The Roles of Hormone Signals Involved in Rhizosphere Pressure Response Induce Corm Expansion in Sagittaria trifolia
Abstract
1. Introduction
2. Results
2.1. Plant Growth Analysis
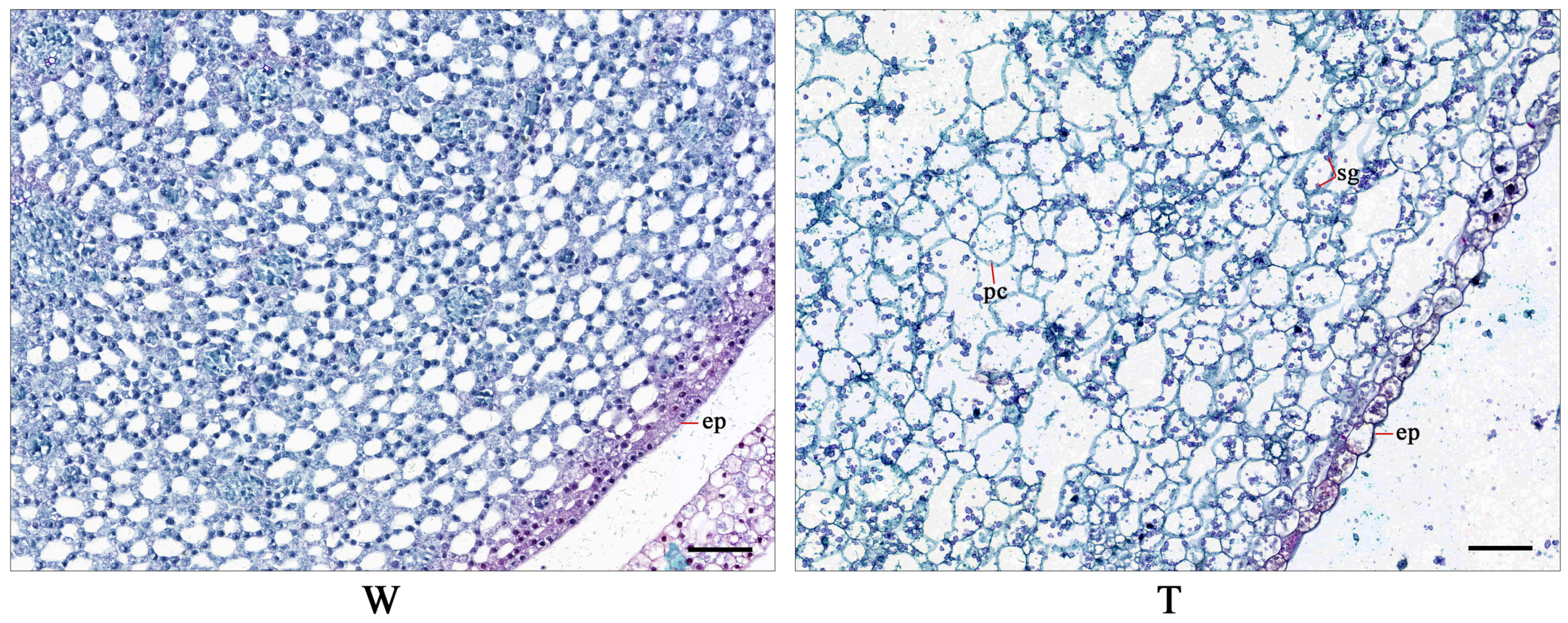
2.2. Anatomical Analysis
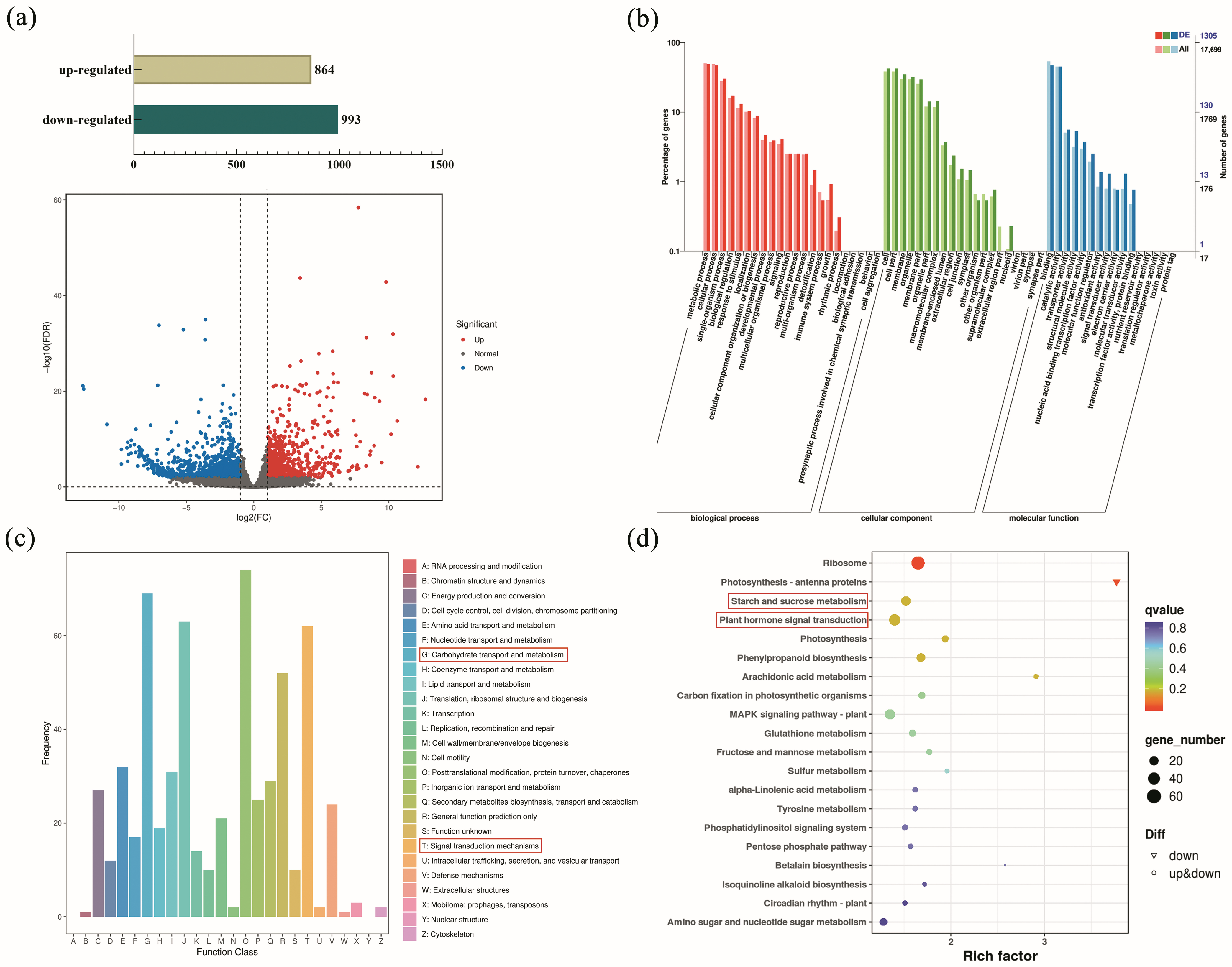
2.3. DEGs Identification and Functional Annotation
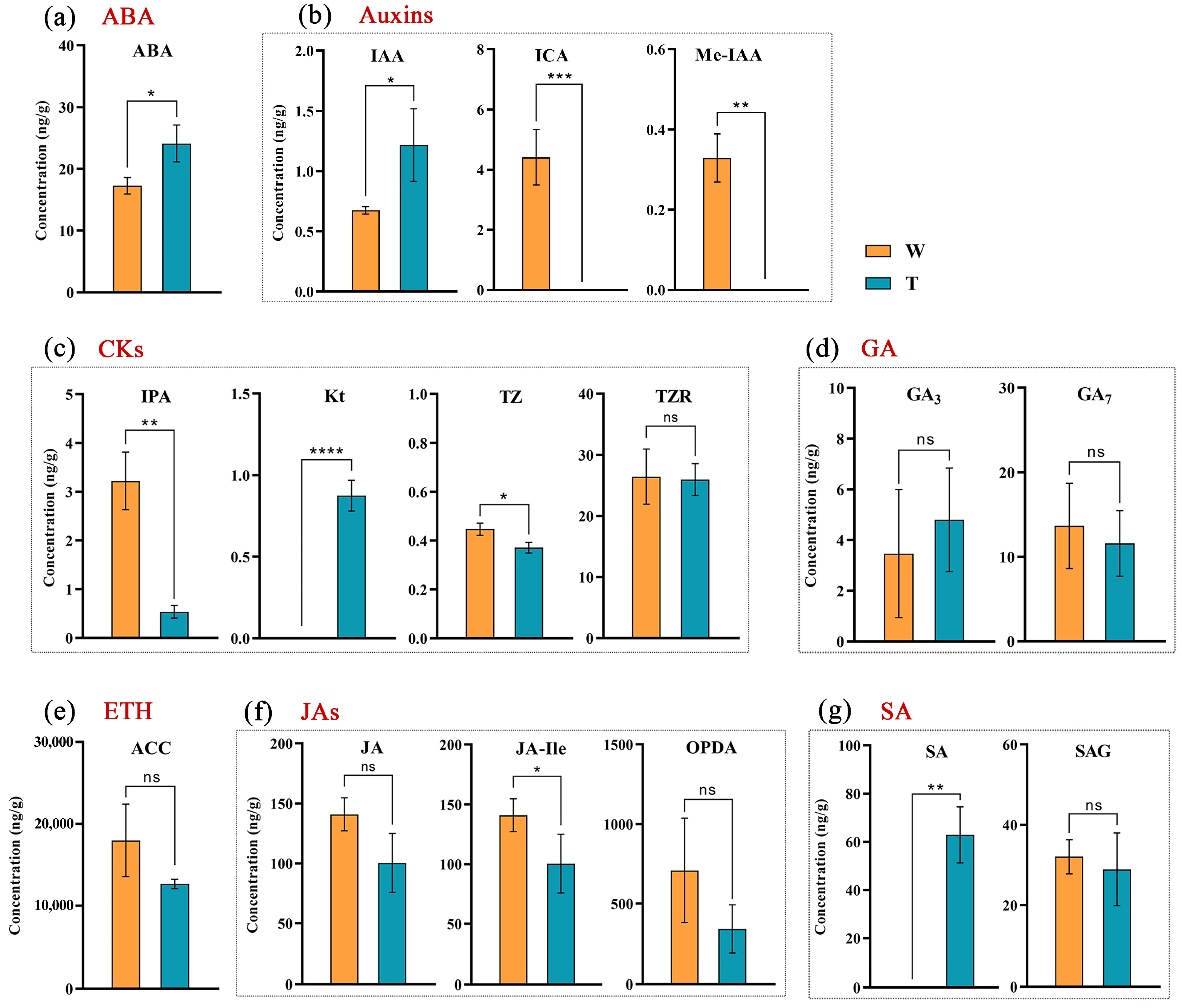
2.4. Hormone Level Determination
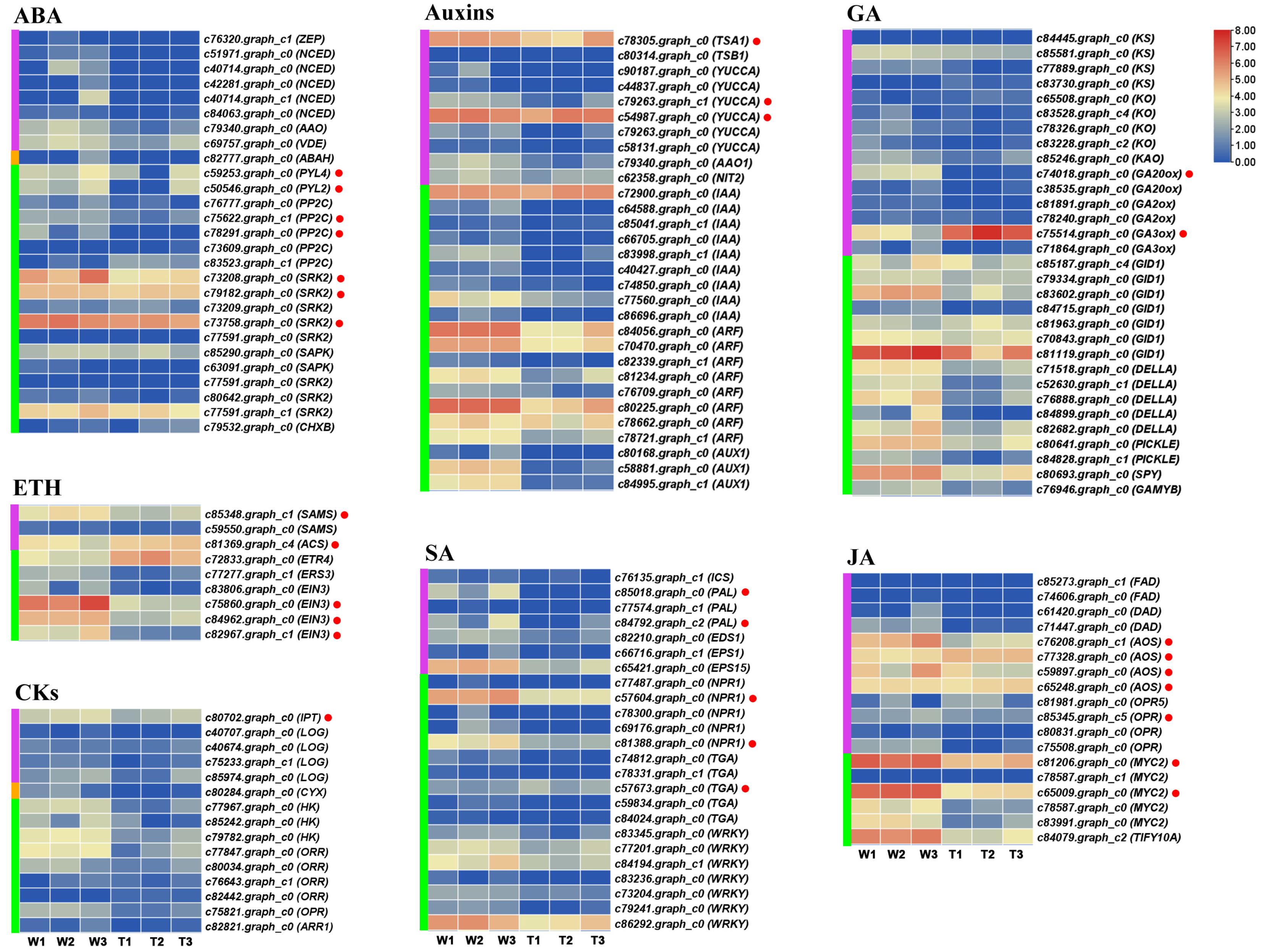
2.5. Expression Profiles of Hormone-Related Genes in RNA-Seq
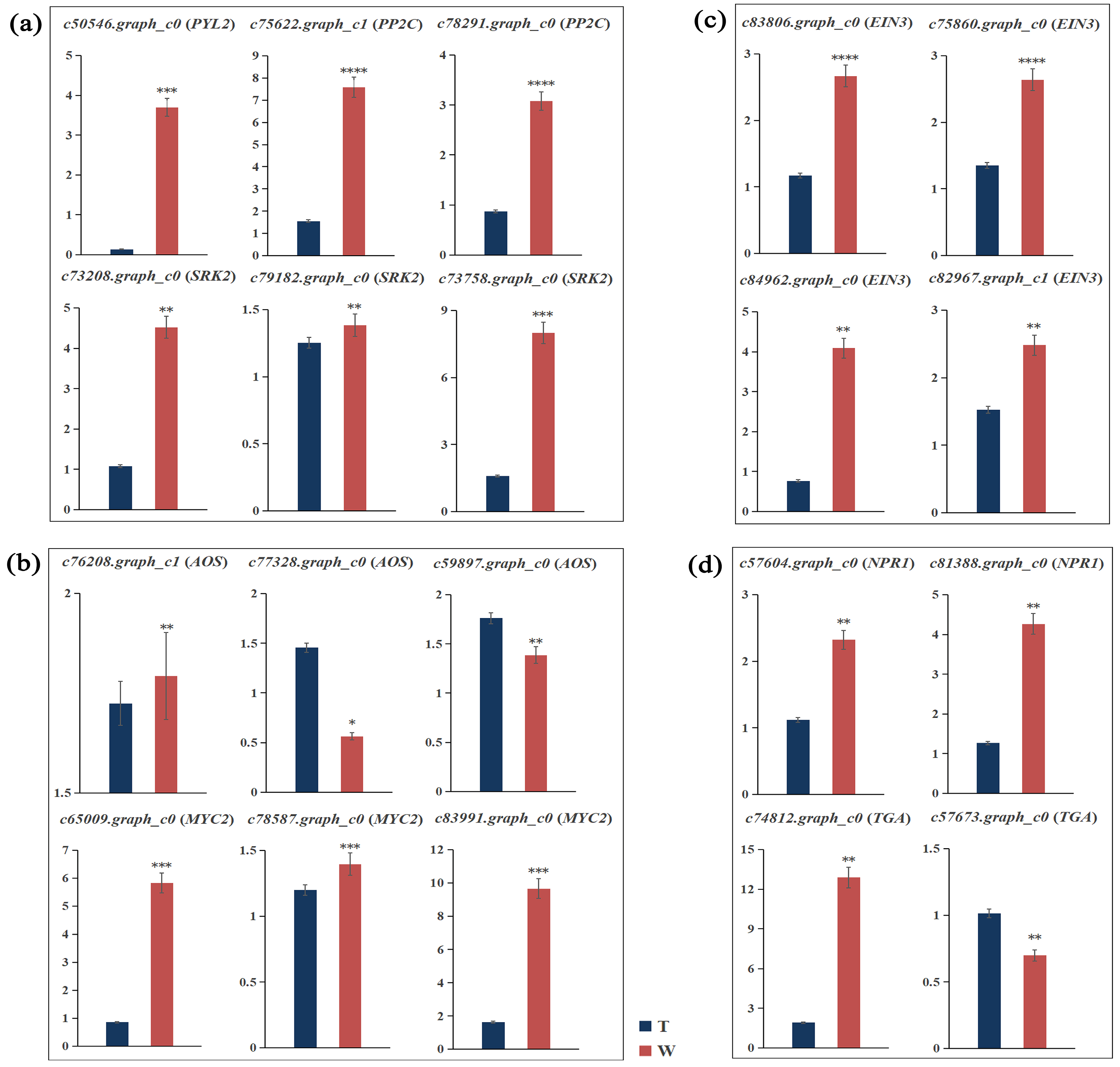
2.6. Verification of Differentially Expressed Genes in Hormone Signaling
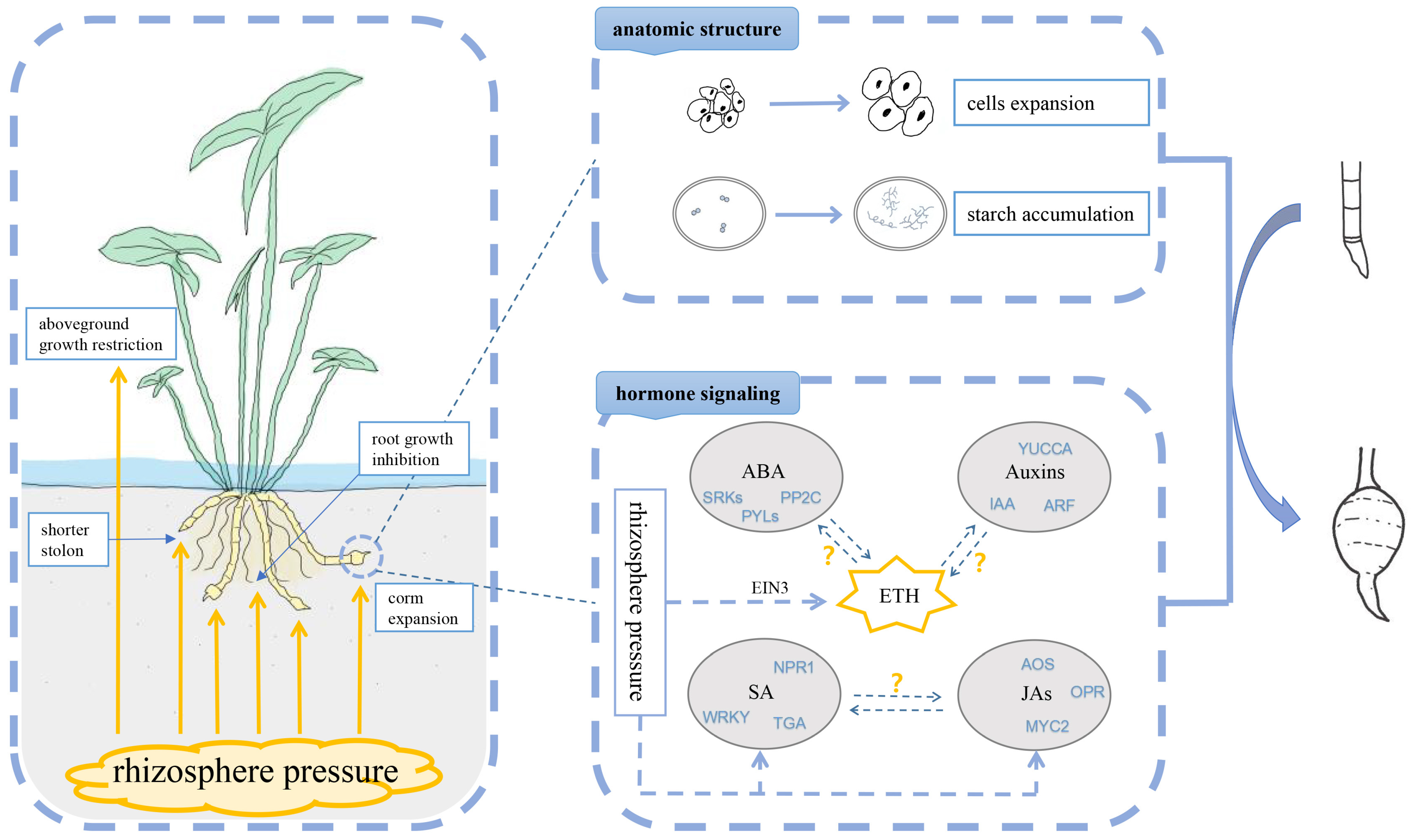
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cultivation Conditions
4.2. Observation of Anatomical Structure of Corms
4.3. RNA Extraction and Detection
4.4. Transcriptome Sequencing, Assembly, and Functional Annotation
4.5. Endogenous Hormones Determination
4.6. Identification and Analysis of Genes Involved in Various Hormones’ Metabolism Based on RNA-Seq Data
4.7. Gene Expression Analysis Using RT-qPCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil compaction effects on soil health and crop productivity: An overview. Environ. Sci. Pollut. Res. Int. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrie, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Alameda, D.; Anten, N.P.R.; Villar, R. Soil compaction effects on growth and root traits of tobacco depend on light, water regime and mechanical stress. Soil Tillage Res. 2012, 120, 121–129. [Google Scholar] [CrossRef]
- Glab, T. Effect of soil compaction on root system morphology and productivity of alfalfa (Medicago sativa L.). Environ. Sci. Pollut. Res. Int. 2011, 20, 1473–1480. [Google Scholar]
- Canher, B.; Lanssens, F.; Zhang, A.; Bisht, A.; Mazumdar, S.; Heyman, J.; Wolf, S.; Melnyk, C.W.; De Veylder, L. The regeneration factors ERF114 and ERF115 regulate auxin-mediated lateral root development in response to mechanical cues. Mol. Plant. 2022, 15, 1543–1557. [Google Scholar] [CrossRef]
- Atwell, B.J. Response of roots to mechanical impedance. Environ. Exp. Bot. 1993, 33, 27–40. [Google Scholar] [CrossRef]
- Iijima, M.; Kato, J. Combined soil physical stress of soil drying, anaerobiosis and mechanical impedance to seedling root growth of four crop species. Plant Prod. Sci. 2007, 10, 451–459. [Google Scholar] [CrossRef]
- Montagu, K.D.; Conroy, J.P.; Atwell, B.J. The position of localized soil compaction determines root and subsequent shoot growth responses. J. Exp. Bot. 2001, 52, 2127–2133. [Google Scholar] [CrossRef]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef]
- Feng, X.; Xiong, J.; Hu, Y.; Pan, L.; Liao, Z.; Zhang, X.; Guo, W.; Wu, F.; Xu, J.; Hu, E.; et al. Lateral mechanical impedance rather than frontal promotes cortical expansion of roots. Int. J. Mol. Sci. 2020, 15, 1757918. [Google Scholar] [CrossRef]
- Whalen, M.C.; Feldman, L.J. Effects of mechanical impedance on root development. In Rhizosphere Dynamics; CRC Press: Boca Raton, FL, USA, 2019; pp. 260–267. [Google Scholar]
- Santisree, P.; Nongmaithem, S.; Sreelakshmi, Y.; Ivanchenko, M.G.; Sharma, R. The root as a drill an ethylene-auxin interaction facilitates root penetration in soil. Plant. Signal. Behav. 2012, 7, 151–156. [Google Scholar] [CrossRef]
- Shi, H.; Liu, R.; Xue, C.; Shen, X.; Wei, N.; Deng, X.W.; Zhong, S. Seedlings transduce the depth and mechanical pressure of covering soil using COP1 and ethylene to regulate EBF1/EBF2 for soil emergence. Curr. Biol. 2016, 26, 139–149. [Google Scholar] [CrossRef]
- Jacobsen, A.G.R.; Jervis, G.; Xu, J.; Topping, J.F.; Lindsey, K. Root growth responses to mechanical impedance are regulated by a network of ROS, ethylene and auxin signalling in Arabidopsis. New. Phytol. 2021, 231, 225–242. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Dodd, I.C.; Mooney, S.J. Using X-ray computed tomography to explore the role of abscisic acid in moderating the impact of soil compaction on root system architecture. Environ. Exp. Bot. 2015, 110, 11–18. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.S.; Park, J.M.; Cho, H.S.; Jeon, J.H. PIN-mediated polar auxin transport facilitates root-obstacle avoidance. New. Phytol. 2020, 225, 1285–1296. [Google Scholar] [CrossRef]
- Kumari, A.; Nongmaithem, S.; Devulapalli, S.; Sreelakshmi, Y.; Sharma, R. Tomato root senses horizontal/vertical mechanical impedance and correspondingly modulates root/shoot metabolome. Environ. Exp. Bot. 2021, 191, 104608. [Google Scholar] [CrossRef]
- Niu, S.; Li, W.; Chen, X. Negative regulation of gibberellin on root tip diameter. J. Beijing For. Univ. 2013, 35, 71–76. [Google Scholar]
- Huang, G.; Kilic, A.; Karady, M.; Zhang, J.; Mehra, P.; Song, X.; Sturrock, C.J.; Zhu, W.; Qin, H.; Hartman, S.; et al. Ethylene inhibits rice root elongation in compacted soil via ABA- and auxin-mediated mechanisms. Proc. Natl. Acad. Sci. USA 2022, 119, e2201072119. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Alonso, J.M. Ethylene signaling and response: Where different regulatory modules meet. Curr. Opin. Plant Biol. 2009, 12, 548–555. [Google Scholar] [CrossRef]
- Promchuea, S.; Zhu, Y.; Chen, Z.; Zhang, J.; Gong, Z. ARF2 coordinates with PLETHORAs and PINs to orchestrate ABA-mediated root meristem activity in Arabidopsis. J. Integr. Plant Biol. 2017, 59, 30–43. [Google Scholar] [CrossRef]
- Sun, L.R.; Wang, Y.B.; He, S.B.; Hao, F.S. Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal. Behav. 2018, 13, e1500069. [Google Scholar] [CrossRef]
- Takatsuka, H.; Umeda, M. ABA inhibits root cell elongation through repressing the cytokinin signaling. Plant Signal. Behav. 2019, 14, e1578632. [Google Scholar] [CrossRef]
- Singh, A.V.; Singh, P.K. An account of Sagittaria sagittifolia with special reference to phytochemical studies and its socio-economic relevance. J. Phytol. Res. 2009, 22, 235–246. [Google Scholar]
- Heffer, P.; Prud’homme, M. Outlook for world fertilizer demand, supply, and supply/demand balance. Turk. J. Agric. For. 2008, 32, 159–164. [Google Scholar]
- Khan, S.; Purohit, A.; Vadsaria, N. Hydroponics: Current and future state of the art in farming. J. Plant Nutr. 2020, 44, 1515–1538. [Google Scholar] [CrossRef]
- Alameda, D.; Villar, R. Linking root traits to plant physiology and growth in Fraxinus angustifolia Vahl. seedlings under soil compaction conditions. Environ. Exp. Bot. 2012, 79, 49–57. [Google Scholar] [CrossRef]
- Li, M.; Pu, Z.; Tan, W.; Zhang, C.; Yang, S.; Feng, J.; Yan, W. Chlorophyll fluorescence of key stages of storage roots formation in grafted overhead-sweetpotato cultivation system. Southwest China J. Agric. Sci. 2015, 28, 2475–2478. [Google Scholar]
- Nhut, D.T.; Nguyen, N.H.; Thuy, D.T.T. A novel in vitro hydroponic culture system for potato (Solanum tuberosum L.) microtuber production. Sci. Hortic. 2006, 110, 230–234. [Google Scholar] [CrossRef]
- Lim, H.T.; Yoon, C.S.; Choi, S.P.; Dhital, S.P. Application of gibberellic acid and paclobutrazol for efficient production of potato (Solanum tuberosum L.) minitubers and their dormancy breaking under soilless culture system. J. Jpn. Soc. Hortic. Sci. 2004, 45, 189–193. [Google Scholar]
- Masuda, J.I.; Ozaki, Y.; Okubo, H. Regulation in rhizome transition to storage organ in lotus (Nelumbo nucifera Gaertn.) with exogenous gibberellin, gibberellin biosynthesis inhibitors or abscisic acid. J. Jpn. Soc. Hortic. Sci. 2012, 81, 67–71. [Google Scholar] [CrossRef]
- Rui, Z.; Xiu-Juan, W.; Wei, G. Regulation mechanism of plant hormones on secondary metabolites. Zhongguo Zhong Yao Za Zhi 2020, 45, 4205–4210. [Google Scholar]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Potocka, I.; Szymanowska-Pulka, J. Morphological responses of plant roots to mechanical stress. Ann. Bot. 2018, 122, 711–723. [Google Scholar] [CrossRef]
- De Smet, S.; Cuypers, A.; Vangronsveld, J.; Remans, T. Gene networks involved in hormonal control of root development in Arabidopsis thaliana: A framework for studying its disturbance by metal stress. Int. J. Mol. Sci. 2015, 16, 19195–19224. [Google Scholar] [CrossRef]
- Okamoto, T.; Takahashi, T. Ethylene signaling plays a pivotal role in mechanical-stress-induced root-growth cessation in Arabidopsis thaliana. Plant Signal. Behav. 2019, 14, 1669417. [Google Scholar] [CrossRef]
- Sarquis, J.I.; Morgan, P.W.; Jordan, W.R. Metabolism of 1-aminocyclopropane-1-carboxylic acid in etiolated maize seedlings grown under mechanical impedance. Plant Physiol. 1992, 98, 1342–1348. [Google Scholar] [CrossRef][Green Version]
- Visser, E.J.W.; Pierik, R. Inhibition of root elongation by ethylene in wetland and non-wetland plant species and the impact of longitudinal ventilation. Plant Cell Environ. 2007, 30, 31–38. [Google Scholar] [CrossRef]
- Pandey, B.K.; Huang, G.; Bhosale, R.; Hartman, S.; Sturrock, C.J.; Jose, L.; Martin, O.C.; Karady, M.; Voesenek, L.A.C.J.; Ljung, K.; et al. Plant roots sense soil compaction through restricted ethylene diffusion. Science 2021, 371, 276–280. [Google Scholar] [CrossRef]
- Lees, J.; Kim, S.; Yoo, H.Y. Future prospects for industrial application of abscisic acid, a stress-resistant phytohormone. Korean J. Chem. Eng. 2020, 58, 514–523. [Google Scholar]
- Wu, Y.; Chang, Y.; Luo, L.; Tian, W.; Gong, Q.; Liu, X. Abscisic acid employs NRP-dependent PIN2 vacuolar degradation to suppress auxin-mediated primary root elongation in Arabidopsis. New Phytol. 2022, 233, 297–312. [Google Scholar] [CrossRef]
- Han, X.; Kui, M.; He, K.; Yang, M.; Du, J.; Jiang, Y.; Hu, Y. Jasmonate-regulated root growth inhibition and root hair elongation. J. Exp. Bot. 2023, 74, 1176–1185. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic acid in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Pal, M.; Janda, T.; Szalai, G. Abscisic acid may alter the salicylic acid-related abiotic stress response in maize. J. Agron. Crop. Sci. 2011, 197, 368–377. [Google Scholar] [CrossRef]
- Lee, Y.; Heo, S.; Lee, S. Inhibition of type 2C protein phosphatases by ABA receptors in abscisic acid-mediated plant stress responses. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2462, pp. 1–16. [Google Scholar]
- Laudert, D.; Weiler, E.W. Allene oxide synthase: A major control point in Arabidopsis thaliana octadecanoid signalling. Plant J. 1998, 15, 675–684. [Google Scholar] [CrossRef]
- Pratiwi, P.; Tanaka, G.; Takahashi, T.; Xie, X.; Yoneyama, K.; Matsuura, H.; Takahashi, K. Identification of jasmonic acid and jasmonoyl-isoleucine, and characterization of AOS, AOC, OPR and JAR1 in the model lycophyte Selaginella moellendorffii. Plant Cell Physiol. 2017, 58, 789–801. [Google Scholar] [CrossRef]
- Zhu, Z.; An, F.; Feng, Y.; Li, P.; Xue, L.; Mu, A.; Jiang, Z.; Kim, J.-M.; To, T.K.; Li, W.; et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 12539–12544. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.P.; Zhang, L.; Yan, P.; Ahammed, G.J.; Han, W.Y. Methyl salicylate enhances flavonoid biosynthesis in tea leaves by stimulating the phenylpropanoid pathway. Molecules 2019, 24, 362. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, X.; Liu, R.; Zou, L. Mushrooms do produce flavonoids: Metabolite profiling and transcriptome analysis of flavonoid synthesis in the medicinal mushroom Sanghuangporus baumii. J. Fungi 2022, 8, 582. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Gene regulation at transcriptional and post-transcriptional levels to combat salt stress in plants. Physiol. Plant 2021, 173, 1556–1572. [Google Scholar] [CrossRef]
- Cheng, L.; Li, S.; Xu, X.; Hussain, J.; Yin, J.; Zhang, Y.; Li, L.; Chen, X. Identification of differentially expressed genes relevant to corm formation in Sagittaria trifolia. PLoS ONE 2013, 8, e54573. [Google Scholar] [CrossRef]
- You, Y.; Huang, X.; Liu, H.; Cheng, T.; Zheng, X.; Diao, Y.; Bao, Z.; Dong, C.; Ke, W.; Hu, Z. Leaf transcriptome analysis and development of EST-SSR markers in arrowhead (Sagittaria trifolia L. Var. Sinensis). Trop. Plant Biol. 2020, 13, 189–200. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Yan, X.; Zhang, J.; Xu, J. Simultaneous analysis of ten phytohormones in Sargassum horneri by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1804–1813. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]

| Annotation Database | Number of Annotated Genes | Percentage of Annotated Genes (%) |
|---|---|---|
| COG Annotation | 5926 | 11.85 |
| GO Annotation | 17,699 | 35.38 |
| KEGG Annotation | 13,816 | 27.62 |
| KOG Annotation | 11,765 | 23.52 |
| Pfam Annotation | 15,924 | 31.83 |
| Swissprot Annotation | 13,673 | 27.33 |
| TrEMBL Annotation | 21,016 | 42.01 |
| eggNOG Annotation | 18,223 | 36.42 |
| Nr Annotation | 21,053 | 42.08 |
| All Annotated | 22,050 | 44.07 |
| Gene ID | Primer Sequences (5′→3′) | |
|---|---|---|
| Forward | Reverse | |
| c50546.graph_c0 (PYL2) | GTCTGGTCCATCGTCCGTAGCT | TTGGTGTCCTCCTCGCTGTTCC |
| c75622.graph_c1 (PP2C) | TCCCTGAGCCAGAGGTGAGATT | TGGAGTGCAAGCCTGGATAGGT |
| c78291.graph_c0 (PP2C) | CCAACCAGGACTCCGCCATACT | CGGTCATTGCCTTGGTCGTCAT |
| c73208.graph_c0 (SRK2) | GCATTCACAGCCTAAGTCAACAGTT | CCGCAGAGATGGATTGCCAACAA |
| c79182.graph_c0 (SRK2) | CCGCTTCTTCTTCCAGCAACTC | CACACGACCATACATCCGCAAT |
| c73758.graph_c0 (SRK2) | ACTCCAAGTCCTCCGTGCTTCA | CAGACTGGGCTGCTTCGGTTAG |
| c76208.graph_c1 (AOS) | CCTCTTCGCCACCTGCTTCAAC | GCCTCGTAGACCACCGACTTCA |
| c77328.graph_c0 (AOS) | AGGACCGCCTTGACTACTTCTACC | TTGACGGCATGTAGGTTCCTGTGA |
| c59897.graph_c0 (AOS) | ATCGAGGAGCAGATCGCCAAGG | TGAAGCCGCCGAAGGAGTTGA |
| c65009.graph_c0 (MYC2) | AGGACCATATCATCGCCGAGAGG | ACTTCTTAACCAGGACAACCGACTC |
| c78587.graph_c0 (MYC2) | CCAACCTGCACAACAACCTCTG | AAGCGGCGTCTTCGTGTAGC |
| c83991.graph_c0 (MYC2) | TCACGCTCCCTACTGGCAAAGA | ACCCTCACTTGGCGGACTATCA |
| c83806.graph_c0 (EIN3) | CATCCACCACCGCCTCAATCCT | CTGCTGCTGCCGTTGCTGTT |
| c75860.graph_c0 (EIN3) | GCAGCAGCAGCAGTTCAATCC | CGGAAGTCACCAGCGGCATT |
| c84962.graph_c0 (EIN3) | CGAACCTCAAGTGCGTGTCCTT | GCGTTGCCCATCACCCAGAA |
| c82967.graph_c1 (EIN3) | AGCACAGTGGTGAGAAGCAGGA | AACTTCCGTTGTCGCTCCCTCT |
| c57604.graph_c0 (NPR1) | TGCGAGAAGTTGCTGGACAAGT | GCGTATGCGTCATCCAAGGTTG |
| c81388.graph_c0 (NPR1) | TGGCAGCGGTGTACTGGTCAA | ACGGTGAGAGGTGAGCATGGAA |
| c74812.graph_c0 (TGA) | CGCTGCTTCATGTGGCTTGGT | CGGTTGTGGTACTCGCCTATGG |
| c57673.graph_c0 (TGA) | CGCCAGAGAATGAGGTGAGGTT | AGTCCTTGCGAGAGTGAATCCT |
| c76930.graph_c0 (SLEEPER) | CCTGTATTGCTTGGAAGTGGTAT | CCGTCGTCTTGATTGAATGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, E.; Tang, J.; Liu, J.; Zhang, Z.; Hua, B.; Jiang, J.; Miao, M. The Roles of Hormone Signals Involved in Rhizosphere Pressure Response Induce Corm Expansion in Sagittaria trifolia. Int. J. Mol. Sci. 2023, 24, 12345. https://doi.org/10.3390/ijms241512345
Li E, Tang J, Liu J, Zhang Z, Hua B, Jiang J, Miao M. The Roles of Hormone Signals Involved in Rhizosphere Pressure Response Induce Corm Expansion in Sagittaria trifolia. International Journal of Molecular Sciences. 2023; 24(15):12345. https://doi.org/10.3390/ijms241512345
Chicago/Turabian StyleLi, Enjiao, Jing Tang, Jiexia Liu, Zhiping Zhang, Bing Hua, Jiezeng Jiang, and Minmin Miao. 2023. "The Roles of Hormone Signals Involved in Rhizosphere Pressure Response Induce Corm Expansion in Sagittaria trifolia" International Journal of Molecular Sciences 24, no. 15: 12345. https://doi.org/10.3390/ijms241512345
APA StyleLi, E., Tang, J., Liu, J., Zhang, Z., Hua, B., Jiang, J., & Miao, M. (2023). The Roles of Hormone Signals Involved in Rhizosphere Pressure Response Induce Corm Expansion in Sagittaria trifolia. International Journal of Molecular Sciences, 24(15), 12345. https://doi.org/10.3390/ijms241512345






