzelmann, A.; Van Der Laan, I.J.; Sterk, G.J.; Thibaut, U.; Timmerman, H. Novel Selective PDE4 Inhibitors. 1. Synthesis, Structure−Activity Relationships, and Molecular Modeling of 4-(3,4-Dimethoxyphenyl)-2H-phthalazin-1-ones and Analogues. J. Med. Chem. 2001, 44, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Van Der Mey, M.; Hatzelmann, A.; Van Klink, G.P.M.; Van Der Laan, I.J.; Sterk, G.J.; Thibaut, U.; Ulrich, W.R.; Timmerman, H. Novel Selective PDE4 Inhibitors. 2. Synthesis and Structure−Activity Relationships of 4-Aryl-Substituted cis-Tetra- and cis-Hexahydrophthalazinones. J. Med. Chem. 2001, 44, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Souness, J.E.; Aldous, D.; Sargent, C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology 2000, 47, 127–162. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Gristwood, R.W.; Cooper, N.; Hellewell, P.G. Phosphodiesterase (PDE)4 inhibitors: Anti-inflammatory drugs of the future? Trends Pharmacol. Sci. 1997, 18, 164–170. [Google Scholar] [CrossRef]
- Veerman, J.; Van Den Bergh, T.; Orrling, K.M.; Jansen, C.; Cos, P.; Maes, L.; Chatelain, E.; Ioset, J.-R.; Edink, E.E.; Tenor, H.; et al. Synthesis and evaluation of analogs of the phenylpyridazinone NPD-001 as potent trypanosomal TbrPDEB1 phosphodiesterase inhibitors and in vitro trypanocidals. Bioorg. Med. Chem. 2016, 24, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- De Heuvel, E.; Singh, A.K.; Boronat, P.; Kooistra, A.J.; Van Der Meer, T.; Sadek, P.; Blaazer, A.R.; Shaner, N.C.; Bindels, D.S.; Caljon, G.; et al. Alkynamide phthalazinones as a new class of TbrPDEB1 inhibitors (Part 2). Bioorg. Med. Chem. 2019, 27, 4013–4029. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Cerami, A. Identification of a novel, thiol-containing co-factor essential for glutathione reductase enzyme activity in trypanosomatids. Mol. Biochem. Parasitol. 1985, 14, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting Trypanothione Reductase, a Key Enzyme in the Redox Trypanosomatid Metabolism, to Develop New Drugs against Leishmaniasis and Trypanosomiases. Molecules 2020, 25, 1924. [Google Scholar] [CrossRef] [PubMed]
- Shames, S.L.; Fairlamb, A.H.; Cerami, A.; Walsh, C.T. Purification and characterization of trypanothione reductase from Crithidia fasciculata, a new member of the family of disulfide-containing flavoprotein reductases. Biochemistry 1986, 25, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Krieger, S.; Schwarz, W.; Ariyanayagam, M.R.; Fairlamb, A.H.; Krauth-Siegel, R.L.; Clayton, C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress: Trypanosomes lacking trypanothione reductase. Mol. Microbiol. 2002, 35, 542–552. [Google Scholar]
- Stoll, V.S.; Simpson, S.J.; Krauth-Siegel, R.L.; Walsh, C.T.; Pai, E.F. Glutathione Reductase Turned into Trypanothione Reductase: Structural Analysis of an Engineered Change in Substrate Specificity. Biochemistry 1997, 36, 6437–6447. [Google Scholar] [CrossRef]
- Zhang, Y.; Bond, C.S.; Bailey, S.; Cunningham, M.L.; Fairlamb, A.H.; Hunter, W.N. The crystal structure of trypanothione reductase from the human pathogen Trypanosoma cruzi at 2.3 Å resolution: Trypanothione reductase structure. Protein Sci. 1996, 5, 52–61. [Google Scholar] [CrossRef]
- Beig, M.; Oellien, F.; Garoff, L.; Noack, S.; Krauth-Siegel, R.L.; Selzer, P.M. Trypanothione Reductase: A Target Protein for a Combined In Vitro and In Silico Screening Approach. PLoS Negl. Trop. Dis. 2015, 9, e0003773. [Google Scholar] [CrossRef]
- Tovar, J.; Cunningham, M.L.; Smith, A.C.; Croft, S.L.; Fairlamb, A.H. Down-regulation of Leishmania donovani trypanothione reductase by heterologous expression of a trans-dominant mutant homologue: Effect on parasite intracellular survival. Proc. Natl. Acad. Sci. USA 1998, 95, 5311–5316. [Google Scholar] [CrossRef]
- Turcano, L.; Battista, T.; De Haro, E.T.; Missineo, A.; Alli, C.; Paonessa, G.; Colotti, G.; Harper, S.; Fiorillo, A.; Ilari, A.; et al. Spiro-containing derivatives show antiparasitic activity against Trypanosoma brucei through inhibition of the trypanothione reductase enzyme. PLoS Negl. Trop. Dis. 2020, 14, e0008339. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Blackburn, P.; Ulrich, P.; Chait, B.T.; Cerami, A. Trypanothione: A Novel Bis(glutathionyl)spermidine Cofactor for Glutathione Reductase in Trypanosomatids. Science 1985, 227, 1485–1487. [Google Scholar] [CrossRef]
- Oza, S.L.; Ariyanayagam, M.R.; Aitcheson, N.; Fairlamb, A.H. Properties of trypanothione synthetase from Trypanosoma brucei. Mol. Biochem. Parasitol. 2003, 131, 25–33. [Google Scholar] [CrossRef]
- Comini, M.A.; Guerrero, S.A.; Haile, S.; Menge, U.; Lünsdorf, H.; Flohé, L. Valdiation of Trypanosoma brucei trypanothione synthetase as drug target. Free Radic. Biol. Med. 2004, 36, 1289–1302. [Google Scholar] [CrossRef]
- Mesías, A.C.; Sasoni, N.; Arias, D.G.; Pérez Brandán, C.; Orban, O.C.F.; Kunick, C.; Robello, C.; Comini, M.A.; Garg, N.J.; Zago, M.P. Trypanothione synthetase confers growth, survival advantage and resistance to anti-protozoal drugs in Trypanosoma cruzi. Free Radic. Biol. Med. 2019, 130, 23–34. [Google Scholar] [CrossRef]
- Benítez, D.; Franco, J.; Sardi, F.; Leyva, A.; Durán, R.; Choi, G.; Yang, G.; Kim, T.; Kim, N.; Heo, J.; et al. Drug-like molecules with anti-trypanothione synthetase activity identified by high throughput screening. J. Enzyme Inhib. Med. Chem. 2022, 37, 912–929. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Henderson, G.B.; Bacchi, C.J.; Cerami, A. In vivo effects of difluoromethylornithine on trypanothione and polyamine levels in bloodstream forms of Trypanosoma brucei. Mol. Biochem. Parasitol. 1987, 24, 185–191. [Google Scholar] [CrossRef]
- Yun, O.; Priotto, G.; Tong, J.; Flevaud, L.; Chappuis, F. NECT is next: Implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e720. [Google Scholar] [CrossRef]
- Heby, O.; Persson, L.; Rentala, M. Targeting the polyamine biosynthetic enzymes: A promising approach to therapy of African sleeping sickness, Chagas’ disease, and leishmaniasis. Amino Acids 2007, 33, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Willert, E.K.; Phillips, M.A. Regulated Expression of an Essential Allosteric Activator of Polyamine Biosynthesis in African Trypanosomes. PLoS Pathog. 2008, 4, e1000183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, M.C.; Kaur, H.; Blessington, B.; Kelly, J.M.; Wilkinson, S.R. Validation of spermidine synthase as a drug target in African trypanosomes. Biochem. J. 2008, 409, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. S-Adenosylmethionine decarboxylase. Essays Biochem. 2009, 46, 25–46. [Google Scholar] [PubMed]
- Volkov, O.A.; Cosner, C.C.; Brockway, A.J.; Kramer, M.; Booker, M.; Zhong, S.; Ketcherside, A.; Wei, S.; Longgood, J.; McCoy, M.; et al. Identification of Trypanosoma brucei AdoMetDC Inhibitors Using a High-Throughput Mass Spectrometry-Based Assay. ACS Infect. Dis. 2017, 3, 512–526. [Google Scholar] [CrossRef]
- Ekstrom, J.L.; Tolbert, W.D.; Xiong, H.; Pegg, A.E.; Ealick, S.E. Structure of a Human S-Adenosylmethionine Decarboxylase Self-Processing Ester Intermediate and Mechanism of Putrescine Stimulation of Processing As Revealed by the H243A Mutant. Biochemistry 2001, 40, 9495–9504. [Google Scholar] [CrossRef]
- Tolbert, W.D.; Zhang, Y.; Cottet, S.E.; Bennett, E.M.; Ekstrom, J.L.; Pegg, A.E.; Ealick, S.E. Mechanism of Human S-Adenosylmethionine Decarboxylase Proenzyme Processing As Revealed by the Structure of the S68A Mutant. Biochemistry 2003, 42, 2386–2395. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Ealick, S.E. Structural biology of S-adenosylmethionine decarboxylase. Amino Acids 2010, 38, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Willert, E.K.; Fitzpatrick, R.; Phillips, M.A. Allosteric regulation of an essential trypanosome polyamine biosynthetic enzyme by a catalytically dead homolog. Proc. Natl. Acad. Sci. USA 2007, 104, 8275–8280. [Google Scholar] [CrossRef]
- Velez, N.; Brautigam, C.A.; Phillips, M.A. Trypanosoma brucei S-Adenosylmethionine Decarboxylase N Terminus Is Essential for Allosteric Activation by the Regulatory Subunit Prozyme. J. Biol. Chem. 2013, 288, 5232–5240. [Google Scholar] [CrossRef] [PubMed]
- Rusché, L.N.; Huang, C.E.; Piller, K.J.; Hemann, M.; Wirtz, E.; Sollner-Webb, B. The two RNA ligases of the Trypanosoma brucei RNA editing complex: Cloning the essential band IV gene and identifying the band V gene. Mol. Cell. Biol. 2001, 21, 979–989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schnaufer, A.; Panigrahi, A.K.; Panicucci, B.; Igo, R.P.; Wirtz, E.; Salavati, R.; Stuart, K. An RNA ligase essential for RNA editing and survival of the bloodstream form of Trypanosoma brucei. Science 2001, 291, 2159–2162. [Google Scholar] [CrossRef]
- Amaro, R.E.; Schnaufer, A.; Interthal, H.; Hol, W.; Stuart, K.D.; McCammon, J.A. Discovery of drug-like inhibitors of an essential RNA-editing ligase in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 2008, 105, 17278–17283. [Google Scholar] [CrossRef]
- Bello, A.R.; Nare, B.; Freedman, D.; Hardy, L.; Beverley, S.M. PTR1: A reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA 1994, 91, 11442–11446. [Google Scholar] [CrossRef]
- Sienkiewicz, N.; Ong, H.B.; Fairlamb, A.H. Trypanosoma brucei pteridine reductase 1 is essential for survival in vitro and for virulence in mice. Mol. Microbiol. 2010, 77, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Robello, C.; Navarro, P.; Castanys, S.; Gamarro, F. A pteridine reductase gene ptr1 contiguous to a P-glycoprotein confers resistance to antifolates in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1997, 90, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Cavazzuti, A.; Paglietti, G.; Hunter, W.N.; Gamarro, F.; Piras, S.; Loriga, M.; Allecca, S.; Corona, P.; McLuskey, K.; Tulloch, L.; et al. Discovery of potent pteridine reductase inhibitors to guide antiparasite drug development. Proc. Natl. Acad. Sci. USA 2008, 105, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, L.B.; Martini, V.P.; Iulek, J.; Huggan, J.K.; Lee, J.H.; Gibson, C.L.; Smith, T.K.; Suckling, C.J.; Hunter, W.N. Structure-Based Design of Pteridine Reductase Inhibitors Targeting African Sleeping Sickness and the Leishmaniases. J. Med. Chem. 2010, 53, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kimuda, M.P.; Laming, D.; Hoppe, H.C.; Tastan Bishop, Ö. Identification of Novel Potential Inhibitors of Pteridine Reductase 1 in Trypanosoma brucei via Computational Structure-Based Approaches and in Vitro Inhibition Assays. Molecules 2019, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Landi, G.; Linciano, P.; Borsari, C.; Bertolacini, C.P.; Moraes, C.B.; Cordeiro-da-Silva, A.; Gul, S.; Witt, G.; Kuzikov, M.; Costi, M.P.; et al. Structural Insights into the Development of Cycloguanil Derivatives as Trypanosoma brucei Pteridine-Reductase-1 Inhibitors. ACS Infect. Dis. 2019, 5, 1105–1114. [Google Scholar] [CrossRef]
- Chow, T.Y.-K.; Alaoui-Jamali, M.A.; Yeh, C.; Yuen, L.; Griller, D. The DNA double-stranded break repair protein endo-exonuclease as a therapeutic target for cancer. Mol. Cancer Ther. 2004, 3, 911–919. [Google Scholar] [CrossRef]
- Jung, H.-J.; Suh, S.-I.; Suh, M.-H.; Baek, W.-K.; Park, J.-W. Pentamidine reduces expression of hypoxia-inducible factor-1α in DU145 and MDA-MB-231 cancer cells. Cancer Lett. 2011, 303, 39–46. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z.; Kou, Z. Pentamidine Inhibits Ovarian Cancer Cell Proliferation and Migration by Maintaining Stability of PTEN in vitro. Drug Des. Devel. Ther. 2021, 15, 2857–2868. [Google Scholar] [CrossRef]
- Martínez-Flórez, A.; Galizzi, M.; Izquierdo, L.; Bustamante, J.M.; Rodriguez, A.; Rodriguez, F.; Rodríguez-Cortés, A.; Alberola, J. Repurposing bioenergetic modulators against protozoan parasites responsible for tropical diseases. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Rohlenova, K.; Sachaphibulkij, K.; Stursa, J.; Bezawork-Geleta, A.; Blecha, J.; Endaya, B.; Werner, L.; Cerny, J.; Zobalova, R.; Goodwin, J.; et al. Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2 high Breast Cancer. Antioxid. Redox Signal. 2017, 26, 84–103. [Google Scholar] [CrossRef]
- Arbon, D.; Ženíšková, K.; Šubrtová, K.; Mach, J.; Štursa, J.; Machado, M.; Zahedifard, F.; Leštinová, T.; Hierro-Yap, C.; Neuzil, J.; et al. Repurposing of MitoTam: Novel Anti-Cancer Drug Candidate Exhibits Potent Activity against Major Protozoan and Fungal Pathogens. Antimicrob. Agents Chemother. 2022, 66, e00727-22. [Google Scholar] [CrossRef]
- Burger, A.; Ludewig, M.H.; Boshoff, A. Investigating the Chaperone Properties of a Novel Heat Shock Protein, Hsp70.c, from Trypanosoma brucei. J. Parasitol. Res. 2014, 2014, 172582. [Google Scholar] [CrossRef]
- Ludewig, M.H.; Boshoff, A.; Horn, D.; Blatch, G.L. Trypanosoma brucei J protein 2 is a stress inducible and essential Hsp40. Int. J. Biochem. Cell Biol. 2015, 60, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.J.; Boshoff, A. Trypanosoma brucei J-Protein 2 Functionally Co-Operates with the Cytosolic Hsp70 and Hsp70.4 Proteins. Int. J. Mol. Sci. 2019, 20, 5843. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.J.; Shapiro, T.A. Cytosolic and Mitochondrial Hsp90 in Cytokinesis, Mitochondrial DNA Replication, and Drug Action in Trypanosoma brucei. Antimicrob. Agents Chemother. 2021, 65, e00632-21. [Google Scholar] [CrossRef]
- Jamabo, M.; Bentley, S.J.; Macucule-Tinga, P.; Tembo, P.; Edkins, A.L.; Boshoff, A. In silico analysis of the HSP90 chaperone system from the African trypanosome, Trypanosoma brucei. Front. Mol. Biosci. 2022, 9, 947078. [Google Scholar] [CrossRef]
- Louw, C.A.; Ludewig, M.H.; Mayer, J.; Blatch, G.L. The Hsp70 chaperones of the Tritryps are characterized by unusual features and novel members. Parasitol. Int. 2010, 59, 497–505. [Google Scholar] [CrossRef]
- Bentley, S.J.; Jamabo, M.; Boshoff, A. The Hsp70/J-protein machinery of the African trypanosome, Trypanosoma brucei. Cell Stress Chaperones 2019, 24, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Andreassend, S.K.; Bentley, S.J.; Blatch, G.L.; Boshoff, A.; Keyzers, R.A. Screening for Small Molecule Modulators of Trypanosoma brucei Hsp70 Chaperone Activity Based upon Alcyonarian Coral-Derived Natural Products. Mar. Drugs 2020, 18, E81. [Google Scholar] [CrossRef]
- Burger, A.; Macucule-Tinga, P.; Bentley, S.J.; Ludewig, M.H.; Mhlongo, N.N.; Shonhai, A.; Boshoff, A. Characterization of an Atypical Trypanosoma brucei Hsp70 Demonstrates Its Cytosolic-Nuclear Localization and Modulation by Quercetin and Methylene Blue. Int. J. Mol. Sci. 2021, 22, 6776. [Google Scholar] [CrossRef] [PubMed]
- Odunuga, O.O.; Longshaw, V.M.; Blatch, G.L. Hop: More than an Hsp70/Hsp90 adaptor protein. BioEssays 2004, 26, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Assimon, V.; Gillies, A.; Rauch, J.; Gestwicki, J. Hsp70 Protein Complexes as Drug Targets. Curr. Pharm. Des. 2013, 19, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y. Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr. Pharm. Des. 2005, 11, 1131–1138. [Google Scholar] [CrossRef]
- Mahalingam, D.; Swords, R.; Carew, J.S.; Nawrocki, S.T.; Bhalla, K.; Giles, F.J. Targeting HSP90 for cancer therapy. Br. J. Cancer 2009, 100, 1523–1529. [Google Scholar] [CrossRef]
- Butler, L.M.; Ferraldeschi, R.; Armstrong, H.K.; Centenera, M.M.; Workman, P. Maximizing the Therapeutic Potential of HSP90 Inhibitors. Mol. Cancer Res. 2015, 13, 1445–1451. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270. [Google Scholar] [CrossRef]
- Pizarro, J.C.; Hills, T.; Senisterra, G.; Wernimont, A.K.; Mackenzie, C.; Norcross, N.R.; Ferguson, M.A.J.; Wyatt, P.G.; Gilbert, I.H.; Hui, R. Exploring the Trypanosoma brucei Hsp83 Potential as a Target for Structure Guided Drug Design. PLoS Negl. Trop. Dis. 2013, 7, e2492. [Google Scholar] [CrossRef]
- Jones, C.; Anderson, S.; Singha, U.K.; Chaudhuri, M. Protein phosphatase 5 is required for Hsp90 function during proteotoxic stresses in Trypanosoma brucei. Parasitol. Res. 2008, 102, 835–844. [Google Scholar] [CrossRef]
- Meyer, K.J.; Shapiro, T.A. Potent antitrypanosomal activities of heat shock protein 90 inhibitors in vitro and in vivo. J. Infect. Dis. 2013, 208, 489–499. [Google Scholar] [CrossRef][Green Version]
- Pacey, S.; Wilson, R.H.; Walton, M.; Eatock, M.M.; Hardcastle, A.; Zetterlund, A.; Arkenau, H.-T.; Moreno-Farre, J.; Banerji, U.; Roels, B.; et al. A Phase I Study of the Heat Shock Protein 90 Inhibitor Alvespimycin (17-DMAG) Given Intravenously to Patients with Advanced Solid Tumors. Clin. Cancer Res. 2011, 17, 1561–1570. [Google Scholar] [CrossRef]
- Anderson, S.; Jones, C.; Saha, L.; Chaudhuri, M. Functional characterization of the serine/threonine protein phosphatase 5 from Trypanosoma brucei. J. Parasitol. 2006, 92, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Ogbunude, P.O.J.; Ikediobi, C.O. Comparative aspects of purine metabolism in some African trypanosomes. Mol. Biochem. Parasitol. 1983, 9, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.J.; Gutteridge, W.E. Purine and pyrimidine metabolism in the trypanosomatidae. Mol. Biochem. Parasitol. 1984, 13, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A. Targeting the nucleotide metabolism of Trypanosoma brucei and other trypanosomatids. FEMS Microbiol. Rev. 2023, 47, fuad020. [Google Scholar] [CrossRef] [PubMed]
- Campagnaro, G.D. Purine Transporters as Efficient Carriers for Anti-kinetoplastid Molecules: 3′-Deoxytubercidin versus Trypanosomes. ACS Infect. Dis. 2022, 8, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P.; Jarvis, S.M. Adenosine Transporters in Bloodstream Forms of Trypanosoma brucei brucei: Substrate Recognition Motifs and Affinity for Trypanocidal Drugs. Mol. Pharmacol. 1999, 56, 1162–1170. [Google Scholar] [CrossRef]
- Mäser, P.; Sütterlin, C.; Kralli, A.; Kaminsky, R. A Nucleoside Transporter from Trypanosoma brucei Involved in Drug Resistance. Science 1999, 285, 242–244. [Google Scholar] [CrossRef]
- Li, J.Y.; Boado, R.J.; Pardridge, W.M. Cloned Blood–Brain Barrier Adenosine Transporter is Identical to the Rat Concentrative Na + Nucleoside Cotransporter CNT2. J. Cereb. Blood Flow Metab. 2001, 21, 929–936. [Google Scholar] [CrossRef]
- Drew, M.E.; Morris, J.C.; Wang, Z.; Wells, L.; Sanchez, M.; Landfear, S.M.; Englund, P.T. The Adenosine Analog Tubercidin Inhibits Glycolysis in Trypanosoma brucei as Revealed by an RNA Interference Library. J. Biol. Chem. 2003, 278, 46596–46600. [Google Scholar] [CrossRef]
- Vodnala, S.K.; Ferella, M.; Lundén-Miguel, H.; Betha, E.; Van Reet, N.; Amin, D.N.; Öberg, B.; Andersson, B.; Kristensson, K.; Wigzell, H.; et al. Preclinical Assessment of the Treatment of Second-Stage African Trypanosomiasis with Cordycepin and Deoxycoformycin. PLoS Negl. Trop. Dis. 2009, 3, e495. [Google Scholar] [CrossRef] [PubMed]
- Kaplinsky, C.; Yeger, H.; Estrov, Z.; Barankiewicz, J.; Pawlin, G.; Freedman, M.H.; Cohen, A. Selective protection of tubercidin toxicity by nitrobenzyl thioinosine in normal tissues but not in human neuroblastoma cells. Cancer Chemother. Pharmacol. 1986, 17, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Hulpia, F.; Mabille, D.; Campagnaro, G.D.; Schumann, G.; Maes, L.; Roditi, I.; Hofer, A.; De Koning, H.P.; Caljon, G.; Van Calenbergh, S. Combining tubercidin and cordycepin scaffolds results in highly active candidates to treat late-stage sleeping sickness. Nat. Commun. 2019, 10, 5564. [Google Scholar] [CrossRef] [PubMed]
- Hulpia, F.; Bouton, J.; Campagnaro, G.D.; Alfayez, I.A.; Mabille, D.; Maes, L.; De Koning, H.P.; Caljon, G.; Van Calenbergh, S. C6–O-alkylated 7-deazainosine nucleoside analogues: Discovery of potent and selective anti-sleeping sickness agents. Eur. J. Med. Chem. 2020, 188, 112018. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, M.E.; Masocha, W.; Ferella, M.; Petitto-Assis, F.; Goto, H.; Kristensson, K.; McCaffrey, R.; Wigzell, H. Treatment of African Trypanosomiasis with Cordycepin and Adenosine Deaminase Inhibitors in a Mouse Model. J. Infect. Dis. 2005, 192, 1658–1665. [Google Scholar] [CrossRef]
- Ziegelbauer, K.; Overath, P. Identification of invariant surface glycoproteins in the bloodstream stage of Trypanosoma brucei. J. Biol. Chem. 1992, 267, 10791–10796. [Google Scholar] [CrossRef]
- Ziegelbauer, K.; Overath, P. Organization of two invariant surface glycoproteins in the surface coat of Trypanosoma brucei. Infect. Immun. 1993, 61, 4540–4545. [Google Scholar] [CrossRef]
- Salmon, D.; Geuskens, M.; Hanocq, F.; Hanocq-Quertier, J.; Nolan, D.; Ruben, L.; Pays, E. A novel heterodimeric transferrin receptor encoded by a pair of VSG expression site-associated genes in T. brucei. Cell 1994, 78, 75–86. [Google Scholar] [CrossRef]
- Nolan, D.P.; Jackson, D.G.; Biggs, M.J.; Brabazon, E.D.; Pays, A.; Van Laethem, F.; Paturiaux-Hanocq, F.; Elliot, J.F.; Voorheis, H.P.; Pays, E. Characterization of a Novel Alanine-rich Protein Located in Surface Microdomains in Trypanosoma brucei. J. Biol. Chem. 2000, 275, 4072–4080. [Google Scholar] [CrossRef]
- Mehlert, A.; Wormald, M.R.; Ferguson, M.A.J. Modeling of the N-Glycosylated Transferrin Receptor Suggests How Transferrin Binding Can Occur within the Surface Coat of Trypanosoma brucei. PLoS Pathog. 2012, 8, e1002618. [Google Scholar] [CrossRef]
- Horn, D. Antigenic variation in African trypanosomes. Mol. Biochem. Parasitol. 2014, 195, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Radwanska, M.; Guirnalda, P.; De Trez, C.; Ryffel, B.; Black, S.; Magez, S. Trypanosomiasis-Induced B Cell Apoptosis Results in Loss of Protective Anti-Parasite Antibody Responses and Abolishment of Vaccine-Induced Memory Responses. PLoS Pathog. 2008, 4, e1000078. [Google Scholar] [CrossRef] [PubMed]
- Lança, A.S.C.; de Sousa, K.P.; Atouguia, J.; Prazeres, D.M.F.; Monteiro, G.A.; Silva, M.S. Trypanosoma brucei: Immunisation with plasmid DNA encoding invariant surface glycoprotein gene is able to induce partial protection in experimental African trypanosomiasis. Exp. Parasitol. 2011, 127, 18–24. [Google Scholar]
- Geiger, A.; Hirtz, C.; Bécue, T.; Bellard, E.; Centeno, D.; Gargani, D.; Rossignol, M.; Cuny, G.; Peltier, J.-B. Exocytosis and protein secretion in Trypanosoma. BMC Microbiol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Magez, S.; Li, Z.; Nguyen, H.T.T.; Pinto Torres, J.E.; Van Wielendaele, P.; Radwanska, M.; Began, J.; Zoll, S.; Sterckx, Y.G.-J. The History of Anti-Trypanosome Vaccine Development Shows That Highly Immunogenic and Exposed Pathogen-Derived Antigens Are Not Necessarily Good Target Candidates: Enolase and ISG75 as Examples. Pathogens 2021, 10, 1050. [Google Scholar] [CrossRef]
- Franco, J.R.; Cecchi, G.; Paone, M.; Diarra, A.; Grout, L.; Kadima Ebeja, A.; Simarro, P.P.; Zhao, W.; Argaw, D. The elimination of human African trypanosomiasis: Achievements in relation to WHO road map targets for 2020. PLoS Negl. Trop. Dis. 2022, 16, e0010047. [Google Scholar] [CrossRef] [PubMed]
- Borlase, A.; Le Rutte, E.A.; Castaño, S.; Blok, D.J.; Toor, J.; Giardina, F.; Davis, E.L.; Aliee, M.; Anderson, R.M.; Ayabina, D.; et al. Evaluating and mitigating the potential indirect effect of COVID-19 on control programmes for seven neglected tropical diseases: A modelling study. Lancet Glob. Health 2022, 10, e1600–e1611. [Google Scholar]
- The Independent. Uganda Eliminates Sleeping Sickness as Public Health Problem: MOH. The Independent Uganda. 22 October 2022. Available online: https://www.independent.co.ug/uganda-eliminates-sleeping-sickness-as-public-health-problem-moh/ (accessed on 3 November 2022).
- Büscher, P.; Bart, J.-M.; Boelaert, M.; Bucheton, B.; Cecchi, G.; Chitnis, N.; Courtin, D.; Figueiredo, L.M.; Franco, J.-R.; Grébaut, P.; et al. Do Cryptic Reservoirs Threaten Gambiense-Sleeping Sickness Elimination? Trends Parasitol. 2018, 34, 197–207. [Google Scholar] [CrossRef]
- Barrett, M.P.; Croft, S.L. Management of trypanosomiasis and leishmaniasis. Br. Med. Bull. 2012, 104, 175–196. [Google Scholar] [CrossRef]
- Abaza, S. Recent advances in identification of potential drug targets and development of novel drugs in parasitic diseases. Part III: Helminths. Parasitol. United J. 2022, 15, 126–143. [Google Scholar] [CrossRef]
- Ross, N.T.; Lohmann, F.; Carbonneau, S.; Fazal, A.; Weihofen, W.A.; Gleim, S.; Salcius, M.; Sigoillot, F.; Henault, M.; Carl, S.H.; et al. CPSF3-dependent pre-mRNA processing as a druggable node in AML and Ewing’s sarcoma. Nat. Chem. Biol. 2020, 16, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.R.; Lai, J.; Chauhan, U.; Beauchamp, E.; Dong, W.-F.; Glubrecht, D.; Sim, Y.-W.; Ghosh, S.; Bigras, G.; Lai, R.; et al. N-myristoyltransferase proteins in breast cancer: Prognostic relevance and validation as a new drug target. Breast Cancer Res. Treat. 2021, 186, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Varikuti, S.; Jha, B.K.; Volpedo, G.; Ryan, N.M.; Halsey, G.; Hamza, O.M.; McGwire, B.S.; Satoskar, A.R. Host-Directed Drug Therapies for Neglected Tropical Diseases Caused by Protozoan Parasites. Front. Microbiol. 2018, 9, 2655. [Google Scholar] [CrossRef] [PubMed]
- Thacker, S.G.; McWilliams, I.L.; Bonnet, B.; Halie, L.; Beaucage, S.; Rachuri, S.; Dey, R.; Duncan, R.; Modabber, F.; Robinson, S.; et al. CpG ODN D35 improves the response to abbreviated low-dose pentavalent antimonial treatment in non-human primate model of cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0008050. [Google Scholar] [CrossRef]
- Arnold, C. Inside the nascent industry of AI-designed drugs. Nat. Med. 2023, 29, 1292–1295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamabo, M.; Mahlalela, M.; Edkins, A.L.; Boshoff, A. Tackling Sleeping Sickness: Current and Promising Therapeutics and Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 12529. https://doi.org/10.3390/ijms241512529
Jamabo M, Mahlalela M, Edkins AL, Boshoff A. Tackling Sleeping Sickness: Current and Promising Therapeutics and Treatment Strategies. International Journal of Molecular Sciences. 2023; 24(15):12529. https://doi.org/10.3390/ijms241512529
Chicago/Turabian StyleJamabo, Miebaka, Maduma Mahlalela, Adrienne L. Edkins, and Aileen Boshoff. 2023. "Tackling Sleeping Sickness: Current and Promising Therapeutics and Treatment Strategies" International Journal of Molecular Sciences 24, no. 15: 12529. https://doi.org/10.3390/ijms241512529
APA StyleJamabo, M., Mahlalela, M., Edkins, A. L., & Boshoff, A. (2023). Tackling Sleeping Sickness: Current and Promising Therapeutics and Treatment Strategies. International Journal of Molecular Sciences, 24(15), 12529. https://doi.org/10.3390/ijms241512529







