Matching Mechanics and Energetics of Muscle Contraction Suggests Unconventional Chemomechanical Coupling during the Actin–Myosin Interaction
Abstract
1. Introduction
2. Results
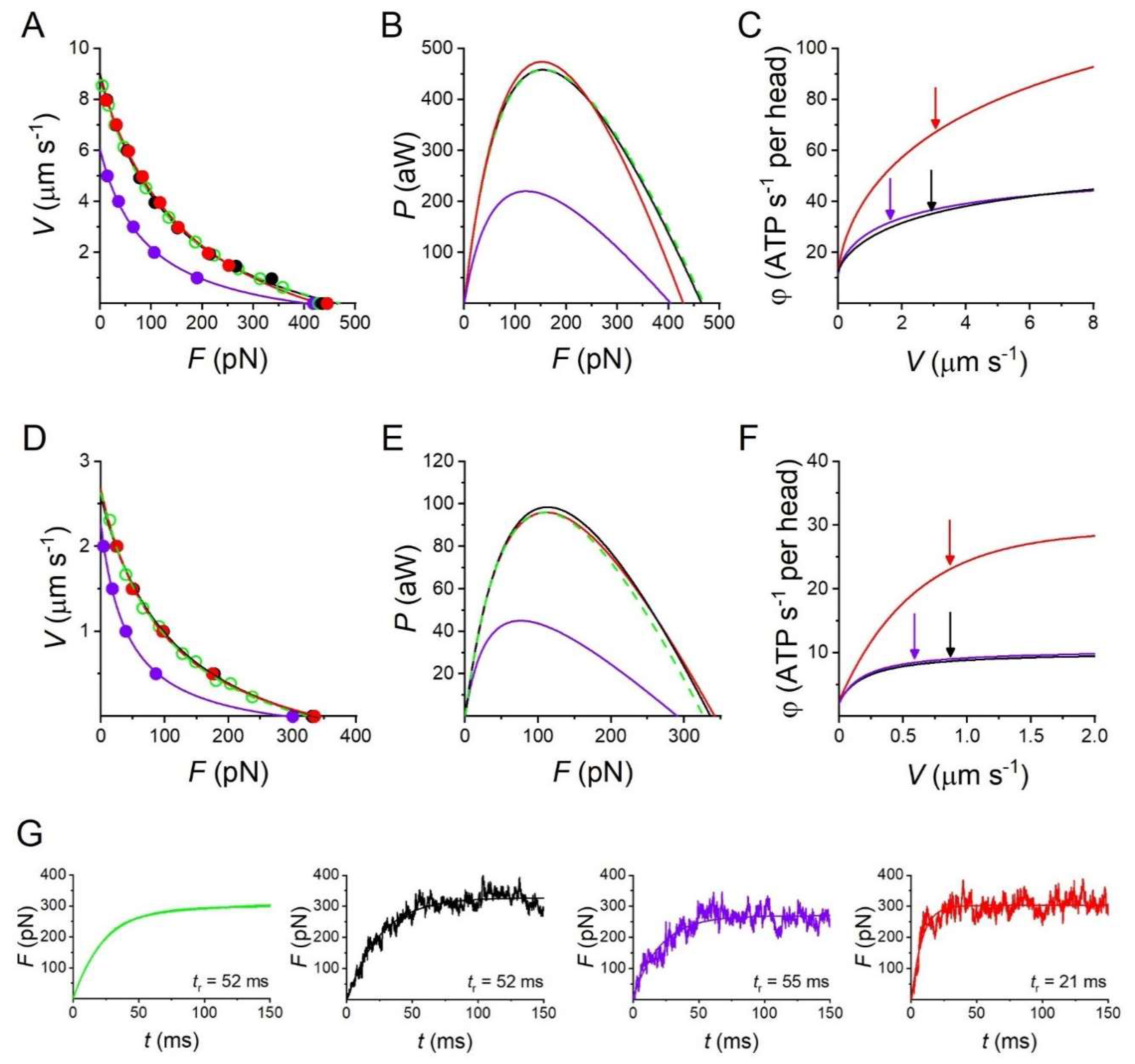
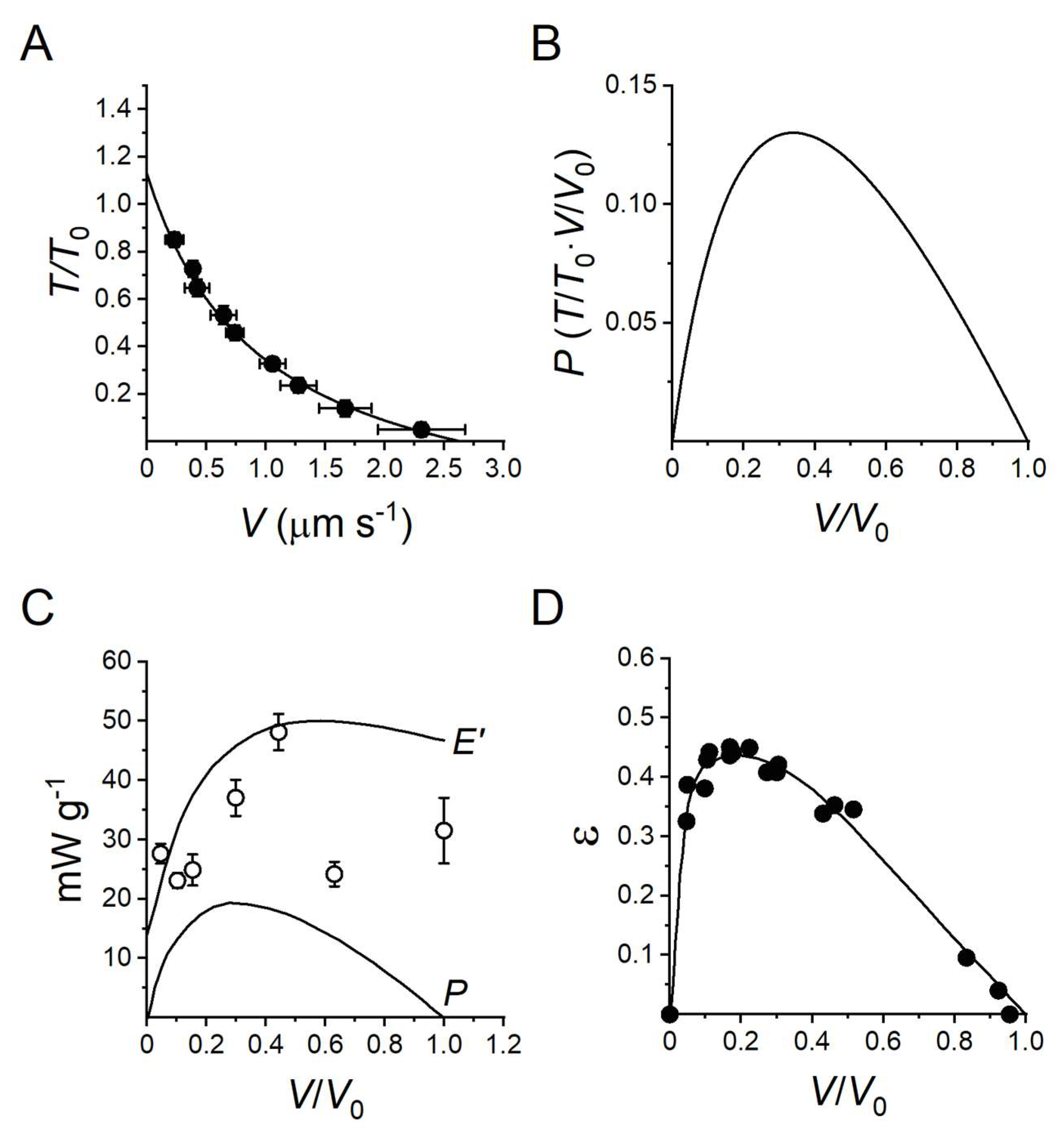
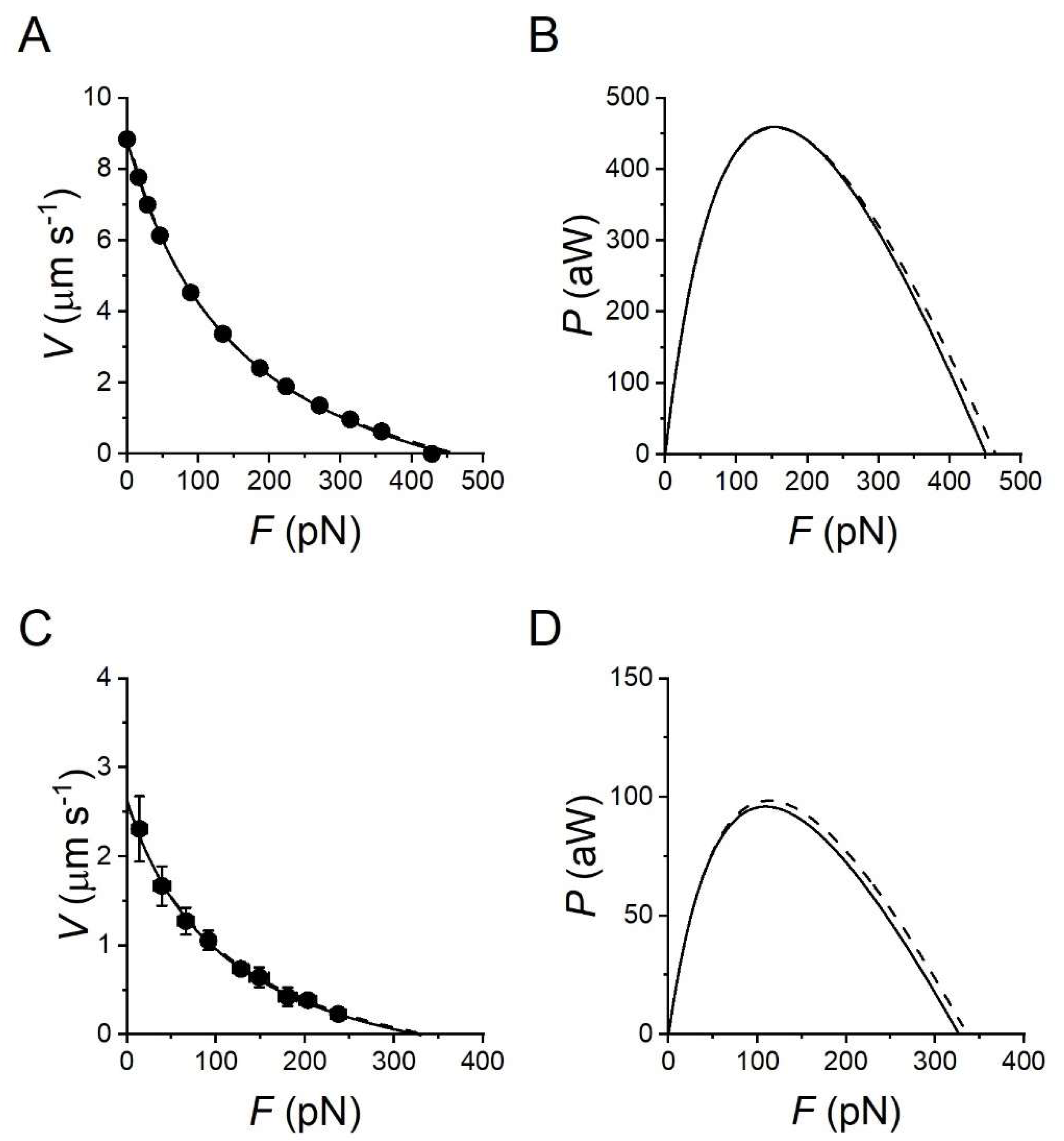
2.1. Mechanics and Energetics of Skeletal Muscle
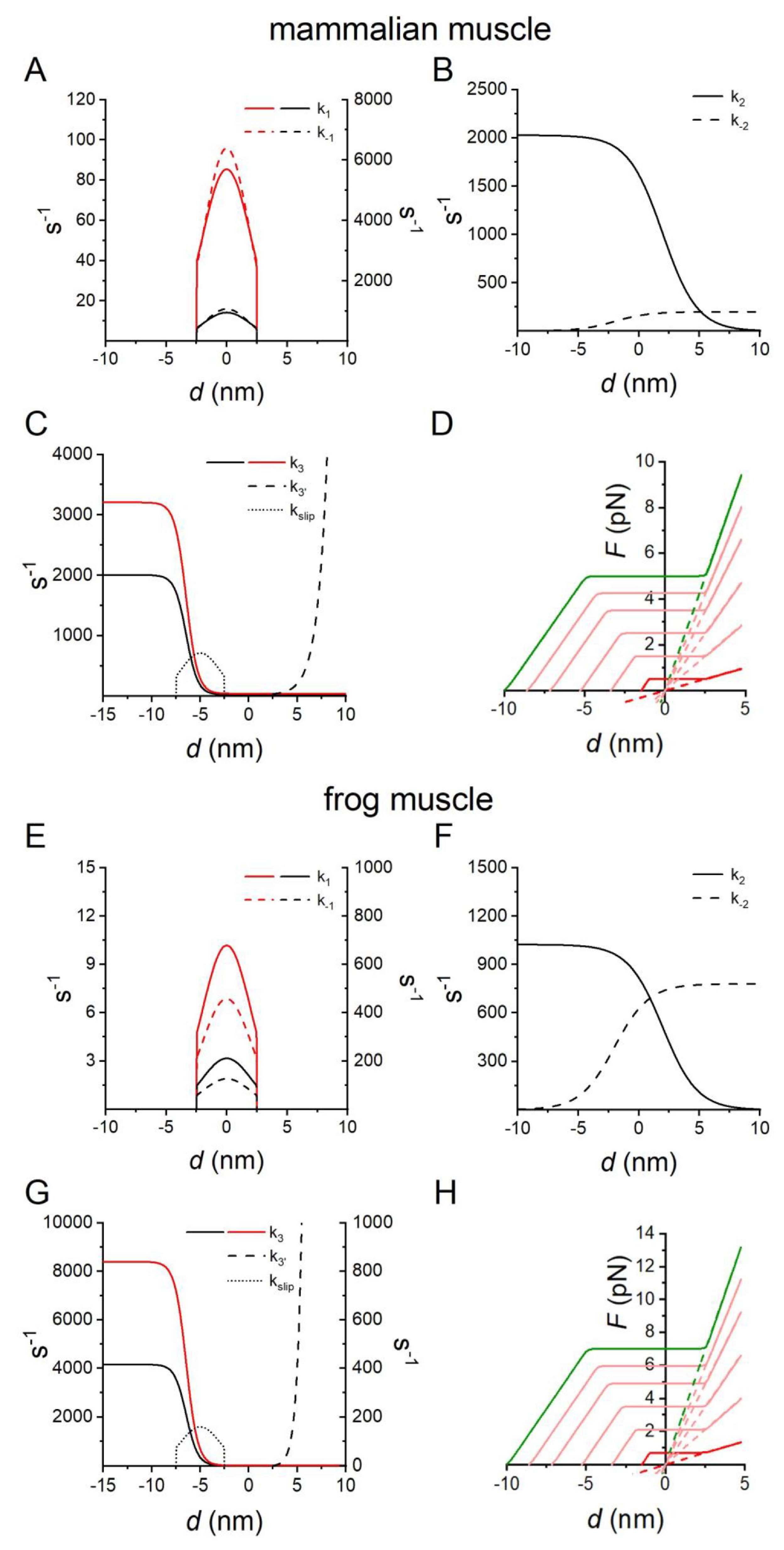
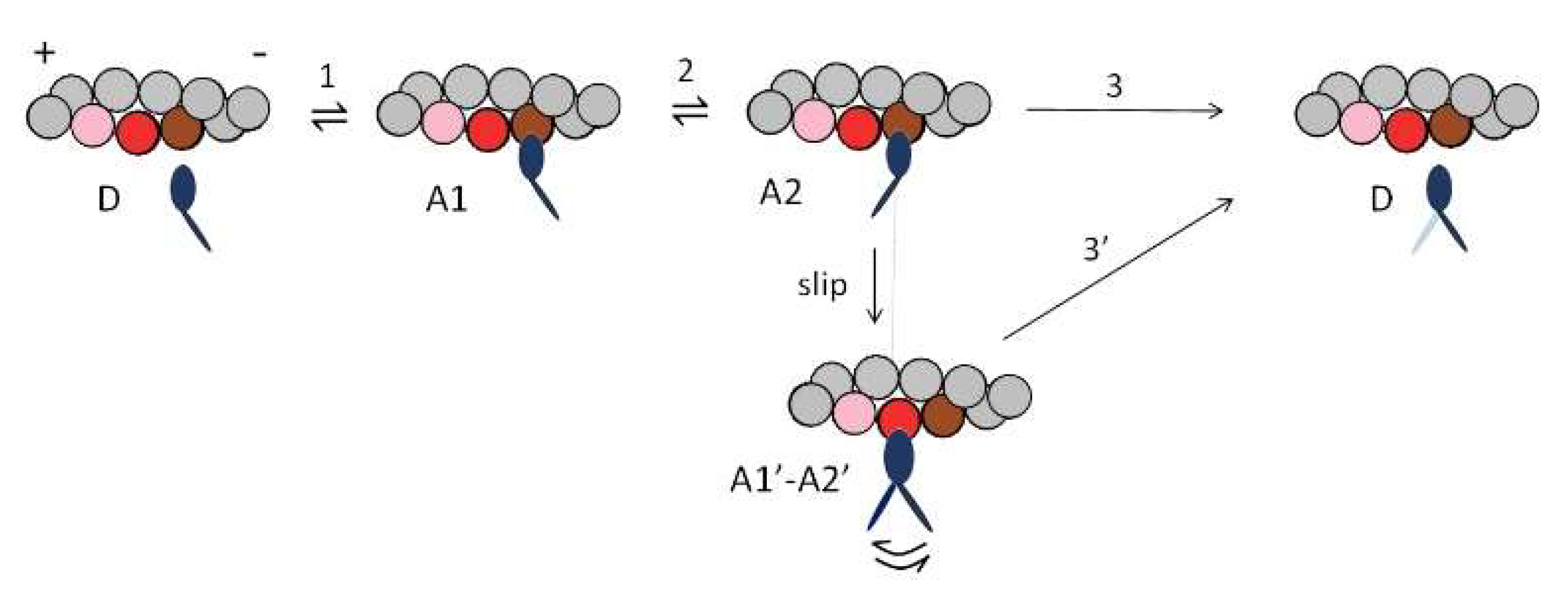
2.2. Fitting the Performance of Muscle Contraction with a Simple Three-State Model
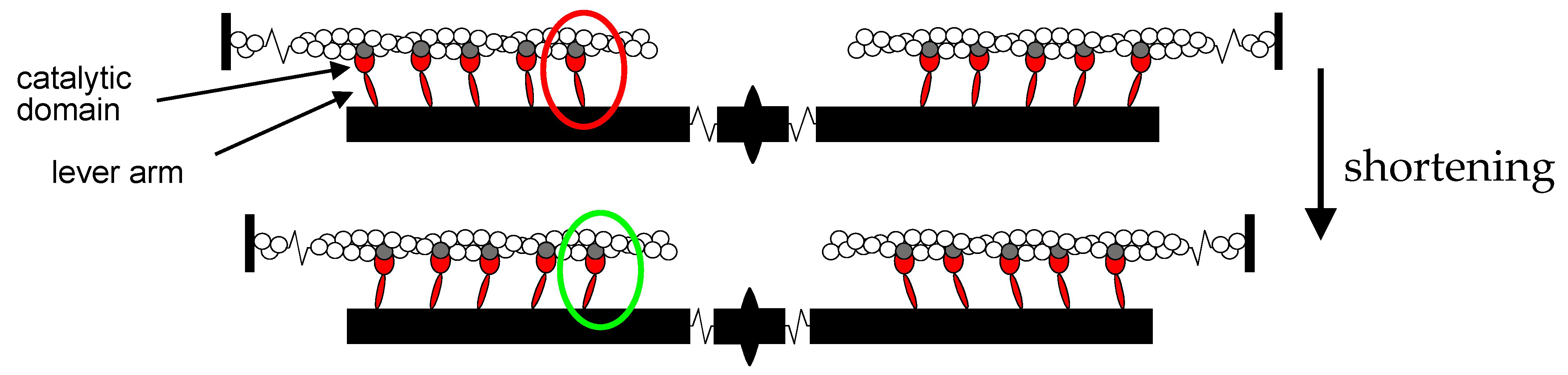
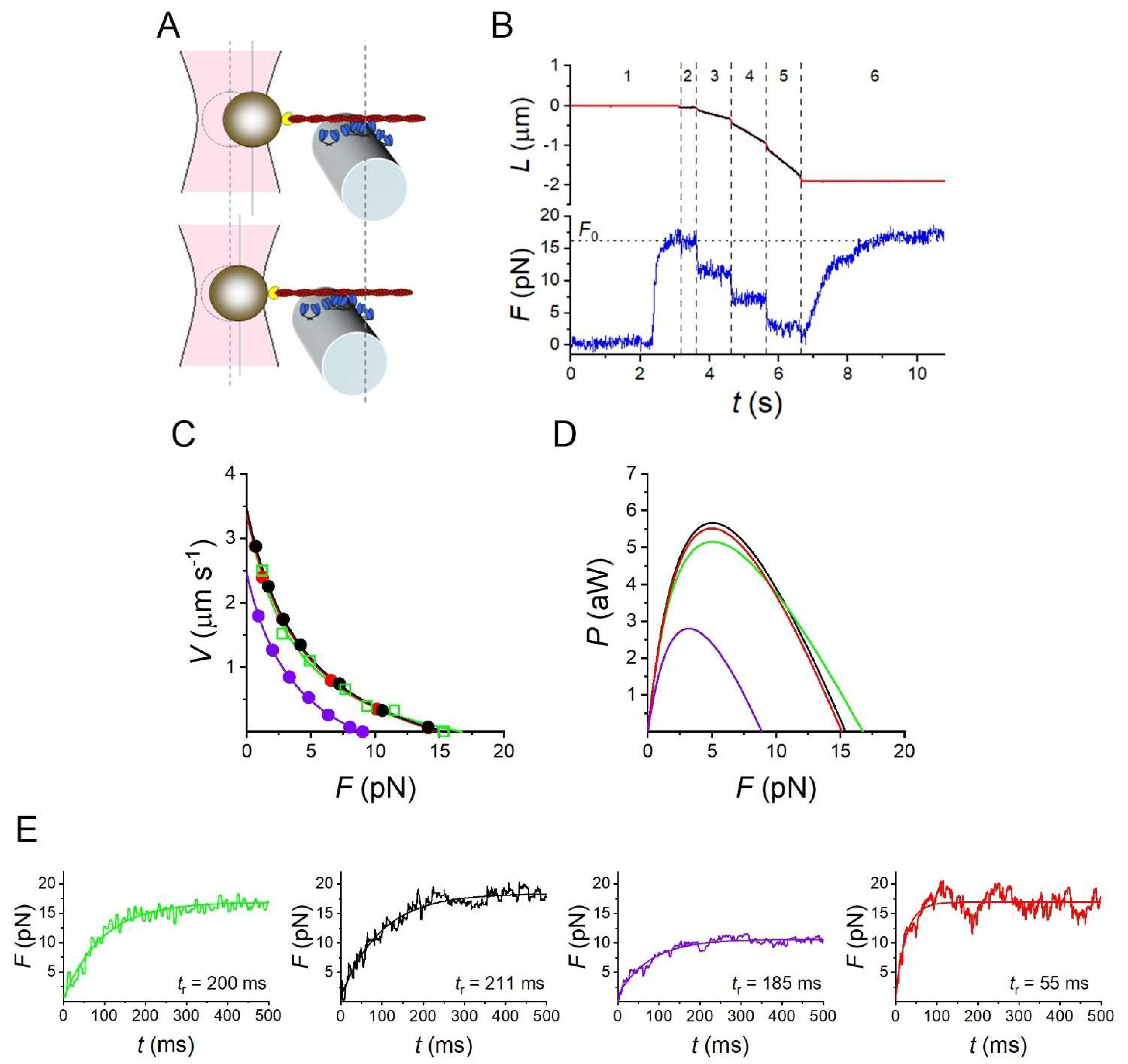
2.3. Mechanics and Energetics of the Sarcomere-like Nanomachine
3. Discussion
4. Material and Methods
4.1. Experimental Data
4.2. Model Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huxley, A.F. Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.E. The mechanism of muscular contraction. Science 1969, 164, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Lymn, R.W.; Taylor, E.W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 1971, 10, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Rayment, I.; Holden, H.M.; Whittaker, M.; Yohn, C.B.; Lorenz, M.; Holmes, K.C.; Milligan, R.A. Structure of the actin-myosin complex and its implications for muscle contraction. Science 1993, 261, 58–65. [Google Scholar] [CrossRef]
- Geeves, M.A.; Holmes, K.C. Structural Mechanism of Muscle Contraction. Annu. Rev. Biochem. 1999, 68, 687–728. [Google Scholar] [CrossRef]
- Brunello, E.; Caremani, M.; Melli, L.; Linari, M.; Fernandez-Martinez, M.; Narayanan, T.; Irving, M.; Piazzesi, G.; Lombardi, V.; Reconditi, M. The contributions of filaments and cross-bridges to sarcomere compliance in skeletal muscle. J. Physiol. 2014, 592, 3881–3899. [Google Scholar] [CrossRef]
- Zeng, W.; Conibear, P.B.; Dickens, J.L.; Cowie, R.A.; Wakelin, S.; Málnási-Csizmadia, A.; Bagshaw, C.R. Dynamics of actomyosin interactions in relation to the cross-bridge cycle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1843–1855. [Google Scholar]
- Hill, A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B Biol. Sci. 1938, 126, 136–195. [Google Scholar]
- Fenn, W.O. The relation between the work performed and the energy liberated in muscular contraction. J. Physiol. 1924, 58, 373–395. [Google Scholar] [CrossRef]
- Woledge, R.C.; Curtin, N.A.; Homsher, E. Energetic aspects of muscle contraction. Monogr. Physiol. Soc. 1985, 41, 1–357. [Google Scholar]
- Smith, D.A.; Geeves, M.A. Strain-dependent cross-bridge cycle for muscle. Biophys. J. 1995, 69, 524–537. [Google Scholar] [CrossRef]
- Smith, D.A.; Geeves, M.A. Strain-dependent cross-bridge cycle for muscle. II. Steady-state behavior. Biophys. J. 1995, 69, 538–552. [Google Scholar] [CrossRef]
- Smith, D.A.; Geeves, M.A.; Sleep, J.; Mijailovich, S.M. Towards a Unified Theory of Muscle Contraction. I: Foundations. Ann. Biomed. Eng. 2008, 36, 1624–1640. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Kayser-Herold, O.; Stojanovic, B.; Nedic, D.; Irving, T.C.; Geeves, M.A. Three-dimensional stochastic model of actin-myosin binding in the sarcomere lattice. J. Gen. Physiol. 2016, 148, 459–488. [Google Scholar] [CrossRef]
- Kushmerick, M.J.; Davies, R.E. The Chemical Energetics of Muscle Contraction. II. The Chemistry, Efficiency and Power of Maximally Working Sartorius Muscles. Proc. R. Soc. Lond. B Biol. Sci. 1969, 174, 315–353. [Google Scholar]
- Hill, A.V. The Effect of Load on the Heat of Shortening of Muscle. Proc. R. Soc. Lond. B Biol. Sci. 1964, 159, 297–318. [Google Scholar]
- Barclay, C.J.; Woledge, R.C.; Curtin, N.A. Inferring crossbridge properties from skeletal muscle energetics. Prog. Biophys. Mol. Biol. 2010, 102, 53–71. [Google Scholar] [CrossRef]
- Pertici, I.; Bongini, L.; Melli, L.; Bianchi, G.; Salvi, L.; Falorsi, G.; Squarci, C.; Bozó, T.; Cojoc, D.; Kellermayer, M.S.Z.; et al. A myosin II nanomachine mimicking the striated muscle. Nat. Commun. 2018, 9, 3532. [Google Scholar]
- Linari, M.; Caremani, M.; Piperio, C.; Brandt, P.; Lombardi, V. Stiffness and fraction of Myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophys. J. 2007, 92, 2476–2490. [Google Scholar] [CrossRef]
- Ranatunga, K.W. Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J. Physiol. 1982, 329, 465–483. [Google Scholar]
- Ranatunga, K.W. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J. Physiol. 1984, 351, 517–529. [Google Scholar] [PubMed]
- Ranatunga, K.W. Temperature Effects on Force and Actin-Myosin Interaction in Muscle: A Look Back on Some Experimental Findings. Int. J. Mol. Sci. 2018, 19, 1538. [Google Scholar] [CrossRef] [PubMed]
- Percario, V.; Boncompagni, S.; Protasi, F.; Pertici, I.; Pinzauti, F.; Caremani, M. Mechanical parameters of the molecular motor myosin II determined in permeabilised fibres from slow and fast skeletal muscles of the rabbit. J. Physiol. 2018, 596, 1243–1257. [Google Scholar] [PubMed]
- Piazzesi, G.; Caremani, M.; Linari, M.; Reconditi, M.; Lombardi, V. Thick Filament Mechano-Sensing in Skeletal and Cardiac Muscles: A Common Mechanism Able to Adapt the Energetic Cost of the Contraction to the Task. Front. Physiol. 2018, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Pertici, I.; Bianchi, G.; Bongini, L.; Lombardi, V.; Bianco, P. A Myosin II-Based Nanomachine Devised for the Study of Ca2+-Dependent Mechanisms of Muscle Regulation. Int. J. Mol. Sci. 2020, 21, 7372. [Google Scholar] [CrossRef] [PubMed]
- Barclay, C.J.; Constable, J.K.; Gibbs, C.L. Energetics of fast- and slow-twitch muscles of the mouse. J. Physiol. 1993, 472, 61–80. [Google Scholar] [PubMed]
- Barclay, C.J.; Wooledge, R.C.; Curtin, N.A. Is the efficiency of mammalian (mouse) skeletal muscle temperature dependent? J. Physiol. 2010, 588 Pt 19, 3819–3831. [Google Scholar]
- Reggiani, C.; Potma, E.J.; Bottinelli, R.; Canepari, M.; Pellegrino, M.A.; Stienen, G.J. Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat. J. Physiol. 1997, 502 Pt 2, 449–460. [Google Scholar] [CrossRef]
- Potma, E.J.; Stienen, G.J. Increase in ATP consumption during shortening in skinned fibres from rabbit psoas muscle: Effects of inorganic phosphate. J. Physiol. 1996, 496 Pt 1, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.B.; Hilber, K.; Irving, M. Effect of active shortening on the rate of ATP utilisation by rabbit psoas muscle fibres. J. Physiol. 2001, 531 Pt 3, 781–791. [Google Scholar] [CrossRef]
- He, Z.H.; Bottinelli, R.; Pellegrino, M.A.; Ferenczi, M.A.; Reggiani, C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys. J. 2000, 79, 945–961. [Google Scholar] [CrossRef]
- Reggiani, C.; Bottinelli, R.; Stienen, G.J. Sarcomeric Myosin Isoforms: Fine Tuning of a Molecular Motor. News Physiol. Sci. 2000, 15, 26–33. [Google Scholar] [CrossRef]
- Lombardi, V.; Piazzesi, G.; Linari, M. Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature 1992, 355, 638–641. [Google Scholar] [CrossRef]
- Piazzesi, G.; Lombardi, V. A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys. J. 1995, 68, 1966–1979. [Google Scholar] [CrossRef]
- Caremani, M.; Melli, L.; Dolfi, M.; Lombardi, V.; Linari, M. The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site. J. Physiol. 2013, 591, 5187–5205. [Google Scholar] [CrossRef]
- Huxley, A.F.; Simmons, R.M. Proposed mechanism of force generation in striated muscle. Nature 1971, 233, 533–538. [Google Scholar] [CrossRef]
- Craig, R.; Offer, G. Axial arrangement of crossbridges in thick filaments of vertebrate skeletal muscle. J. Mol. Biol. 1976, 102, 325–332. [Google Scholar] [CrossRef]
- Linari, M.; Brunello, E.; Reconditi, M.; Fusi, L.; Caremani, M.; Narayanan, T.; Piazzesi, G.; Lombardi, V.; Irving, M. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 2015, 528, 276–279. [Google Scholar] [CrossRef]
- Fusi, L.; Percario, V.; Brunello, E.; Caremani, M.; Bianco, P.; Powers, J.D.; Reconditi, M.; Lombardi, V.; Piazzesi, G. Minimum number of myosin motors accounting for shortening velocity under zero load in skeletal muscle. J. Physiol. 2017, 595, 1127–1142. [Google Scholar] [CrossRef]
- Bianco, P.; Bongini, L.; Melli, L.; Dolfi, M.; Lombardi, V. PicoNewton-millisecond force steps reveal the transition kinetics and mechanism of the double-stranded DNA elongation. Biophys. J. 2011, 101, 866–874. [Google Scholar] [CrossRef][Green Version]
- Woodhead, J.L.; Zhao, F.Q.; Craig, R.; Egelman, E.H.; Alamo, L.; Padrón, R. Atomic model of a myosin filament in the relaxed state. Nature 2005, 436, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.A.; Franks-Skiba, K.; Chen, S.; Cooke, R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 2010, 107, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Homsher, E.; Irving, M.; Wallner, A. High-energy phosphate metabolism and energy liberation associated with rapid shortening in frog skeletal muscle. J. Physiol. 1981, 321, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, L.; Fukunaga, H.; Yanagida, T.; Iwaki, M. The Synergic Role of Actomyosin Architecture and Biased Detachment in Muscle Energetics: Insights in Cross Bridge Mechanism Beyond the Lever-Arm Swing. Int. J. Mol. Sci. 2021, 22, 7037. [Google Scholar] [CrossRef] [PubMed]
- Mansson, A. Hypothesis: Single Actomyosin Properties Account for Ensemble Behavior in Active Muscle Shortening and Isometric Contraction. Int. J. Mol. Sci. 2020, 21, 8399. [Google Scholar] [CrossRef]
- Ford, L.E.; Huxley, A.F.; Simmons, R.M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J. Physiol. 1977, 269, 441–515. [Google Scholar] [CrossRef]
- Irving, M.; Lombardi, V.; Piazzesi, G.; Ferenczi, M.A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature 1992, 357, 156–158. [Google Scholar] [CrossRef]
- Lombardi, V.; Piazzesi, G.; Ferenczi, M.A.; Thirlwell, H.; Dobbie, I.; Irving, M. Elastic distortion of myosin heads and repriming of the working stroke in muscle. Nature 1995, 374, 553–555. [Google Scholar] [CrossRef]
- Caremani, M.; Melli, L.; Dolfi, M.; Lombardi, V.; Linari, M. Force and number of myosin motors during muscle shortening and the coupling with the release of the ATP hydrolysis products. J. Physiol. 2015, 593, 3313–3332. [Google Scholar] [CrossRef]
- Yanagida, T.; Ishijima, A. Forces and Steps Generated by Single Myosin Molecules. Biophys. J. 1995, 68 (Suppl. 4), 312S–320S. [Google Scholar]
- Ishijima, A.; Kojima, H.; Higuchi, H.; Harada, Y.; Funatsu, T.; Yanagida, T. Multiple- and single-molecule analysis of the actomyosin motor by nanometer-piconewton manipulation with a microneedle: Unitary steps and forces. Biophys. J. 1996, 70, 383–400. [Google Scholar] [CrossRef]
- Kaya, M.; Higuchi, H. Nonlinear elasticity and an 8-nm working stroke of single myosin molecules in myofilaments. Science 2010, 329, 686–689. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishijima, A.; Honda, M.; Saito, K.; Yanagida, T. Orientation dependence of displacements by a single one-headed myosin relative to the actin filament. Biophys. J. 1998, 75, 1886–1894. [Google Scholar] [CrossRef]
- Nyitrai, M.; Geeves, M.A. Adenosine diphosphate and strain sensitivity in myosin motors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1867–1877. [Google Scholar]
- Piazzesi, G.; Reconditi, M.; Linari, M.; Lucii, L.; Bianco, P.; Brunello, E.; Decostre, V.; Stewart, A.; Gore, D.B.; Irving, T.C.; et al. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 2007, 131, 784–795. [Google Scholar] [CrossRef]

| T0 | 1/3 T0 | |
|---|---|---|
| E′ (mW g−1) | 11.2 | 45.0 |
| P (mW g−1) | 0 | 22.0 (Pmax) |
| ATPase rate (μmol s−1 g−1) | 0.23 | 0.90 |
| φ (s−1) | 1.1 | 4.3 |
| E′0 (mW g−1) | E′Pmax (mW g−1) | Pmax (mW g−1) | ε, η | φ0 (s−1) | φPmax (s−1) | φPmax/φ0 | ||
|---|---|---|---|---|---|---|---|---|
| Mouse EDL, intact [26] | 21 °C | 134 | 214 | 57.2 | 0.28 | 12.4 | 19.8 | 1.6 |
| Mouse EDL, intact [27] | 25 °C | 144 | 269 | 70 | 0.26 | 13.3 | 25 | 1.87 |
| Rat IIB, skinned [28] | 12 °C | 15 | 40.2 | 9.59 | 0.24 | 1.39 | 3.7 | 2.7 |
| Rabbit psoas, skinned [29] | 15 °C | 2.1 | 6 | 2.8 | ||||
| Rabbit psoas, skinned [30] | 10 °C | 11.4 | 30 | (10) | 0.33 | 1.05 | 2.77 | 2.6 |
| Human IIA, skinned [31] | 20 °C | 48 | 96 | 30 | 0.31 | 4.4 | 8.89 | 2 |
| N = 294 | F0 (pN) | r0 | φ0 (s−1) | a/F0 | V0 (µm s−1) | Pmax (aW) | φPmax (s−1) |
|---|---|---|---|---|---|---|---|
| Model 1 | 433 ± 5 | 0.32 | 11.65 | 0.36 | 8.61 ± 0.16 | 462 | 35.5 |
| Model 2 | 418 ± 6 | 0.31 | 11.71 | 0.22 | 6.02 ± 0.12 | 220 | 31.9 |
| Model 3 | 445 ± 9 | 0.33 | 11.41 | 0.39 | 8.79 ± 0.14 | 474 | 67.2 |
| N = 294 | F0 (pN) | r0 | φ0 (s−1) | a/F0 | V0 (µm s−1) | Pmax (aW) | φPmax (s−1) |
|---|---|---|---|---|---|---|---|
| Model 1 | 336 ± 12 | 0.24 | 2.30 | 0.34 | 2.60 ± 0.07 | 98.5 | 8.63 |
| Model 2 | 291 ± 23 | 0.23 | 2.10 | 0.14 | 2.25 ± 0.03 | 45.0 | 8.45 |
| Model 3 | 342 ± 21 | 0.24 | 2.40 | 0.30 | 2.67 ± 0.11 | 96.0 | 23.1 |
| F0 (pN) | r0 | φ0 (s−1) | a/F0 | V0 (µm s−1) | Pmax (aW) | φPmax (s−1) | |
|---|---|---|---|---|---|---|---|
| N = 294 Compliance 0.01 nm pN−1 | 433 ± 5 | 0.32 | 11.65 | 0.36 | 8.61 ± 0.16 | 462 | 35.50 |
| N = 16 Compliance 3.7 nm pN−1 + random | 15.8 ± 0.4 | 0.40 | 18.21 | 0.24 | 3.45 ± 0.13 | 5.45 | 26.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertici, I.; Bongini, L.; Caremani, M.; Reconditi, M.; Linari, M.; Piazzesi, G.; Lombardi, V.; Bianco, P. Matching Mechanics and Energetics of Muscle Contraction Suggests Unconventional Chemomechanical Coupling during the Actin–Myosin Interaction. Int. J. Mol. Sci. 2023, 24, 12324. https://doi.org/10.3390/ijms241512324
Pertici I, Bongini L, Caremani M, Reconditi M, Linari M, Piazzesi G, Lombardi V, Bianco P. Matching Mechanics and Energetics of Muscle Contraction Suggests Unconventional Chemomechanical Coupling during the Actin–Myosin Interaction. International Journal of Molecular Sciences. 2023; 24(15):12324. https://doi.org/10.3390/ijms241512324
Chicago/Turabian StylePertici, Irene, Lorenzo Bongini, Marco Caremani, Massimo Reconditi, Marco Linari, Gabriella Piazzesi, Vincenzo Lombardi, and Pasquale Bianco. 2023. "Matching Mechanics and Energetics of Muscle Contraction Suggests Unconventional Chemomechanical Coupling during the Actin–Myosin Interaction" International Journal of Molecular Sciences 24, no. 15: 12324. https://doi.org/10.3390/ijms241512324
APA StylePertici, I., Bongini, L., Caremani, M., Reconditi, M., Linari, M., Piazzesi, G., Lombardi, V., & Bianco, P. (2023). Matching Mechanics and Energetics of Muscle Contraction Suggests Unconventional Chemomechanical Coupling during the Actin–Myosin Interaction. International Journal of Molecular Sciences, 24(15), 12324. https://doi.org/10.3390/ijms241512324









